Comprehensive Volatilome Signature of Various Brassicaceae Species
Abstract
1. Introduction
2. Results and Discussion
2.1. Volatile Compounds from the Mevalonic Acid (MVA) and Methylerythritol Phosphate (MEP) Pathways
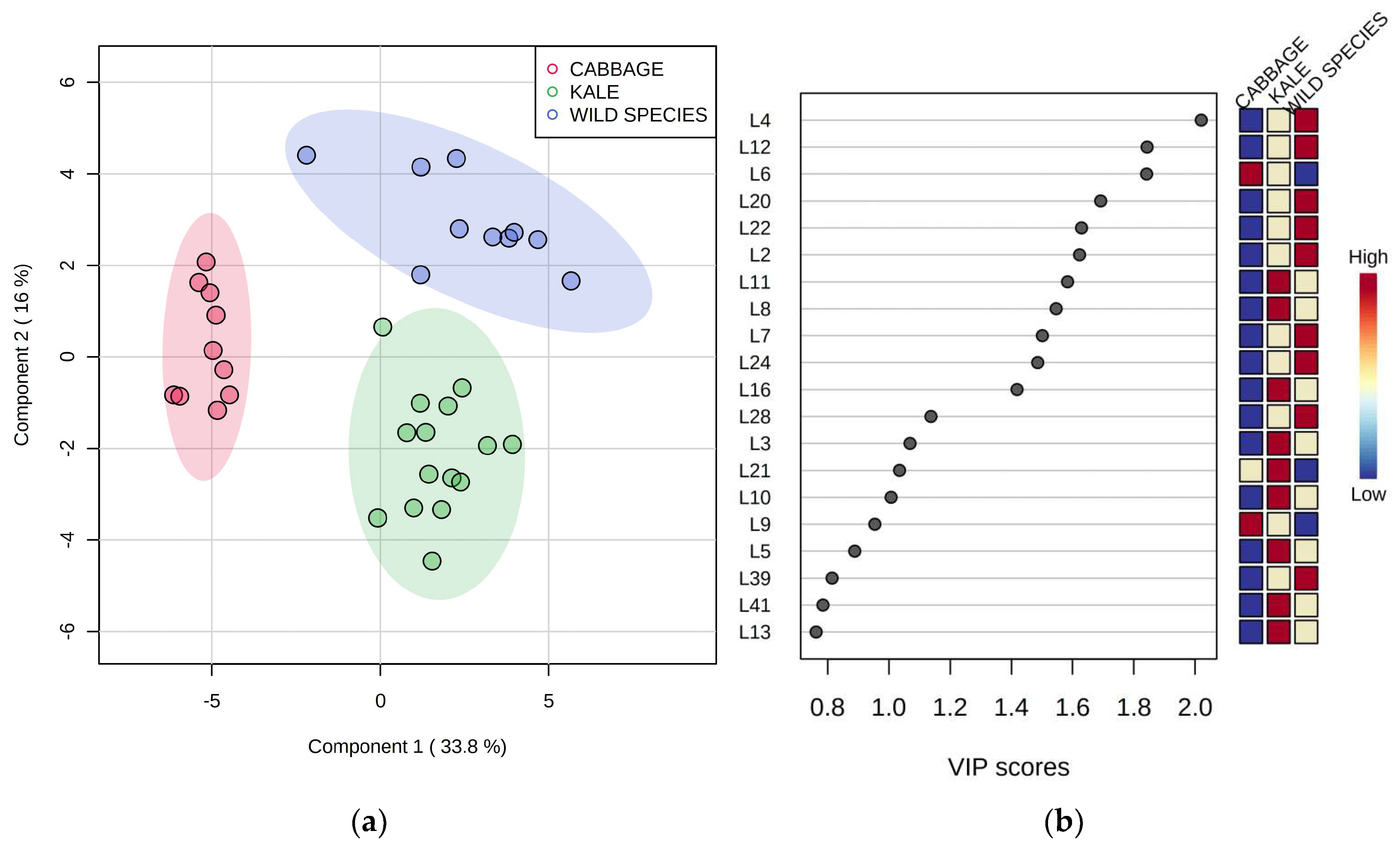
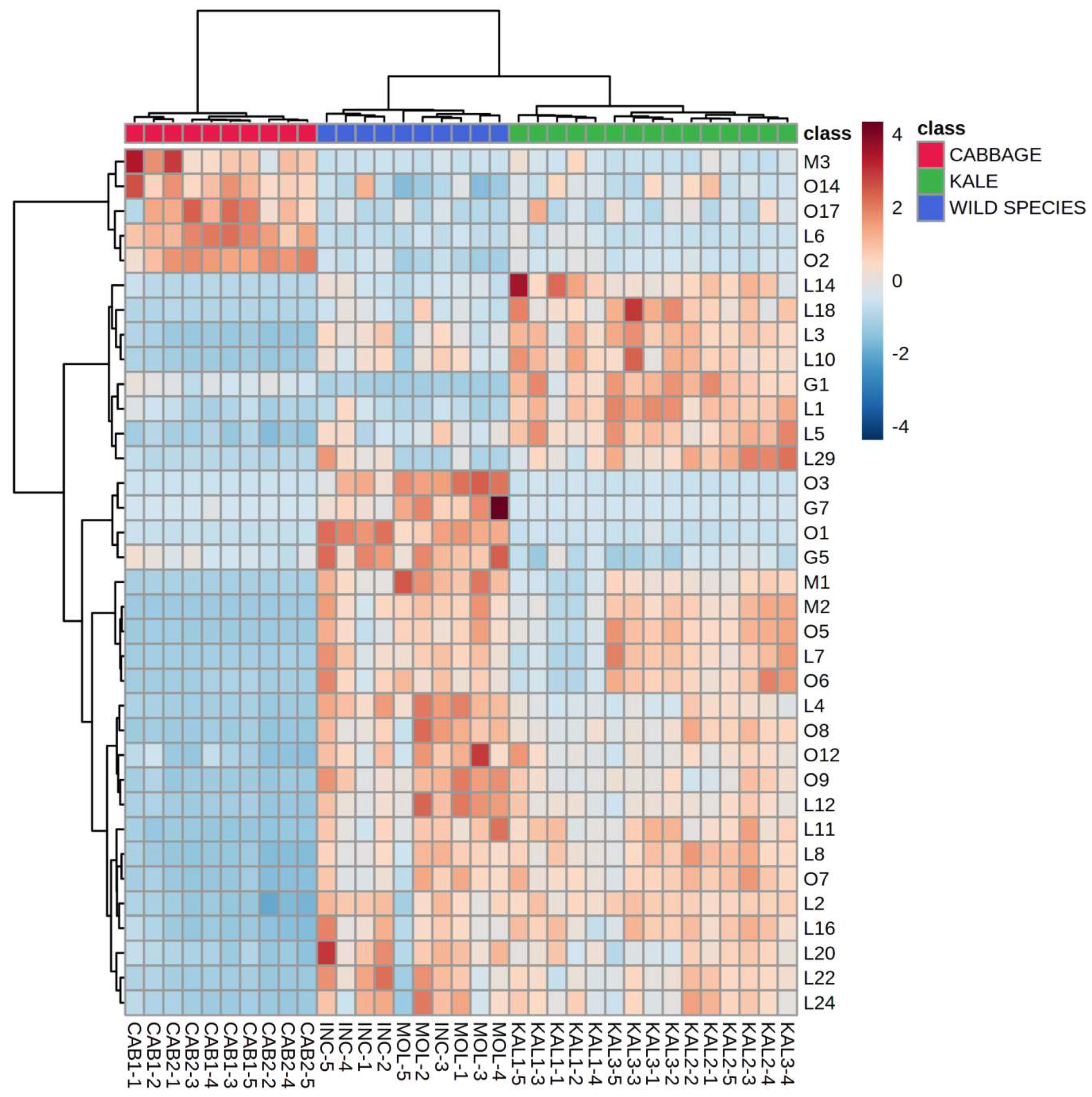
2.2. Volatile Compounds from the Lipoxygenase (LOX) Pathway
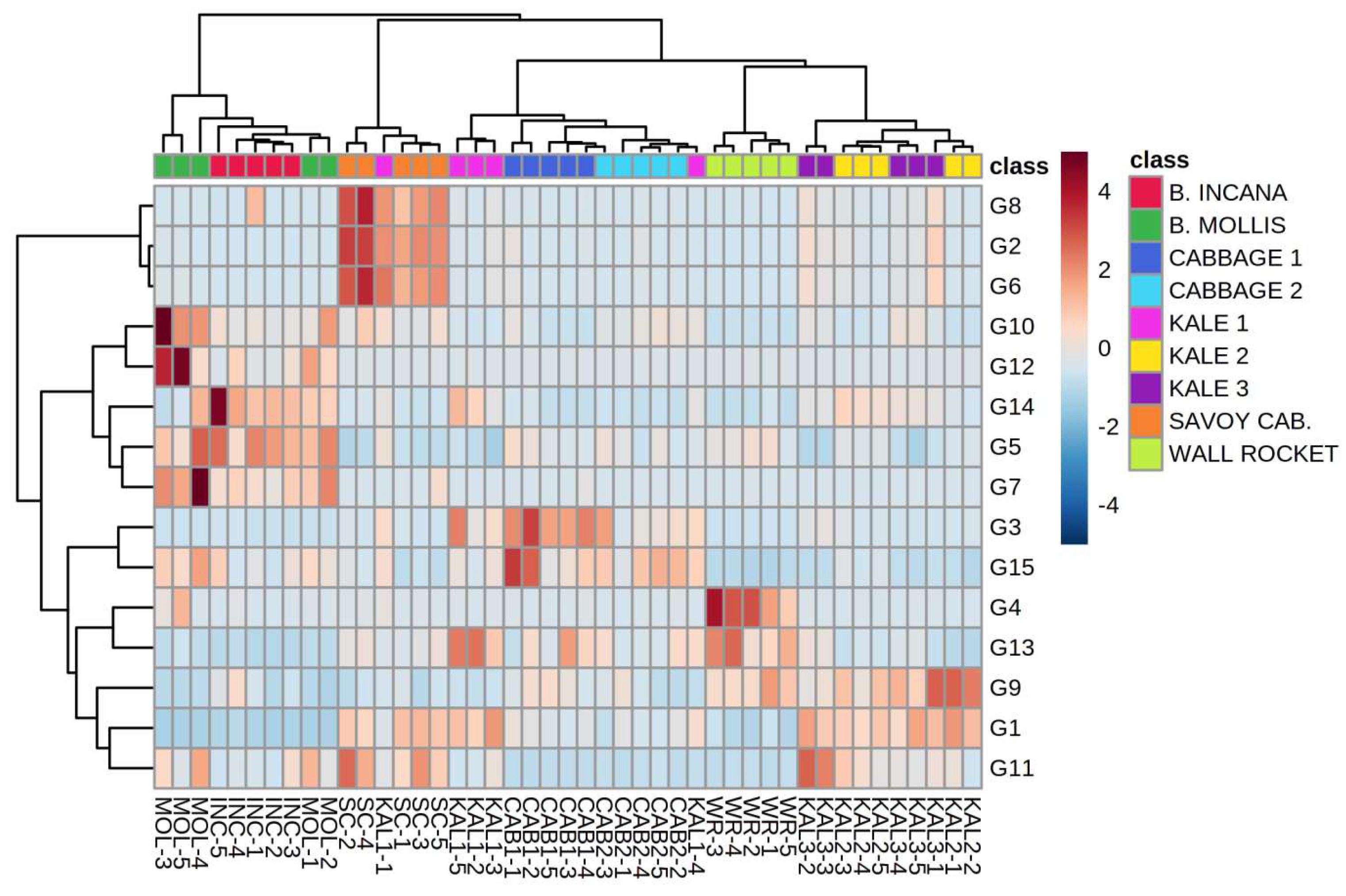
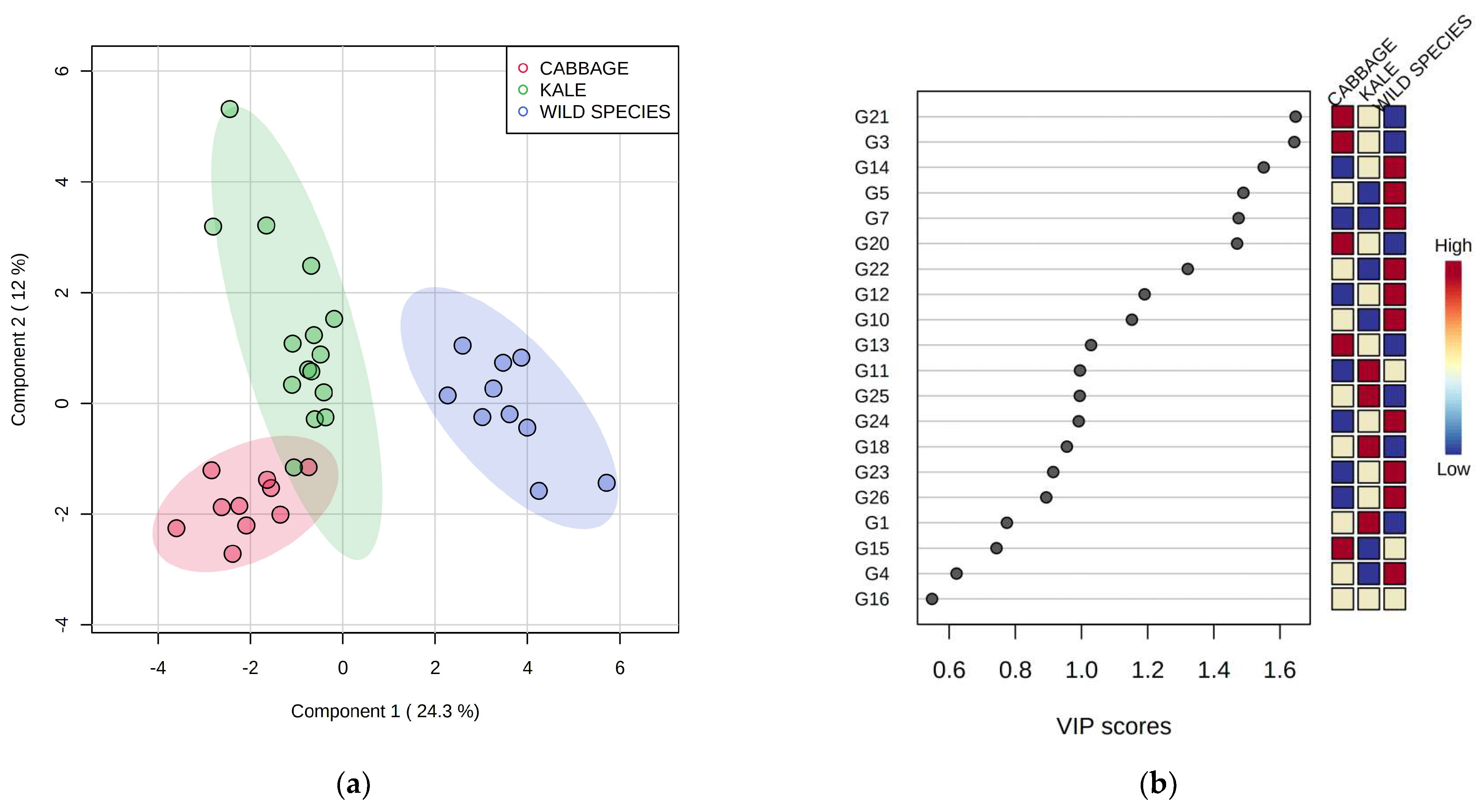
2.3. Volatile Compounds from the Glucosinolate (GSL) Pathway
2.4. Other Volatile Compounds
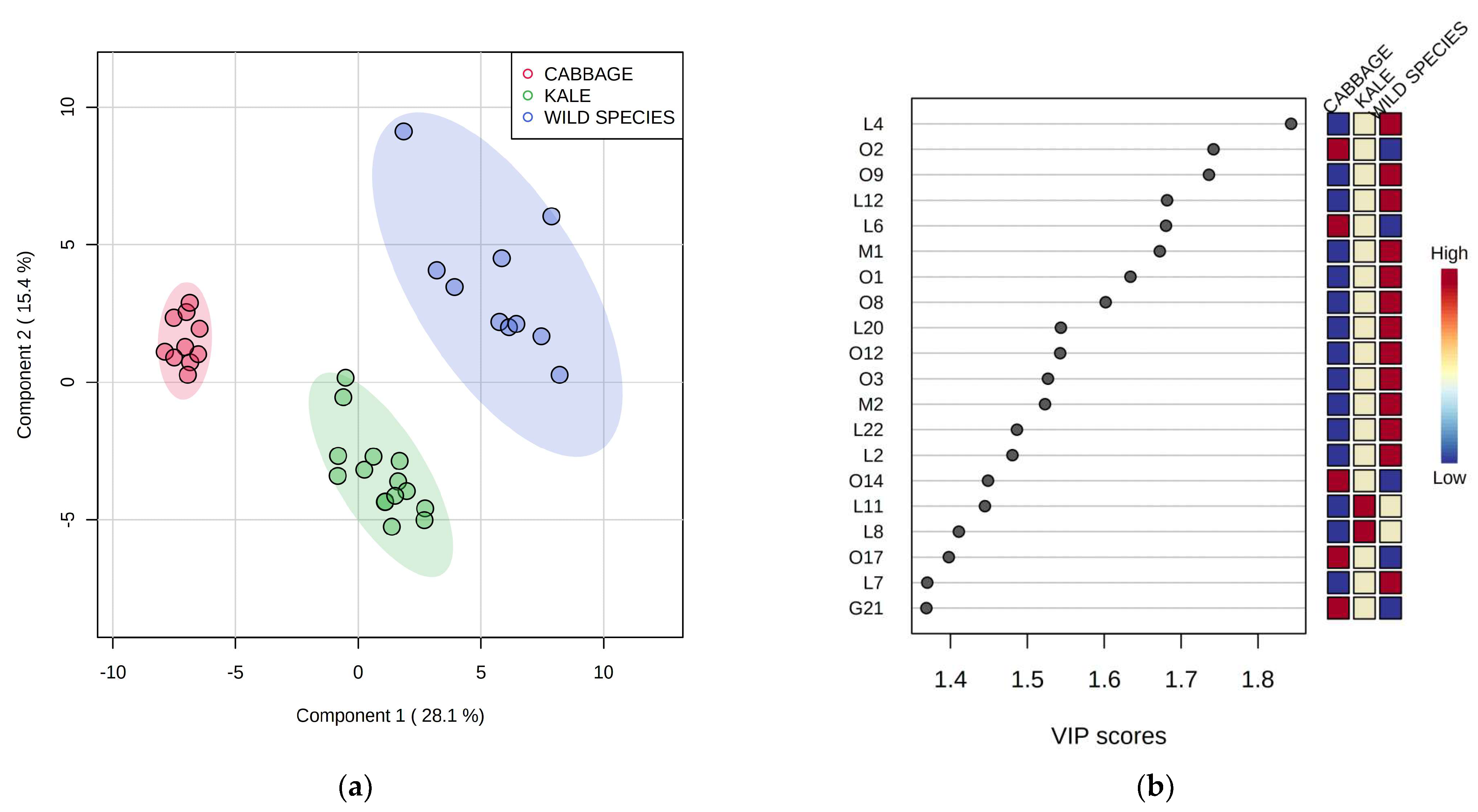
2.5. Differentiation Based on the Overall Volatilome
3. Materials and Methods
3.1. Brassicaceae Species, Landraces, and Accessions
3.2. Analysis of Volatile Compounds
3.3. Statistical Data Elaboration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katche, E.; Quezada-Martinez, D.; Katche, E.I.; Vasquez-Teuber, P.; Mason, A.S. Interspecific Hybridization for Brassica Crop Improvement. Crop Breed Genet Genom. 2019. [Google Scholar] [CrossRef]
- Rakow, G. Species Origin and Economic Importance of Brassica. In Biotechnology in Agriculture and Forestry; Pua, E.-C., Douglas, C.J., Eds.; Springer: Berlin, Germany, 2004; pp. 3–11. [Google Scholar] [CrossRef]
- Miceli, N.; Cavò, E.; Ragusa, M.; Cacciola, F.; Mondello, L.; Dugo, L.; Acquaviva, R.; Malfa, G.A.; Marino, A.; D’Arrigo, M.; et al. Brassica Incana Ten. (Brassicaceae): Phenolic Constituents, Antioxidant and Cytotoxic Properties of the Leaf and Flowering Top Extracts. Molecules 2020, 25, 1461. [Google Scholar] [CrossRef] [PubMed]
- Manchali, S.; Chidambara Murthy, K.N.; Patil, B.S. Crucial Facts about Health Benefits of Popular Cruciferous Vegetables. J. Funct. Foods 2012, 4, 94–106. [Google Scholar] [CrossRef]
- Singh, J.; Upadhyay, A.K.; Prasad, K.; Bahadur, A.; Rai, M. Variability of Carotenes, Vitamin C, E and Phenolics in Brassica Vegetables. J. Food Compost. Anal. 2007, 20, 106–112. [Google Scholar] [CrossRef]
- Baldwin, I.T.; Halitschke, R.; Paschold, A.; von Dahl, C.C.; Preston, C.A. Volatile Signaling in Plant-Plant Interactions: “Talking Trees” in the Genomics Era. Science 2006, 311, 812–815. [Google Scholar] [CrossRef]
- Clavijo McCormick, A.; Unsicker, S.B.; Gershenzon, J. The Specificity of Herbivore-Induced Plant Volatiles in Attracting Herbivore Enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of Plant Volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef]
- Heil, M.; Silva Bueno, J.C. Within-Plant Signaling by Volatiles Leads to Induction and Priming of an Indirect Plant Defense in Nature. Proc. Natl. Acad. Sci. USA 2007, 104, 5467–5472. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Umashankar, S.; Liang, X.; Lee, H.W.; Swarup, S.; Ong, C.N. Characterization of Plant Volatiles Reveals Distinct Metabolic Profiles and Pathways among 12 Brassicaceae Vegetables. Metabolites 2018, 8, 94. [Google Scholar] [CrossRef]
- Unsicker, S.B.; Kunert, G.; Gershenzon, J. Protective Perfumes: The Role of Vegetative Volatiles in Plant Defense against Herbivores. Curr. Opin. Plant Biol. 2009, 12, 479–485. [Google Scholar] [CrossRef]
- Pasković, I.; Soldo, B.; Goreta Ban, S.; Radić, T.; Lukić, M.; Urlić, B.; Mimica, M.; Brkić Bubola, K.; Colla, G.; Rouphael, Y.; et al. Fruit Quality and Volatile Compound Composition of Processing Tomato as Affected by Fertilisation Practices and Arbuscular Mycorrhizal Fungi Application. Food Chem. 2021, 359, 129961. [Google Scholar] [CrossRef] [PubMed]
- Hinge, V.R.; Shaikh, I.M.; Chavhan, R.L.; Deshmukh, A.S.; Shelake, R.M.; Ghuge, S.A.; Dethe, A.M.; Suprasanna, P.; Kadam, U.S. Assessment of Genetic Diversity and Volatile Content of Commercially Grown Banana (Musa spp.) Cultivars. Sci. Rep. 2022, 12, 7979. [Google Scholar] [CrossRef] [PubMed]
- Pare, P.; Tumlinson, J. Plant Volatiles as a Defense against Insect Herbivores. Plant Physiol. 1999, 121, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Veeranki, O.L.; Bhattacharya, A.; Tang, L.; Marshall, J.R.; Zhang, Y. Cruciferous Vegetables, Isothiocyanates, and Prevention of Bladder Cancer. Curr. Pharmacol. Rep. 2015, 1, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Blažević, I.; Mastelić, J. Free and Bound Volatiles of Rocket (Eruca sativa Mill.). Flavour Fragr. J. 2008, 23, 278–285. [Google Scholar] [CrossRef]
- Melchini, A.; Traka, M.H. Biological Profile of Erucin: A New Promising Anticancer Agent from Cruciferous Vegetables. Toxins 2010, 2, 593–612. [Google Scholar] [CrossRef]
- Ragaert, P.; Verbeke, W.; Devlieghere, F.; Debevere, J. Consumer Perception and Choice of Minimally Processed Vegetables and Packaged Fruits. Food Qual. Prefer. 2004, 15, 259–270. [Google Scholar] [CrossRef]
- Lončarić, A.; Marček, T.; Šubarić, D.; Jozinović, A.; Babić, J.; Miličević, B.; Sinković, K.; Šubarić, D.; Ačkar, Đ. Comparative Evaluation of Bioactive Compounds and Volatile Profile of White Cabbages. Molecules 2020, 25, 3696. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Schreiner, M. Isothiocyanates, Nitriles, and Epithionitriles from Glucosinolates Are Affected by Genotype and Developmental Stage in Brassica Oleracea Varieties. Front. Plant. Sci. 2017, 8, 1095. [Google Scholar] [CrossRef]
- Rajkumar, G.; Shanmugam, S.; de Galvâo, M.S.; Dutra Sandes, R.D.; Leite Neta, M.T.S.; Narain, N.; Mujumdar, A.S. Comparative Evaluation of Physical Properties and Volatiles Profile of Cabbages Subjected to Hot Air and Freeze Drying. LWT 2017, 80, 501–509. [Google Scholar] [CrossRef]
- Raffo, A.; Masci, M.; Moneta, E.; Nicoli, S.; Sánchez Del Pulgar, J.; Paoletti, F. Characterization of Volatiles and Identification of Odor-Active Compounds of Rocket Leaves. Food Chem. 2018, 240, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Kitsopanou, E.; Oloyede, O.O.; Lignou, S. Important Odorants of Four Brassicaceae Species, and Discrepancies between Glucosinolate Profiles and Observed Hydrolysis Products. Foods 2021, 10, 1055. [Google Scholar] [CrossRef] [PubMed]
- Luca, A.; Mahajan, P.V.; Edelenbos, M. Changes in Volatile Organic Compounds from Wild Rocket (Diplotaxis tenuifolia L.) during Modified Atmosphere Storage. Postharvest Biol. Technol. 2016, 114, 1–9. [Google Scholar] [CrossRef]
- Vincenti, S.; Mariani, M.; Alberti, J.-C.; Jacopini, S.; Brunini-Bronzini de Caraffa, V.; Berti, L.; Maury, J. Biocatalytic Synthesis of Natural Green Leaf Volatiles Using the Lipoxygenase Metabolic Pathway. Catalysts 2019, 9, 873. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant Volatiles: Recent Advances and Future Perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Nagegowda, D.A. Plant Volatile Terpenoid Metabolism: Biosynthetic Genes, Transcriptional Regulation and Subcellular Compartmentation. FEBS Lett. 2010, 584, 2965–2973. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, Function and Metabolic Engineering of Plant Volatile Organic Compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Rohdich, F.; Zepeck, F.; Adam, P.; Hecht, S.; Kaiser, J.; Laupitz, R.; Gräwert, T.; Amslinger, S.; Eisenreich, W.; Bacher, A.; et al. The Deoxyxylulose Phosphate Pathway of Isoprenoid Biosynthesis: Studies on the Mechanisms of the Reactions Catalyzed by IspG and IspH Protein. Proc. Natl. Acad. Sci. USA 2003, 100, 1586–1591. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of Plant-Derived Flavor Compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Fernandes, F.; Guedes de Pinho, P.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Volatile Constituents throughout Brassica oleracea L. Var. Acephala Germination. J. Agric. Food Chem. 2009, 57, 6795–6802. [Google Scholar] [CrossRef]
- ul Hassan, M.N.; Zainal, Z.; Ismail, I. Green Leaf Volatiles: Biosynthesis, Biological Functions and Their Applications in Biotechnology. Plant Biotechnol. J. 2015, 13, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, S.; Sugimoto, K.; Koeduka, T.; Matsui, K. Arabidopsis Lipoxygenase 2 Is Essential for Formation of Green Leaf Volatiles and Five-Carbon Volatiles. FEBS Lett. 2016, 590, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Kissen, R.; Rossiter, J.; Bones, A. The “Mustard Oil Bomb”: Not so Easy to Assemble?! Localization, Expression and Distribution of the Components of the Myrosinase Enzyme System. Phytochem. Rev. 2009, 8, 69–86. [Google Scholar] [CrossRef]
- Bell, L.; Wagstaff, C. Enhancement of Glucosinolate and Isothiocyanate Profiles in Brassicaceae Crops: Addressing Challenges in Breeding for Cultivation, Storage, and Consumer-Related Traits. J. Agric. Food Chem. 2017, 65, 9379–9403. [Google Scholar] [CrossRef]
- Matusheski, N.V.; Swarup, R.; Juvik, J.A.; Mithen, R.; Bennett, M.; Jeffery, E.H. Epithiospecifier Protein from Broccoli (Brassica oleracea L. ssp. Italica) Inhibits Formation of the Anticancer Agent Sulforaphane. J. Agric. Food Chem. 2006, 54, 2069–2076. [Google Scholar] [CrossRef] [PubMed]

| Code | Coumpound Class and Name | LRI-Exp | LRI-Lit | F-Ratio | CAB1 | CAB2 | KAL1 | KAL2 | KAL3 | SC | INC | MOL | WR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mevalonic acid (MVA) and methylerythritol phosphate (MEP) pathways volatile compounds | |||||||||||||
| M1 | β-Ionone | 1940 | 1952 | 26.80 | 11.4 d | 14.2 d | 111.8 cd | 298.2 b | 315.4 b | 178.6 c | 347.6 b | 578.2 a | 367.2 b |
| M2 | 5,6-Epoxy-β-ionone | 1992 | 1989 | 15.71 | 2.1 c | 2.5 c | 39.4 b | 93.4 a | 99.4 a | 55.8 b | 85.1 a | 98.9 a | 93.4 a |
| M3 | Isophorone | 1594 | 1593 | 8.91 | 169.5 a | 129.1 a | 43.8 b | 16.2 b | 9.8 b | 123.7 a | 10.9 b | 6.0 b | 10.3 b |
| M4 | Perhydrofarnesyl acetone | 2133 | 2129 | 8.31 | 1.6 bc | 1.3 bc | 7.7 a | 0.9 bc | 1.5 bc | 0.7 c | 2.6 bc | 3.4 b | 7.2 a |
| M5 | Eucalyptol | 1210 | 1209 | 1.21 | 7.0 | 5.0 | 1.1 | 5.6 | 3.6 | 0.7 | 0.6 | 4.2 | 1.5 |
| M6 | Lilial | 2045 | n.a. | 0.89 | 3.1 | 2.8 | 3.0 | 2.0 | 2.5 | 2.0 | 2.4 | 2.5 | 2.0 |
| Lipoxygenase (LOX) pathway volatile compounds | |||||||||||||
| L1 | cis-3-Hexen-1-ol | 1385 | 1386 | 43.53 | 42,875 de | 23,776 e | 137,317 b | 142,105 b | 206,377 a | 144,606 b | 63,475 cd | 25,835 e | 73,140 c |
| L2 | 1-Penten-3-ol | 1153 | 1155 | 40.19 | 512 e | 369 e | 1143 bc | 1186 ab | 1260 ab | 1240 ab | 1332 a | 988 cd | 885 d |
| L3 | trans-2-Penten-1-ol | 1309 | 1307 | 29.75 | 23.5 d | 10.4 d | 142.6 ab | 144.6 ab | 164.5 a | 171.9 a | 122.4 b | 70.1 c | 66.0 c |
| L4 | trans-2-Butenal | 1045 | 1037 | 29.21 | 2.8 d | 1.6 d | 11.8 c | 18.2 b | 10.9 c | 11.0 c | 26.2 a | 27.3 a | 19.0 b |
| L5 | 3,5,5-Trimethyl-2-hexene | 1482 | n.a. | 28.35 | 6.4 ef | 4.7 f | 18.9 bc | 19.2 bc | 22.4 b | 17.1 bcd | 14.1 cd | 11.8 de | 39.8 a |
| L6 | 2-Ethyl-1-hexanol | 1487 | 1486 | 27.56 | 158.0 a | 139.9 a | 38.3 bc | 17.5 c | 19.6 bc | 23.8 bc | 11.7 c | 16.5 c | 49.6 b |
| L7 | 6-Methyl-5-hepten-2-ol | 1620 | n.a. | 23.37 | 0.5 e | 0.5 e | 6.1 de | 21.9 c | 29.8 ab | 13.2 d | 23.3 bc | 21.1 c | 33.0 a |
| L8 | trans,cis-2,4-Heptadienal | 1462 | 1460 | 22.00 | 102.1 c | 45.9 c | 448.4 b | 615.1 a | 490.6 b | 534.8 ab | 468.0 b | 481.4 b | 531.4 ab |
| L9 | 3,5,5-Trimethyl-3-cyclohexanone | 1411 | 1410 | 19.95 | 8.5 b | 4.2 bcd | 6.9 bc | 1.1 cd | 1.0 cd | 29.7 a | 0.5 d | 0.3 d | 0.2 d |
| L10 | trans-3-Penten-2-one | 1120 | 1121 | 19.10 | 1.4 e | 0.8 e | 13.9 b | 11.9 b | 13.2 b | 21.1 a | 9.6 bc | 6.5 cd | 3.7 de |
| L11 | 3,4-Nonadiene | 2054 | n.a. | 18.92 | 3.2 c | 2.9 c | 31.2 b | 32.3 b | 35.9 ab | 43.7 a | 29.9 b | 37.4 ab | 7.7 c |
| L12 | trans,trans-2,4-Heptadienal | 1491 | 1487 | 18.52 | 204 d | 101 d | 1019 bc | 1100 bc | 897 bc | 1186 b | 1151 b | 1828 a | 795 c |
| L13 | 2-Methyl-2-pentenal | <1000 | 982 | 17.94 | 3.8 d | 0.6 d | 18.6 d | 74.2 b | 51.8 bc | 16.2 d | 48.6 c | 12.1 d | 110.3 a |
| L14 | 3-Ethyl-1,5-octadiene | 1037 | 1027 | 15.27 | 2.4 e | 0.0 e | 79.6 a | 52.9 b | 29.9 cd | 49.5 bc | 19.6 de | 10.8 de | 3.5 e |
| L15 | trans-3-Hexen-1-ol | 1361 | 1358 | 15.06 | 1910 b | 1893 b | 16,505 b | 2336 b | 9488 b | 70,947 a | 716 b | 2624 b | 2347 b |
| L16 | cis-4-Heptenal | 1240 | 1240 | 13.83 | 2.6 c | 1.4 c | 8.6 ab | 10.4 a | 9.0 ab | 10.5 a | 10.1 a | 6.9 b | 8.0 ab |
| L17 | Alkene, n.i. m/z 55, 70, 41 | 1246 | n.a. | 13.55 | 28.0 c | 33.7 bc | 39.0 b | 34.4 bc | 44.2 ab | 53.5 a | 27.6 c | 16.7 d | 8.4 d |
| L18 | 3-Pentanone | 1002 | 1002 | 13.10 | 29.5 c | 28.8 c | 434.2 b | 411.8 b | 739.6 a | 472.8 b | 198.3 c | 196.7 c | 432.4 b |
| L19 | Hexanoic acid | 1846 | 1848 | 12.85 | 14.0 d | 23.3 d | 44.6 bc | 83.2 a | 57.0 b | 56.6 b | 47.2 b | 28.7 cd | 20.7 d |
| L20 | trans-2-Hexenal | 1218 | 1219 | 12.08 | 38,717 ef | 30,052 f | 72,222 bcd | 85,546 b | 56,715 de | 63,388 cd | 110,718 a | 81,749 bc | 89,090 b |
| L21 | 1-Hexanol | 1351 | 1353 | 11.76 | 6266 cd | 10,344 b | 9036 bc | 4689 de | 15,905 a | 10,719 b | 1788 ef | 375 f | 5176 cde |
| L22 | trans-2-Pentenal | 1123 | 1121 | 11.67 | 67.5 d | 36.6 d | 283.0 c | 414.8 ab | 309.3 bc | 369.6 bc | 530.1 a | 317.9 bc | 264.5 c |
| L23 | cis-3-Hexenal | 1132 | 1134 | 10.70 | 945 a | 299 bcde | 282 cde | 285 cde | 147 e | 166 de | 498 b | 359 bcd | 425 bc |
| L24 | 1-Penten-3-one | 1031 | 1031 | 10.57 | 331 d | 185 d | 1654 ab | 2194 a | 1274 bc | 2367 a | 2075 a | 1765 ab | 719 cd |
| L25 | Nonanoic acid | 2164 | 2168 | 9.30 | 11.0 b | 6.7 bc | 3.9 bc | 27.8 a | 3.6 c | 2.9 c | 2.8 c | 4.7 bc | 5.4 bc |
| L26 | trans-2-Hexen-1-ol | 1403 | 1406 | 8.86 | 352.6 cd | 286.5 cd | 2218.1 b | 1096.6 bcd | 3917.1 a | 1343.3 bc | 706.7 cd | 97.4 d | 874.0 cd |
| L27 | 5-Methyl-3-heptene | 1114 | n.a. | 8.33 | 0.7 c | 0.3 c | 1.5 bc | 2.4 b | 1.1 bc | 2.3 b | 0.5 c | 0.5 c | 4.8 a |
| L28 | cis-2-Hexenal | 1198 | 1196 | 7.16 | 315 cd | 252 d | 376 cd | 447 bc | 257 d | 296 d | 651 a | 374 cd | 529 ab |
| L29 | trans-3-Hexenoic acid | 1947 | 1948 | 7.13 | 0.5 d | 0.4 d | 3.4 cd | 8.5 ab | 6.4 bc | 2.9 cd | 4.2 cd | 0.6 d | 10.6 a |
| L30 | trans-6-Nonenal | 1535 | 1535 | 6.75 | 4.0 bc | 3.8 bc | 6.0 a | 4.8 abc | 3.7 bcd | 5.1 ab | 1.7 e | 3.5 cd | 2.3 de |
| L31 | Octanal | 1288 | 1289 | 6.54 | 1.3 bc | 2.4 ab | 3.6 a | 0.8 c | 3.5 a | 0.9 c | 0.8 c | 0.8 c | 3.4 a |
| L32 | trans-2-Octenal | 1429 | 1427 | 6.35 | 26.2 bcd | 19.7 cd | 42.5 a | 27.9 bc | 23.1 cd | 36.1 ab | 15.8 de | 26.8 bcd | 7.4 e |
| L33 | trans,trans-2,4-Hexadienal | 1395 | 1391 | 5.19 | 220 bc | 195 bcd | 241 bc | 176 cd | 183 cd | 126 d | 318 a | 180 cd | 254 ab |
| L34 | Heptanal | 1182 | 1179 | 5.08 | 5.6 d | 6.2 d | 9.7 ab | 7.6 bcd | 6.8 cd | 12.2 a | 7.4 bcd | 5.7 d | 9.2 bc |
| L35 | cis-2-Heptenal | 1323 | 1322 | 4.46 | 27.4 cd | 37.1 bcd | 53.5 ab | 54.0 ab | 58.3 a | 44.6 abc | 42.2 abc | 52.4 ab | 19.3 d |
| L36 | cis-3-Hexenyl isovalerate | 1475 | 1480 | 4.30 | 0.0 b | 0.0 b | 2.4 b | 1.1 b | 7.5 a | 0.9 b | 2.3 b | 0.0 b | 0.9 b |
| L37 | trans,trans-2,4-Nonadienal | 1714 | 1712 | 3.77 | 8.3 b | 10.5 b | 15.9 b | 13.4 b | 11.2 b | 15.8 b | 8.1 b | 15.6 b | 106.9 a |
| L38 | Hexanal | 1080 | 1079 | 3.57 | 12,152 bc | 20,079 a | 13,801 abc | 15,286 abc | 13,547 bc | 15,379 ab | 16,562 ab | 8898 cd | 5543 d |
| L39 | 2,2-Dimethyl-3-hexene | 1515 | n.a. | 3.40 | 4.8 b | 4.2 b | 8.0 b | 7.7 b | 4.6 b | 5.6 b | 10.4 b | 5.2 b | 18.8 a |
| L40 | trans-3-Hexenal | 1138 | 1138 | 2.82 | 1383 abc | 1377 abc | 1057 bc | 825 bc | 433 c | 560 c | 2177 a | 684 bc | 1534 ab |
| L41 | cis-2-Penten-1-ol | 1316 | 1318 | 2.56 | 1498 b | 1984 b | 13,820 a | 10,297 ab | 13,541 a | 7816 ab | 14,686 a | 5781 ab | 4044 b |
| L42 | cis-2-Pentenal | 1100 | 1105 | 1.42 | 7.5 | 5.7 | 24.7 | 31.8 | 81.4 | 27.4 | 57.6 | 22.3 | 26.2 |
| L43 | trans,cis-2,4-Hexadienal | 1401 | 1395 | 1.35 | 475 | 412 | 438 | 498 | 653 | 362 | 790 | 498 | 554 |
| Total LOX compounds | 108,713 de | 92,044 e | 272,937 b | 270,227 b | 326,842 a | 322,559 a | 219,390 c | 133,442 d | 187,727 c | ||||
| Glucosinolate pathway (GSL) volatile compounds | |||||||||||||
| G1 | Methyl thiocyanate | 1269 | 1274 | 29.73 | 129.0 b | 104.6 bc | 252.0 a | 292.1 a | 308.8 a | 279.9 a | 14.9 d | 0.0 d | 44.4 cd |
| G2 | 4-Methylthiazole | 1282 | 1287 | 21.42 | 8.5 bc | 6.1 bc | 34.2 b | 8.0 bc | 34.2 b | 160.7 a | 0.0 c | 3.7 bc | 0.1 c |
| G3 | 4-Methylpentanenitrile | 1243 | 1253 | 21.06 | 416.1 a | 149.4 b | 200.9 b | 40.1 c | 41.7 c | 24.2 c | 8.0 c | 3.2 c | 8.1 c |
| G4 | 4-Methylpentyl isothiocyanate | 1544 | 1533 | 18.96 | 2.2 b | 1.6 b | 3.5 b | 0.6 b | 1.6 b | 2.7 b | 1.9 b | 12.6 b | 77.7 a |
| G5 | 4-Mercaptophenol | 1897 | n.a. | 17.32 | 1.8 b | 1.6 bc | 1.2 bcd | 1.5 bc | 0.8 d | 0.8 cd | 3.8 a | 3.6 a | 1.9 b |
| G6 | Allyl isothiocyanate | 1359 | 1353 | 14.83 | 615.9 bc | 403.3 bc | 3962.4 b | 754.1 bc | 3228.0 bc | 15,951.0 a | 20.8 c | 597.0 bc | 5.3 c |
| G7 | 2,4-Pentadienenitrile | 1262 | n.a. | 14.34 | 1.9 c | 0.0 c | 0.0 c | 0.0 c | 0.0 c | 4.9 c | 27.7 b | 90.3 a | 0.5 c |
| G8 | Isothiocyanate derivative n.i. m/z 99, 41, 39, 72 | 1449 | n.a. | 13.25 | 4.4 b | 5.3 b | 23.7 b | 5.9 b | 18.7 b | 102.8 a | 13.5 b | 2.9 b | 2.3 b |
| G9 | 1-Allyl-1-cyclohexanol | 1571 | n.a. | 9.64 | 7.9 b | 5.0 bc | 4.0 bc | 16.6 a | 13.9 a | 3.8 bc | 5.7 bc | 1.9 c | 12.8 a |
| G10 | Benzenepropanenitrile | 2034 | 2041 | 8.68 | 210.1 b | 637.4 b | 476.6 b | 93.1 b | 625.2 b | 824.2 b | 704.1 b | 2864.8 a | 16.8 b |
| G11 | Unsatur. aliph. thiol, n.i. m/z 41, 68, 69, 39 | 1555 | n.a. | 8.35 | 3.3 e | 5.1 e | 17.3 de | 32.1 cd | 56.4 ab | 70.3 a | 18.5 de | 45.3 bc | 4.6 e |
| G12 | 3-Butenyl isothiocyanate | 1455 | 1453 | 8.25 | 6.5 b | 1.5 b | 79.8 b | 7.2 b | 135.2 b | 286.4 b | 9516.0 b | 74,056.8 a | 163.8 b |
| G13 | Carbon disulfide | <1000 | 735 | 8.13 | 65.5 bc | 46.6 cd | 103.4 ab | 16.0 de | 39.3 cde | 47.1 cd | 5.9 e | 13.0 de | 115.2 a |
| G14 | 2-Ethylthiophene | 1171 | 1173 | 8.03 | 52.2 c | 38.9 c | 217.4 b | 177.0 bc | 155.9 bc | 65.2 c | 502.3 a | 216.7 b | 37.2 c |
| G15 | Hexanenitrile | 1297 | 1303 | 7.91 | 32.6 a | 25.6 ab | 16.8 bcd | 7.2 def | 4.0 ef | 6.9 def | 13.5 cde | 23.8 abc | 1.7 f |
| G16 | Erucin | 2137 | 2132 | 7.80 | 0.0 b | 0.0 b | 0.0 b | 0.0 b | 0.0 b | 0.0 b | 0.0 b | 47.3 b | 12,891.0 a |
| G17 | Cycl. sulfur compound, n.i. m/z 88, 116, 117, 60 | 1846 | n.a. | 7.50 | 0.2 b | 0.5 b | 0.0 b | 0.6 b | 0.9 b | 0.2 b | 0.0 b | 0.0 b | 196.9 a |
| G18 | 2-Methylbutyl isothiocyanate | 1423 | 1412 | 7.00 | 27.2 bcd | 28.0 bcd | 48.9 b | 14.9 cd | 31.5 bc | 79.2 a | 0.0 d | 0.1 d | 0.0 d |
| G19 | Iberverin | 1982 | 1979 | 6.62 | 14.4 b | 2.9 b | 23.8 b | 2.7 b | 11.7 b | 21.8 b | 0.4 b | 5.0 b | 300.0 a |
| G20 | 3-(Methylthio)butylnitrile | 1799 | 1806 | 5.36 | 206.7 a | 102.6 bc | 118.1 b | 91.7 bc | 60.6 bcd | 21.5 cd | 0.4 d | 3.4 d | 0.7 d |
| G21 | Allyl nitrile | 1173 | 1186 | 5.31 | 934.2 ab | 1096.5 a | 673.4 ab | 488.2 bc | 501.7 bc | 808.0 ab | 0.5 c | 3.8 c | 0.6 c |
| G22 | 4-(Methylthio)butylnitrile | 1937 | 1931 | 5.08 | 1.1 bc | 0.4 c | 0.8 c | 0.1 c | 0.4 c | 0.3 c | 16.0 a | 9.5 ab | 14.3 a |
| G23 | Phenethyl isothiocyanate | 2222 | 2216 | 4.29 | 0.7 b | 3.4 b | 28.6 b | 1.5 b | 25.6 b | 395.0 a | 17.3 b | 352.1 a | 23.1 b |
| G24 | Benzyl isothiocyanate | 2098 | 2107 | 3.88 | 15.1 b | 3.9 b | 60.4 b | 3.7 b | 16.4 b | 17.0 b | 64.5 b | 46.5 b | 377.8 a |
| G25 | Isothiocyanate derivative, n.i. m/z 99, 71, 72, 59 | 1837 | n.a. | 3.20 | 1010.9 abc | 1298.2 a | 1748.2 a | 1213.7 ab | 1184.1 ab | 1456.8 a | 19.1 c | 152.8 bc | 0.0 c |
| G26 | Benzyl nitrile | 1923 | 1927 | 2.76 | 94.5 b | 11.8 b | 533.1 a | 54.0 b | 92.4 b | 14.7 b | 570.7 a | 268.2 ab | 3.2 b |
| G27 | Dimethyl disulfide | 1073 | 1075 | 2.51 | 89.4 abc | 15.6 c | 237.1 a | 45.6 bc | 130.3 abc | 197.9 ab | 22.2 c | 6.6 c | 13.1 c |
| G28 | Isothiocyanatocyclopropane | 1229 | 1223 | 0.84 | 1839.5 | 7.5 | 54.4 | 10.3 | 52.8 | 254.9 | 4268.7 | 4.7 | 1135.1 |
| Total isothiocyanates (ITCs) | 3537 d | 1756 d | 6034 cd | 2015 d | 4706 d | 18,568 b | 13,922 bc | 75,278 a | 14,976 bc | ||||
| Total nitriles | 1897.1 bc | 2023.7 b | 2019.7 b | 774.5 c | 1326.0 bc | 1704.7 bc | 1341.0 bc | 3267.0 a | 46.0 d | ||||
| Total ITCs/total nitriles | 1.92 c | 0.88 c | 2.57 c | 2.31 c | 3.62 c | 11.43 c | 10.06 c | 31.09 b | 315.34 a | ||||
| Other compounds | |||||||||||||
| O1 | n.i. m/z 113, 45, 73, 86 | 1938 | 105.30 | 17.2 c | 7.3 c | 81.6 c | 26.1 c | 95.1 c | 50.0 c | 1848.5 a | 1267.1 b | 1.9 c | |
| O2 | 1-Butoxy-2-propanol | 1341 | 1364 | 77.16 | 28.0 b | 35.4 a | 11.9 c | 9.1 cd | 9.5 cd | 9.4 cd | 9.1 cd | 2.3 e | 6.9 d |
| O3 | N-isobutylidene cyclopropylamine | 1070 | na | 55.28 | 4.1 c | 2.9 c | 10.1 c | 1.3 c | 7.8 c | 4.0 c | 259.2 b | 471.4 a | 1.4 c |
| O4 | 2-Phenylethanol | 1907 | 1910 | 22.00 | 9.4 bc | 12.8 bc | 56.3 bc | 21.5 bc | 194.6 a | 240.5 a | 68.8 b | 232.9 a | 6.5 c |
| O5 | 1-Cyclohexene-1-carboxaldehyde | 1627 | 1631 | 21.83 | 1.4 e | 1.5 e | 14.2 d | 33.4 ab | 40.2 a | 21.6 cd | 24.5 bc | 33.2 ab | 36.8 a |
| O6 | Dihydroactinolide | 2354 | 2332 | 18.43 | 0.6 d | 0.1 d | 3.0 cd | 11.3 ab | 12.5 ab | 4.9 c | 10.8 ab | 9.3 b | 13.2 a |
| O7 | 2-(2-Propenyl)furan | 1615 | n.a. | 18.23 | 2.9 c | 1.5 c | 12.2 b | 15.5 a | 12.0 b | 14.2 ab | 11.0 b | 13.0 ab | 11.3 b |
| O8 | Cyclic ketone, n.i. m/z 68, 39, 98 | 1705 | n.a. | 13.59 | 9.3 e | 5.3 e | 42.4 d | 66.4 ab | 45.7 cd | 54.1 abcd | 60.8 abc | 69.9 a | 50.3 bcd |
| O9 | trans-2-(2-Pentenyl)furan | 1302 | 1282 | 13.18 | 0.8 d | 0.4 d | 4.2 bc | 4.4 bc | 4.4 bc | 4.7 bc | 6.0 ab | 7.4 a | 3.0 c |
| O10 | 3-Cyclohex-1-enyl-prop-2-enal | 1890 | n.a. | 10.45 | 3.5 ef | 2.2 f | 14.4 bc | 8.6 cde | 9.5 bcd | 22.9 a | 11.7 bc | 15.3 b | 4.6 def |
| O11 | Benzeneacetaldehyde | 1646 | 1648 | 9.85 | 10.4 c | 29.4 c | 33.1 c | 19.7 c | 108.6 bc | 260.0 b | 163.6 bc | 623.3 a | 8.9 c |
| O12 | Benzaldehyde | 1522 | 1521 | 9.23 | 49.9 cd | 27.8 d | 92.4 bc | 92.0 bc | 76.5 cd | 111.4 ab | 101.9 abc | 122.1 a | 77.0 cd |
| O13 | Furanoid, n.i. m/z 81, 53, 96 | <1000 | na | 6.54 | 183 cde | 152 e | 257 bc | 247 bc | 165 de | 169 de | 359 a | 237 bcd | 274 b |
| O14 | Phenol | 1995 | 1995 | 5.29 | 15.3 a | 13.5 ab | 10.7 c | 11.2 bc | 10.1 cd | 10.8 c | 10.2 cd | 7.8 d | 11.6 bc |
| O15 | 4-Ethyl-4-methyl-1-hexene | 1955 | na | 4.92 | 170 cde | 139 e | 214 bcd | 215 bcd | 128 e | 155 de | 287 a | 237 abc | 240 ab |
| O16 | 1-Octen-3-ol | 1447 | 1447 | 4.29 | 35.0 bcd | 49.5 a | 48.1 a | 38.3 abc | 44.1 ab | 46.6 ab | 24.2 d | 31.0 cd | 31.3 cd |
| O17 | 3-Methylbutanal | <1000 | 924 | 3.48 | 1.2 a | 1.2 a | 0.4 b | 0.3 b | 0.3 b | 0.7 ab | 0.2 b | 0.1 b | 0.3 b |
| O18 | 2-Phenoxyethanol | 2144 | 2144 | 2.11 | 6.8 | 6.6 | 115.8 | 7.8 | 8.7 | 6.8 | 27.7 | 30.4 | 24.0 |
| O19 | Isopropyl myristate | 2038 | 2040 | 1.76 | 18.1 | 17.5 | 11.8 | 10.5 | 9.8 | 8.4 | 9.3 | 234.1 | 15.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukić, I.; Išić, N.; Ban, D.; Salopek Sondi, B.; Goreta Ban, S. Comprehensive Volatilome Signature of Various Brassicaceae Species. Plants 2023, 12, 177. https://doi.org/10.3390/plants12010177
Lukić I, Išić N, Ban D, Salopek Sondi B, Goreta Ban S. Comprehensive Volatilome Signature of Various Brassicaceae Species. Plants. 2023; 12(1):177. https://doi.org/10.3390/plants12010177
Chicago/Turabian StyleLukić, Igor, Nina Išić, Dean Ban, Branka Salopek Sondi, and Smiljana Goreta Ban. 2023. "Comprehensive Volatilome Signature of Various Brassicaceae Species" Plants 12, no. 1: 177. https://doi.org/10.3390/plants12010177
APA StyleLukić, I., Išić, N., Ban, D., Salopek Sondi, B., & Goreta Ban, S. (2023). Comprehensive Volatilome Signature of Various Brassicaceae Species. Plants, 12(1), 177. https://doi.org/10.3390/plants12010177








