Future Climate CO2 Reduces the Tungsten Effect in Rye Plants: A Growth and Biochemical Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Growth and Treatments
2.2. Quantification of Photosynthetic Related Parameters
2.3. Organic Acids and Phenolic Content in Soil Samples
2.4. Quantification of the Tungsten in Soil and Plant
2.5. Quantification of Oxidative Damage Markers
2.6. Quantification of Antioxidant Parameters
2.7. Quantification of Detoxification-Related Parameters
2.8. Statistical Analysis Experiments
3. Results
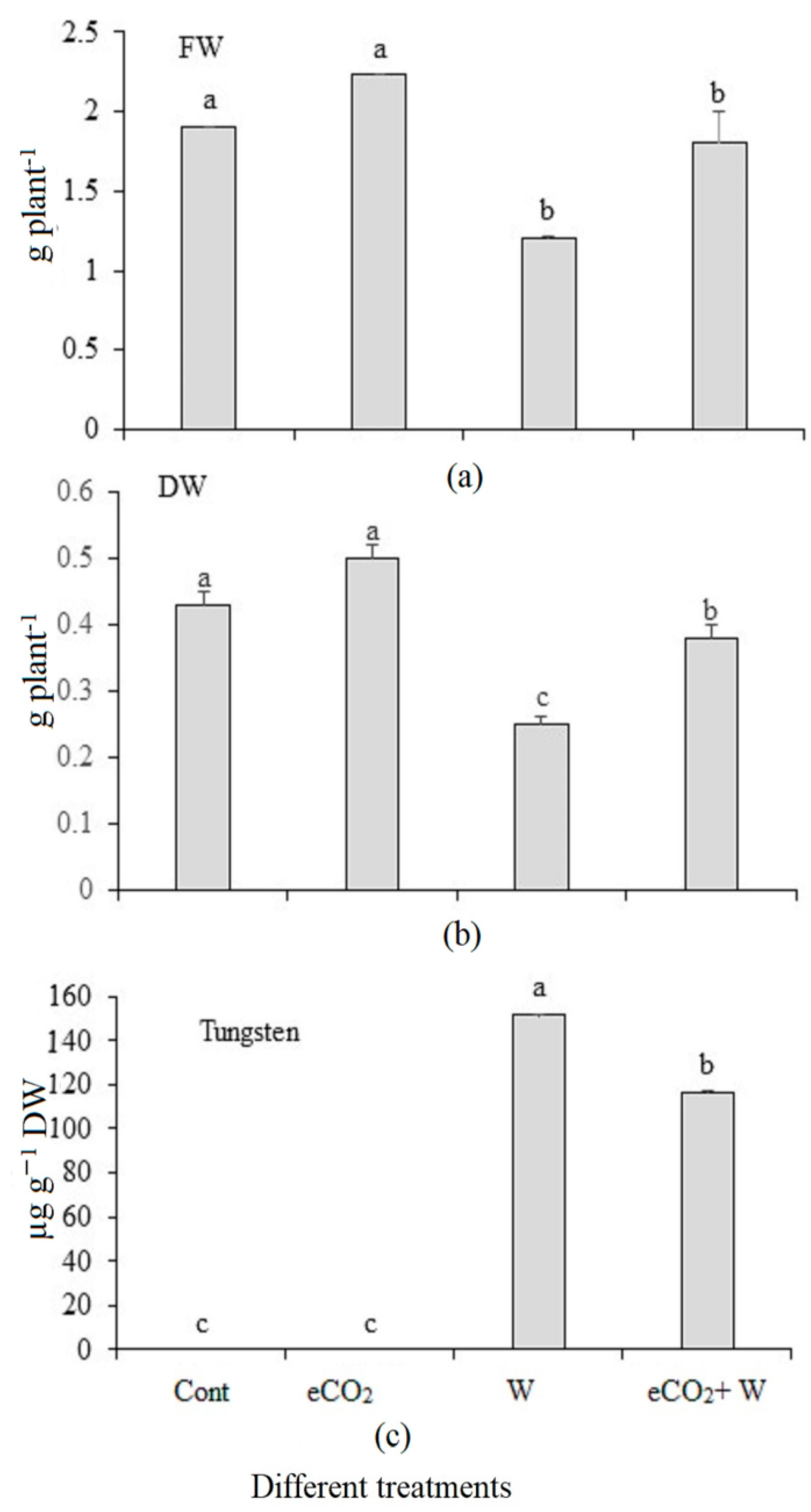
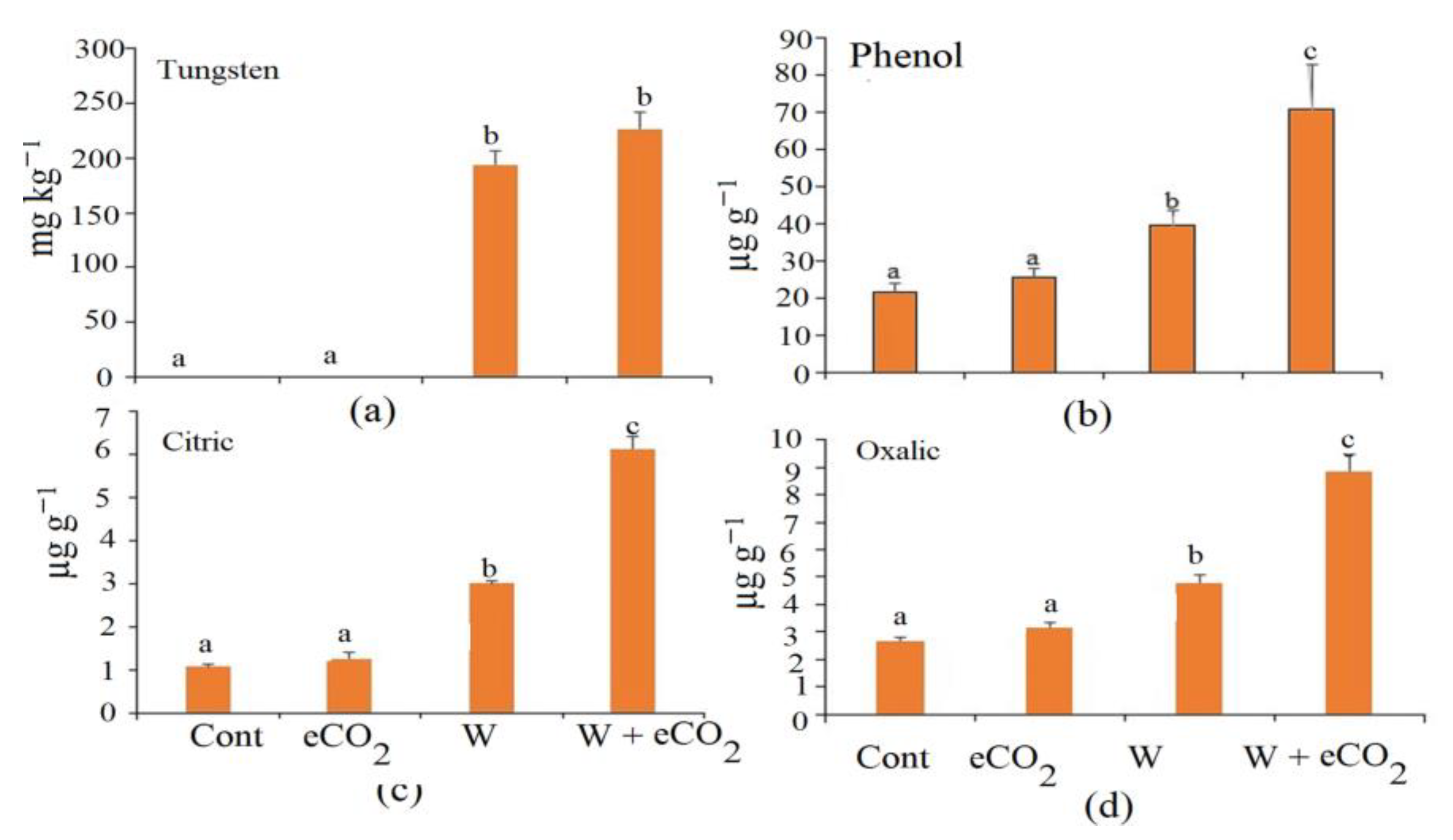
3.1. Growth and Tungsten Accumulation
3.2. Photosynthesis, Gas Exchange and Pigments
3.3. Organic Acids and Phenolic Content in Soil
3.4. Quantification of Oxidative Markers
3.5. Nonenzymatic Antioxidants
3.6. Antioxidant Enzymes
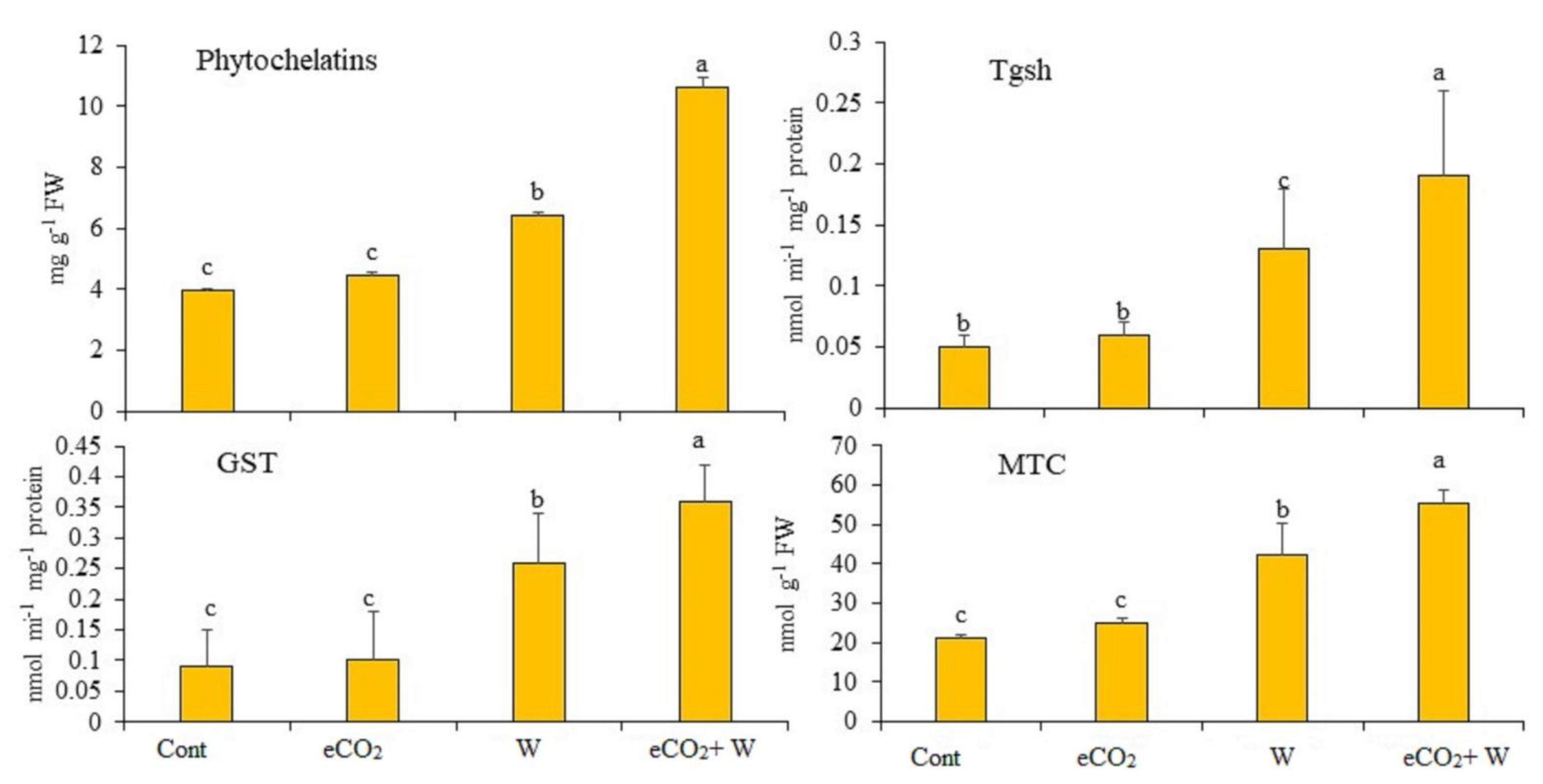
3.7. Detoxification Metabolties and Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, C.; Sheteiwy, M.S.; Han, J.; Dong, Z.; Pan, R.; Guan, Y.; Alhaj Hamoud, Y.; Hu, J. Polyamine biosynthetic pathways and their relation with the cold tolerance of maize (Zea mays L.) seedlings. Plant Signal. Behav. 2020, 15, 1807722. [Google Scholar] [CrossRef]
- Matthews, S.; Mila, I.; Scalbert, A.; Pollet, B.; Lapierre, C.; du Penhoat, C.L.M.; Rolando, C.; Donnelly, D.M.X. Method for estimation of proanthocyanidins based on their acid depolymerization in the presence of nucleophiles. J. Agric. Food Chem. 1997, 45, 1195–1201. [Google Scholar] [CrossRef]
- Yang, S.; Ulhassan, Z.; Shah, A.M.; Khan, A.R.; Azhar, W.; Hamid, Y.; Hussain, S.; Sheteiwy, M.S.; Salam, A.; Zhou, W. Salicylic acid underpins silicon in ameliorating chromium toxicity in rice by modulating antioxidant defense, ion homeostasis and cellular ultrastructure. Plant Physiol. Biochem. 2021, 166, 1001–1013. [Google Scholar] [CrossRef]
- NOAA, National Oceanic and Atmospheric Administration. Recent Global Monthly Mean CO2 [WWW Document]. Earth System Research Laboratories. 2020. Available online: https://www.esrl.noaa.gov/gmd/ccgg/trends/global.html (accessed on 2 November 2022).
- Lhotka, O.; Kyselý, J.; Farda, A. Climate change scenarios of heat waves in Central Europe and their uncertainties. Theor. Appl. Climatol. 2018, 131, 1043–1054. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Wittwer, S. Rising Carbon Dioxide Is Great for Plants. Policy Review, Autumn. 1992. Available online: www.purgit.com/co2ok.html (accessed on 29 March 2022).
- IPCC (Intergovernmental Panel on Climate Change). Special Report: The REGIONAL Impacts of Climate Change: An Assessment of Vulnerability; Watson, R., Zinyowera, M., Moss, R., Eds.; Cambridge University Press: Cambridge, UK, 1997; p. 517.
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Saralabai, V.C.; Vivekanandan, M.; Babu, S.R. Plant responses to high CO2 concentration in the atmosphere. Photosynthetica 1997, 33, 7–37. [Google Scholar] [CrossRef]
- Albert, K.R.; Mikkelsen, T.N.; Michelsen, A.; Ro-Poulsen, H.; van der Linden, L. Interactive effects of drought, elevated CO2 and warming on photosynthetic capacity and photosystem performance in temperate heath plants. J. Plant Physiol. 2011, 168, 1550–1561. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Uribelarrea, M.; Ainsworth, E.A.; Naidu, S.L.; Rogers, A.; Ort, D.R.; Long, S.P. Photosynthesis, productivity, and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought. Plant Physiol. 2006, 140, 779–790. [Google Scholar] [CrossRef] [Green Version]
- Selim, S.; Abuelsoud, W.; Al-Sanea, M.M.; AbdElgawad, H. Elevated CO2 differently suppresses the arsenic oxide nanoparticles-induced stress in C3 (Hordeum vulgare) and C4 (Zea maize) plants via altered homeostasis in metabolites specifically proline and anthocyanin metabolism. Plant Physiol. Biochem. 2021, 166, 235–245. [Google Scholar] [CrossRef]
- Bauweraerts, I.; Wertin, T.M.; Ameye, M.; McGuire, M.A.; Teskey, R.O.; Steppe, K. The effect of heat waves elevated [CO2] and low soil water availability on northern red oak (Quercus rubra L.) seedlings. Global Change Biol. 2013, 19, 517–528. [Google Scholar] [CrossRef]
- Saleh, A.M.; Hassan, Y.M.; Selim, S.; AbdElgawad, H. NiO-nanoparticles induce reduced phytotoxic hazards in wheat (Triticum aestivum L.) grown under future climate CO2. Chemosphere 2019, 220, 1047–1057. [Google Scholar] [CrossRef]
- Alsherif, E.A.; AbdElgawad, H. Elevated CO2 Suppresses the Vanadium Stress in Wheat Plants under the Future Climate CO2. Plants 2023, 12, 1535. [Google Scholar] [CrossRef]
- Shabbaj, I.I.; Abdelgawad, H.; Balkhyour, M.A.; Tammar, A.; Madany, M.M.Y. Elevated CO2 differentially mitigated oxidative stress induced by indium oxide nanoparticles in young and old leaves of C3 and C4 crops. Antioxidants 2022, 11, 308. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef] [Green Version]
- AbdElgawad, H.; Hassan, Y.M.; Alotaibi, M.O.; Mohammed, A.E.; Saleh, A.M. C3 and C4 plant systems respond differently to the concurrent challenges of mercuric oxide nanoparticles and future climate CO2. Sci. Total Environ. 2020, 749, 142356. [Google Scholar] [CrossRef]
- Cho, J.; Oki, T. Application of temperature, water stress, CO2 in rice growth models. Rice 2012, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zinta, G.; Abdelgawad, H.; Domagalska, M.A.; Vergauwen, L.; Knapen, D.; Nijs, I.; Janssens, I.A.; Beemster, G.T.S.; Asard, H. Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Glob. Chang. Biol. 2014, 20, 3670–3685. [Google Scholar] [CrossRef]
- Zinta, G.; Khan, A.; AbdElgawad, H.; Verma, V.; Srivastava, A.K. Unveiling the redox control of plant reproductive development during abiotic stress. Front. Plant Sci. 2016, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Pandita, S.; Sharma, A.; Bakshi, P.; Sharma, P.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Cerda, A. Ecological and human health risks appraisal of metal (loid)s in agricultural soils: A review. Geol. Ecol. Landsc. 2019, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Rashmi, I.; Roy, T.; Kartika, K.S.; Pal, R.; Coumar, V.; Kala, S.; Shinoji, K.C. Organic and inorganic fertilizer contaminants in agriculture: Impact on soil and water resources. In Contaminants in Agriculture: Sources, Impacts and Management; Naeem, M., Ansari, A.A., Gill, S.S., Eds.; Springer: Cham, Switzerland, 2020; pp. 3–41. [Google Scholar] [CrossRef]
- Hogy, P.; Kottmann, L.; Schmid, I.; Fangmeier, A. Heat, wheat and CO2: The relevance of timing and the mode of temperature stress on biomass and yield. J. Agron. Crop Sci. 2019, 205, 1–8. [Google Scholar] [CrossRef]
- Ziska, L.H.; Bunce, J.A.; Shimono, H.; Gealy, D.R.; Baker, J.T.; Newton, P.C.D.; Reynolds, M.P.; Jagadish, K.S.V.; Zhu, C.; Howden, M.; et al. Security and climate change: On the potential to adapt global crop production by active selection to rising atmospheric carbon dioxide. Proc. R. Soc. B Biol. Sci. 2012, 279, 4097–4105. [Google Scholar] [CrossRef]
- Biswas, B.; Qi, F.; Biswas, J.K.; Wijayawardena, A.; Khan, M.A.I.; Naidu, R. The fate of chemical pollutants with soil properties and processes in the climate change paradigm—A review. Soil Syst. 2018, 2, 51. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.S.; Zhang, D.; Hu, Z.; Liu, C.; Zhao, Z.; Sun, W.; Fang, X.; Fan, P. Effects of elevated carbon dioxide on metal transport in soil-crop system: Results from a field rice and wheat experiment. J. Soils Sediments 2019, 19, 3742–3748. [Google Scholar] [CrossRef]
- Rodriguez, J.H.; Klumpp, A.; Fangmeier, A.; Pignata, M.L. Effects of elevated CO2 concentrations and fly ash amended soils on trace element accumulation and translocation among roots, stems and seeds of Glycine max (L.). Merr. J. Hazard Mater. 2011, 187, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Shah, F.U.R.; Ahmad, N.; Masood, K.R.; Peralta-Videa, J.R. Heavy metal toxicity in plants. In Plant Adaptation and Phytoremediation; Springer: Dordrecht, The Netherlands, 2010; pp. 71–97. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Koutsospyros, A.; Braida, W.; Christodoulatos, C.; Dermatas, D.; Strigul, N. A review of tungsten: From environmental obscurity to scrutiny. J. Hazard Mater. 2006, 136, 1–19. [Google Scholar] [CrossRef]
- Wilson, B.; Pyatt, F.B. Bio-availability of tungsten in the vicinity of an abandoned mine in the English Lake District and some potential health implications. Sci. Total Environ. 2006, 370, 401–408. [Google Scholar] [CrossRef]
- Adamakis, I.D.S.; Panteris, E.; Eleftheriou, E.P. Tungsten toxicity in plants. Plants 2012, 1, 82–99. [Google Scholar] [CrossRef]
- Kumar, A.; Aery, N.C. Effect of tungsten on growth, biochemical constituents, molybdenum and tungsten contents in wheat. Plant Soil Environ. 2011, 57, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Preiner, J.; Wienkoop, S.; Weckwerth, W.; Oburger, E. Molecular mechanisms of tungsten toxicity differ for Glycine max depending on nitrogen regime. Front. Plant Sci. 2019, 10, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A reevaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Hemphill, J.K.; Venketeswaran, S. Chlorophyll and carotenoid accumulation in three chlorophyllous callus phenotypes of Glycine max. Am. J. Bot. 1978, 65, 1055. [Google Scholar] [CrossRef]
- Sulpice, R.; Tschoep, H.; Von Korff, M.; Büssis, D.; Usadel, B.; Hohne, M.; Witucka Wall, H.; Altmann, T.; Stitt, M.; Gibon, Y. Description and applications of a rapid and sensitive non-radioactive microplate-based assay for maximum and initial activity of D-ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Cell Environ. 2007, 30, 1163–1175. [Google Scholar] [CrossRef]
- De Sousa, A.; AbdElgawad, H.; Asard, H.; Pinto, A.; Soares, C.; Branco-Neves, S.; Braga, T.; Azenha, M.; Selim, S.; Al Jaouni, S.; et al. Metalaxyl effects on antioxidant defenses in leaves and roots of Solanum nigrum L. Front. Plant Sci. 2017, 8, 1967. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Quin, F.; Brooks, R.R. The rapid determination of tungsten in soils, stream sediments, rocks and vegetation. Anal. Chim. Acta 1972, 58, 301–309. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Woollard, A.C.S.; Wolff, S.P. Hydrogen peroxide production during experimental protein glycation. FEBS Lett. 1990, 268, 69–71. [Google Scholar] [CrossRef] [Green Version]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Steczko, J.; Donoho, G.A.; Dixon, J.E.; Sugimoto, T.; Axelrod, B. Effect of ethanol and low-temperature culture on expression of soybean lipoxygenase L-1 in Escherichia coli. Protein Expr. Purif. 1991, 2, 221–227. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; pp. 15–27. [Google Scholar]
- Lowe, L.E. Soil Sampling and Methods of Analysis; Lewis Publisher: Boca Raton, FL, USA, 1993. [Google Scholar]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575. [Google Scholar] [CrossRef]
- Kumar, K.B.; Khan, P.A. Peroxidase & polyphenol oxidase in excised ragi (Eleusine corocana cv PR 202) leaves during senescence. Indian J. Exp. Biol. 1982, 20, 412–416. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Drotar, A.; Phelps, P.; Fall, R. Evidence for glutathione peroxidase activities in cultured plant cells. Plant Sci. 1985, 42, 35–40. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Mozer, T.J.; Tiemeier, D.C.; Jaworski, E.G. Purification and characterization of corn glutathione S-transferase. Biochemistry 1983, 22, 1068–1072. [Google Scholar] [CrossRef]
- Diopan, V.; Shestivska, V.; Adam, V.; Macek, T.; Mackova, M.; Havel, L.; Kizek, R. Determination of content of metallothionein and low molecular mass stress peptides in transgenic tobacco plants. Plant Cell Tissue Organ Cult. 2008, 94, 291–298. [Google Scholar] [CrossRef]
- De Knecht, J.A.; Koevoets, P.L.M.; Verkleij, J.A.C.; Ernst, W.H.O. Evidence against a role for phytochelatins in naturally selected increased cadmium tolerance in Silene vulgaris (Moench) Garcke. New Phytol. 1992, 122, 681–688. [Google Scholar] [CrossRef]
- Adamakis, I.D.S.; Eleftheriou, E.P.; Rost, T.L. Effects of sodium tungstate on the ultrastructure and growth of pea (Pisum sativum) and cotton (Gossypium hirsutum) seedlings. Environ. Exp. Bot. 2008, 63, 416–425. [Google Scholar] [CrossRef]
- Johnson, D.R.; Inouye, L.S.; Bednar, A.J.; Clarke, J.U.; Winfield, L.E.; Boyd, R.E.; Ang, C.Y.; Goss, J. Tungsten bioavailability and toxicity in sunflowers (Helianthus annuus L.). Land Contam. Reclamat. 2009, 17, 141–151. [Google Scholar] [CrossRef]
- Imran, M.; Hussain, S.; Rana, M.S.; Saleem, M.H.; Rasul, F.; Ali, K.H.; Potcho, M.P.; Pan, S.; Duan, M.; Tang, X. Molybdenum improves 2-acetyl-1-pyrroline, grain quality traits and yield attributes in fragrant rice through efficient nitrogen assimilation under cadmium toxicity. Ecotoxicol. Environ. Saf. 2021, 211, 111911. [Google Scholar] [CrossRef] [PubMed]
- Souri, Z.; Cardoso, A.A.; Da-Silva, C.J.; De Oliveira, L.M.; Dari, B.; Sihi, D.; Karimi, N. Heavy metals and photosynthesis: Recent developments. In Photosynthesis, Productivity and Environmental Stress; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 107–134. [Google Scholar] [CrossRef]
- Bednar, A.J.; Jones, W.T.; Boyd, R.E.; Ringelberg, D.B.; Larson, S.L. Geochemical parameters influencing tungsten mobility in soils. J. Environ. Qual. 2008, 37, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Jain, M.; Mathur, G.; Koul, S.; Sarin, N.B. Ameliorative effects of proline on salt stress induced lipid peroxidation in cell lines of groundnut (Arachis hypogaea L.). Plant Cell Rep. 2001, 20, 463–468. [Google Scholar] [CrossRef]
- Madany, M.M.Y.; Saleh, A.M.; Habeeb, T.H.; Hozzein, W.N.; AbdElgawad, H. Silicon dioxide nanoparticles alleviate the threats of broomrape infection in tomato by inducing cell wall fortification and modulating ROS homeostasis. Environ. Sci. Nano 2020, 7, 1415–1430. [Google Scholar] [CrossRef]
- Xu, J.; Yin, H.X.; Liu, X.J.; Yuan, T.; Mi, Q.; Yang, L.L.; Xie, Z.X.; Wang, W.Y. Nitric oxide alleviates Fe deficiency-induced stress in Solanum nigrum. Biol. Plant. 2009, 53, 784–788. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Cadmium stress tolerance in crop plants: Probing the role of sulfur. Plant Signal. Behav. 2011, 6, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Izbianska, K.; Arasimowicz-Jelonek, M.; Deckert, J. Phenylpropanoid pathway metabolites promote tolerance response of lupine roots to lead stress. Ecotoxicol. Environ. Saf. 2014, 110, 61–67. [Google Scholar] [CrossRef]
- Da Costa, M.V.J.; Sharma, P.K. Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 2016, 54, 110–119. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, J.; Zhou, H.; Sun, Y.; Yin, Y.; Pei, D.; Ji, R.; Wu, J.; Wang, X. Elevated CO2 levels affects the concentrations of copper and cadmium in crops grown in soil contaminated with heavy metals under fully open-air field conditions. Environ. Sci. Technol. 2011, 45, 6997–7003. [Google Scholar] [CrossRef]
- Rajkumar, M.; Prasad, M.N.V.; Swaminathan, S.; Freitas, H. Climate change driven plant–metal–microbe interactions. Environ. Int. 2013, 53, 74–86. [Google Scholar] [CrossRef] [PubMed]
- AbdElgawad, H.; Zinta, G.; Beemster, G.T.S.; Janssens, I.A.; Asard, H. Future climate CO2 levels mitigate stress impact on plants: Increased defense or decreased challenge? Front. Plant Sci. 2016, 7, 556. [Google Scholar] [CrossRef] [Green Version]
- Al Jaouni, S.; Saleh, A.M.; Wadaan, M.A.M.; Hozzein, W.N.; Selim, S.; AbdElgawad, H. Elevated CO2 induces a global metabolic change in basil (Ocimum basilicum L.) and peppermint (Mentha piperita L.) and improves their biological activity. J. Plant Physiol. 2018, 224, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Di Toppi, L.S.; Fossati, F.; Musetti, R.; Mikerezi, I.; Favali, M.A. Effects of Hexavalent Chromium on Maize, Tomato, and Cauliflower Plants. J. Plant Nutr. 2002, 25, 701–717. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakeel, A.; Ali, I.; Wu, M.; Kkan, A.R.; Jan, M.; Ali, A.; Liu, Y.; Ge, S.; Wu, J.; Gan, Y.; et al. Ethylene mediates dichromate-induced oxidative stress and regulation of the enzymatic antioxidant system-related transcriptome in Arabidopsis thaliana. Environ. Exp. Bot. 2019, 161, 166–179. [Google Scholar] [CrossRef]
- Bencze, S.; Bamberger, Z.; Janda, T.; Balla, K.; Varga, B.; BedHo, Z.; Veisz, O. Physiological response of wheat varieties to elevated atmospheric CO2 and low water supply levels. Photosynthetica 2014, 52, 71–82. [Google Scholar] [CrossRef]
- Soares, S.S.; Martins, H.; Duarte, R.O.; Moura, J.J.; Coucelo, J.; Gutiérrez-Merino, C.; Aureliano, M. Vanadium distribution, lipid peroxidation and oxidative stress markers upon decavanadate in vivo administration. J. Inorg. Biochem. 2007, 101, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Kováčik, J.; Babula, P.; Klejdus, B.; Hedbavny, J. Chromium uptake and consequences for metabolism and oxidative stress in chamomile plants. J. Agric. Food Chem. 2013, 61, 7864–7873. [Google Scholar] [CrossRef]
- Duarte, B.; Delgado, M.; Caçador, I. The role of citric acid in cadmium and nickel uptake and translocation, in Halimione portulacoides. Chemosphere 2007, 69, 836–840. [Google Scholar] [CrossRef]
- Rojek, J.; Kozieradzka-Kiszkurno, M.; Kapusta, M.; Aksmann, A.; Jacewicz, D.; Drzeżdżon, J.; Tesmar, A.; Żamojć, K.; Wyrzykowski, D.; Chmurzyński, L. The effect of vanadium (IV) complexes on development of Arabidopsis thaliana subjected to H2O2-induced stress. Funct. Plant Biol. 2019, 46, 942–961. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, O. C4 photosynthesis and water stress. Ann. Bot. 2009, 103, 635–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| H2O2 | MDA | LOX | |
|---|---|---|---|
| N mol g−1 FW | |||
| Control (ambient CO2—410 ppm) | 291 ± 5.7 c | 3.34 ± 0.06 c | 1.94 ± 0.04 c |
| eCO2 (620 ppm) | 308 ± 19.0 c | 3.14 ± 0.08 c | 1.79 ± 0.15 c |
| W (350 mg kg−1) | 827 ± 14.0 a | 8.15 ± 0.38 a | 4 ± 0.06 a |
| eCO2 + W | 680 ± 12.0 b | 5.22 ± 0.11 b | 3.08 ± 0.10 b |
| TAC | Pphenol | Flav | Ttoco | |
|---|---|---|---|---|
| mg g−1 FW | ||||
| Control (ambient CO2—410 ppm) | 34.30 ± 0.9 d | 1.22 ± 0.04 c | 0.56 ± 0.01 a | 21.3 ± 0.43 c |
| eCO2 (620 ppm) | 40.39 ± 1.3 c | 1.44 ± 0.06 c | 0.66 ± 0.01 a | 25.7 ± 0.92 c |
| W (350 mg kg−1) | 52.87 ± 0.8 b | 2.80 ± 0.07 b | 2.28 ± 0.06 b | 35.4 ± 0.96 b |
| eCO2 + W | 87.55 ± 2.4 a | 4.81 ± 0.40 a | 1.87 ± 0.02 a | 57.8 ± 1.34 a |
| GR | GPX | POX | CAT | SOD | APX | DHAR | ASC | GSH | |
|---|---|---|---|---|---|---|---|---|---|
| N mol min−1 mg−1 Protein | mg g−1 FW | ||||||||
| Control (ambient CO2—410 ppm) | 0.070 ± 0.01 c | 0.142 ± 0.012 a | 0.546 ± 0.03 d | 3.703 ± 0.104 b | 100 ± 2.2 b | 0.14 ± 0.01 c | 0.066 ± 0.003 b | 0.089 ± 0.002 b | 0.021 ± 0 c |
| eCO2 (620 ppm) | 0.082 ± 0.01 c | 0.185 ± 0.01 a | 0.743 ± 0.01 c | 4.126 ± 0.24 b | 118 ± 11.2 b | 0.16 ± 0.02 c | 0.078 ± 0.01 a | 0.105 ± 0.010 b | 0.024 ± 0.0 c |
| W (350 mg kg−1) | 0.150 ± 0.01 b | 0.334 ± 0.03 b | 1.124 ± 0.06 b | 9.199 ± 0.13 a | 253 ± 2.7 a | 0.33 ± 0.04 b | 0.145 ± 0.01 a | 0.182 ± 0.002 a | 0.055 ± 0.0 b |
| eCO2 + W | 0.281 ± 0.01 a | 0.479 ± 0.01 a | 1.849 ± 0.10 a | 8.973 ± 0.16 a | 224 ± 10.0 a | 0.48 ± 0.02 a | 0.178 ± 0.01 a | 0.176 ± 0.001 a | 0.084 ± 0.0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsherif, E.A.; Hajjar, D.; AbdElgawad, H. Future Climate CO2 Reduces the Tungsten Effect in Rye Plants: A Growth and Biochemical Study. Plants 2023, 12, 1924. https://doi.org/10.3390/plants12101924
Alsherif EA, Hajjar D, AbdElgawad H. Future Climate CO2 Reduces the Tungsten Effect in Rye Plants: A Growth and Biochemical Study. Plants. 2023; 12(10):1924. https://doi.org/10.3390/plants12101924
Chicago/Turabian StyleAlsherif, Emad A., Dina Hajjar, and Hamada AbdElgawad. 2023. "Future Climate CO2 Reduces the Tungsten Effect in Rye Plants: A Growth and Biochemical Study" Plants 12, no. 10: 1924. https://doi.org/10.3390/plants12101924
APA StyleAlsherif, E. A., Hajjar, D., & AbdElgawad, H. (2023). Future Climate CO2 Reduces the Tungsten Effect in Rye Plants: A Growth and Biochemical Study. Plants, 12(10), 1924. https://doi.org/10.3390/plants12101924








