Reproductive Biology and Breeding Systems of Two Opisthopappus Endemic and Endangered Species on the Taihang Mountains
Abstract
1. Introduction
2. Results
2.1. Flowering Phenology and Floral Traits
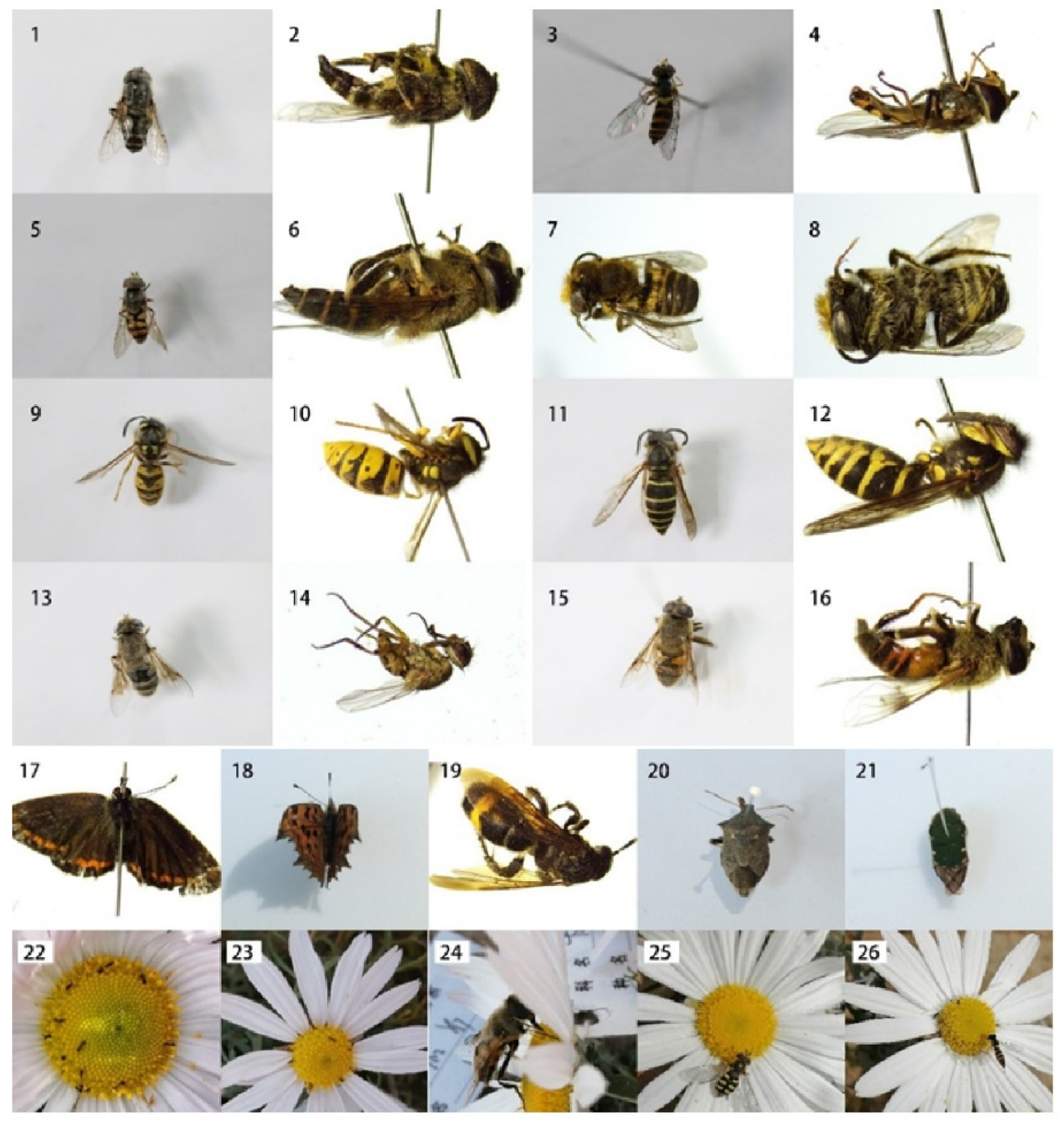
2.2. Floral Visitors
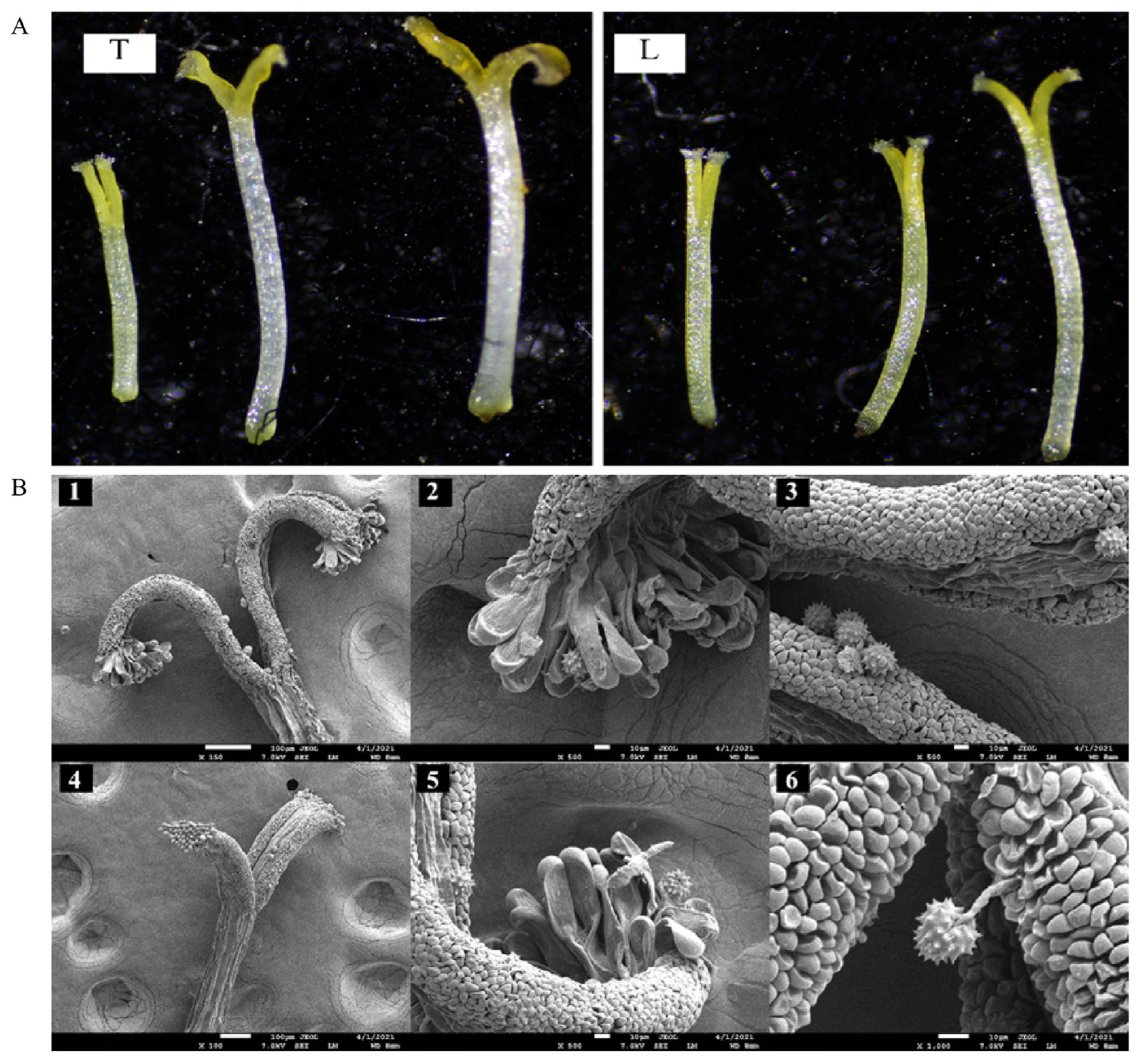
2.3. Pollen Viability and Stigma Receptivity
2.4. Outcrossing Index and Pollen/Ovule Ratio
2.5. Seed Set and Germination Rates
3. Discussion
3.1. Floral Syndromes and Effective Pollination
3.2. Breeding System
4. Materials and Methods
4.1. Study Species and Sites
4.2. Observation of Flowering Phenology and Floral Traits
4.3. Pollinator Observations and Pollination Experiments
4.4. Pollen Viability and Stigma Receptivity
4.5. Outcrossing Index and Pollen/Ovule Ratio
4.6. Seed and Germination Rates
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Epps, M.J.; Allison, S.E.; Wolfe, L.M. Reproduction in Flame Azalea (Rhododendron calendulaceum, Ericaceae): A Rare Case of Insect Wing Pollination. Am. Nat. 2015, 186, 294–301. [Google Scholar] [CrossRef]
- Kuswantoro, F. Flower-Insect Visitor Interaction: Case Study on Rhododendron inundatum Sleumer in Bali Botanic Garden. J. Trop. Biodivers. Biotechnol. 2017, 2, 35–38. [Google Scholar] [CrossRef]
- Wani, I.A.; Verma, S.; Ahmad, P.; El-Serehy, H.A.; Hashim, M.J. Reproductive Biology of Rheum webbianum Royle, a Vulnerable Medicinal Herb from Alpines of North-Western Himalaya. Front. Plant Sci. 2022, 13, 699645. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-M.; Shen, X.-L.; Tong, L.; Lei, F.-W.; Xia, X.-F.; Mu, X.-Y.; Zhang, Z.-X. Reproductive Biology of an Endangered Lithophytic Shrub and Implications for Its Conservation. BMC Plant Biol. 2022, 22, 80. [Google Scholar] [CrossRef] [PubMed]
- Escaravage, N.; Wagner, J. Pollination Effectiveness and Pollen Dispersal in a Rhododendron ferrugineum (Ericaceae) Population. Plant Biol. 2004, 6, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, X.; Li, Z.; Ma, H.; Wan, Y.; Liu, X.; Fu, L. Study on Reproductive Biology of Rhododendron longipedicellatum: A Newly Discovered and Special Threatened Plant Surviving in Limestone Habitat in Southeast Yunnan, China. Front. Plant Sci. 2018, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Wani, I.A.; Khan, S.; Sharma, S.; Kumari, P.; Kaushik, P.; El-Serehy, H.A. Reproductive Biology and Pollination Ecology of Berberis lycium Royle: A Highly Valued Shrub of Immense Medicinal Significance. Plants 2021, 10, 1907. [Google Scholar] [CrossRef]
- Simón-Porcar, V.I.; Muñoz-Pajares, A.J.; de Castro, A.; Arroyo, J. Direct Evidence Supporting Darwin’s Hypothesis of Cross-Pollination Promoted by Sex Organ Reciprocity. New Phytol. 2022, 235, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.; Matthes, U.; Kelly, P. Cliff Ecology: Pattern and Process in Cliff Ecosystems; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Antonsson, H. Plant Species Composition and Diversity in Cliff and Mountain Ecosystems. Ph.D. Thesis, University of Gothenburg, Gothenburg, Sweden, 2012. [Google Scholar]
- Memmott, J.; Craze, P.G.; Waser, N.M.; Price, M.V. Global Warming and the Disruption of Plant-Pollinator Interactions. Ecol. Lett. 2007, 10, 710–717. [Google Scholar] [CrossRef]
- Hegland, S.J.; Nielsen, A.; Lázaro, A.; Bjerknes, A.-L.; Totland, Ø. How Does Climate Warming Affect Plant-Pollinator Interactions? Ecol. Lett. 2009, 12, 184–195. [Google Scholar] [CrossRef]
- Davidson, J.B.; Durham, S.L.; Wolf, P.G. Breeding System of the Threatened Endemic PRimula cusickiana var. maguirei (Primulaceae): Breeding System of Maguire Primrose. Plant Species Biol. 2014, 29, E55–E63. [Google Scholar] [CrossRef]
- Castro, S.; Silveira, P.; Navarro, L. How Flower Biology and Breeding System Affect the Reproductive Success of the Narrow Endemic Polygala Vayredae Costa (Polygalaceae)? Bot. J. Linn. Soc. 2008, 157, 67–81. [Google Scholar] [CrossRef]
- Dellinger, A.S.; Paun, O.; Baar, J.; Temsch, E.M.; Fernández-Fernández, D.; Schönenberger, J. Population structure in Neotropical plants: Integrating pollination biology, topography and climatic niches. Mol. Ecol. Resour. 2022, 31, 2264–2280. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H. Initial Formation and Mesozoic Tectonic Exhumation of an Intracontinental Tectonic Belt of the Northern Part of the Taihang Mountain Belt, Eastern Asia. J. Geol. 2008, 116, 155–172. [Google Scholar] [CrossRef]
- Mu, X.Y.; Wu, Y.M.; Shen, X.L.; Tong, L.; Lei, F.-W.; Xia, X.-F.; Ning, Y. Genomic Data Reveals Profound Genetic Structure and Multiple Glacial Refugia in Lonicera oblata (Caprifoliaceae), a Threatened Montane Shrub Endemic to North China. Front. Plant Sci. 2022, 13, 832559. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fu, W.L.; Du, W.; Zhang, Q.; Li, Y.; Lyu, Y.S.; Wang, X.F. Nectary Tracks as Pollinator Manipulators: The Pollination Ecology of Swertia bimaculata (Gentianaceae). Ecol. Evol. 2018, 8, 3187–3207. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Wang, Z.; Hou, H.; Wu, J.; Gao, Y.; Han, W.; Ru, W.; Sun, G.; Wang, Y. Localized Environmental Heterogeneity Drives the Population Differentiation of Two Endangered and Endemic Opisthopappus shih Species. BMC Ecol. Evol. 2021, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-S.; Zhang, Y.-L.; Huang, J.-S.; Wu, Z.-F.; Zhao, S.-L.; Wang, H.-S.; Zhang, Z.-W.; Chen, Y.-S.; Zhang, C.-L. A Floristic Study on The Seed Plants in The North China Region. Plant Divers. 1995, 17, 1. [Google Scholar]
- López-Pujol, J.; Zhang, F.; Song, G. Population Genetics and Conservation of the Critically Endangered Clematis acerifolia (Ranunculaceae). Can. J. Bot. 2005, 83, 1248–1256. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Ding, Z.; Wang, G.; Zhang, Y.; Gong, H.; Chang, T.; Zhang, Y. De Novo Sequencing and Comparative Transcriptome Analysis of the Male and Hermaphroditic Flowers Provide Insights into the Regulation of Flower Formation in Andromonoecious Taihangia Rupestris. BMC Plant Biol. 2017, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.; Wang, S.; He, J.; Chen, W.; Fan, Z.; Li, J.; Wang, Y. De Novo Assembly and Transcriptome Characterization of Opisthopappus (Asteraceae) for Population Differentiation and Adaption. Front. Genet. 2018, 9, 371. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.; Ye, H.; Wang, Z.; Zhou, Y.; Wu, J.; Gao, Y.; Han, W.; Zang, E.; Zhang, H.; Ru, W.; et al. Genetic Divergence and Relationship Among Opisthopappus Species Identified by Development of EST-SSR Markers. Front. Genet. 2020, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Cao, L.; Zhang, X.; Li, H. Floral Biology, Breeding System and Pollination Ecology of an Endangered Tree Tetracentron sinense Oliv. (Trochodendraceae). Bot. Stud. 2013, 54, 50. [Google Scholar] [CrossRef]
- Dafni, A. Pollination Ecology: A Practical Approach; Oxford University Press: Oxford, UK, 1994; p. 46. [Google Scholar]
- Cruden, R.W. Pollen-Ovule ratios: A conservative indictor of breeding syetems in flowering plants. Evolution 1977, 31, 32–46. [Google Scholar] [CrossRef]
- Fenster, C.; Armbruster, W.; Wilson, P.; Dudash, M.; Thomson, J. Pollination Syndromes and Floral Specialization. Annu. Rev. Ecol. Evol. Syst. 2004, 12, 375–403. [Google Scholar] [CrossRef]
- Conner, J.; Sterling, A. Testing Hypotheses of Functional Relationships: A Comparative Survey of Correlation Patterns Among Floral Traits in Five Insect-Pollinated Plants. Am. J. Bot. 1995, 82, 1399–1406. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Wu, T. Characteristics and Genesis of Geoheritage Resources of Taihang Mountain. Beijing Daxue Xuebao (Ziran Kexue Ban)/Acta Sci. Nat. Univ. Pekin. 2018, 54, 546–554. [Google Scholar]
- Hong, L.; Shen, H.; Ye, W.; Cao, H. Study on pollinating insects of Mikania micrantha HBK and their foraging behavior. J. South China Norm. Univ. (Nat. Sci. Ed.) 2011, 1, 98–102. [Google Scholar]
- Korpela, E.; Hyvönen, T.; Kuussaari, M. Logging in Boreal Field-Forest Ecotones Promotes Flower-Visiting Insect Diversity and Modifies Insect Community Composition. Insect Conserv. Divers. 2015, 8, 152–162. [Google Scholar] [CrossRef]
- Liu, Z.X.; Lan, Y.X.; Zhang, H.; Hao, W.L.; He, S.; Liu, L.; Feng, X.L.; Qie, Q.Y.; Chai, M.; Wang, Y.L. Responses of Aroma Related Metabolic Attributes of Opisthopappus longilobus Flowers to Environmental Changes. Plants 2023, 12, 1592. [Google Scholar] [CrossRef]
- Steinacher, G.; Wagner, J. Flower Longevity and Duration of Pistil Receptivity in High Mountain Plants. Flora 2010, 205, 376–387. [Google Scholar] [CrossRef]
- Lloyd, D.G.; Yates, J.M.A. Intrasexual selection and the segregation of pollen and stigmas in hermaphrodite plants, exemplified by Wahlenbergia albomarginata (Campanulaceae). Evolution 1982, 36, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Magotra, R.; Koul, A.K. Restoration of Eremostachys Superba Royle Ex Benth—A Critically Endangered Species. Curr. Sci. 2003, 84, 1307–1308. [Google Scholar]
- Akeroyd, J.R.; Briggs, D. Genecological Studies of Rumex Crispusl. New Phytol. 1983, 94, 325–343. [Google Scholar] [CrossRef]
- Pansarin, E.R.; Pansarin, L.M. Reproductive Biology of Trichocentrum pumilum: An Orchid Pollinated by Oil-Collecting Bees. Plant Biol. 2011, 13, 576–581. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, Y.Z.; Huang, S.-Q. Generalist Passerine Pollination of a Winter-Flowering Fruit Tree in Central China. Ann. Bot. 2012, 109, 379–384. [Google Scholar] [CrossRef]
- García, M.; Espadaler, X.; Olesen, J. Extreme Reproduction and Survival of a True Cliffhanger: The Endangered Plant Borderea chouardii (Dioscoreaceae). PLoS ONE 2012, 7, e44657. [Google Scholar] [CrossRef]
- Grant, V. Plant Speciation; Columbia University Press: New York, NY, USA, 1971. [Google Scholar]
- Agrawal, A.F. Evolution of Sex: Why Do Organisms Shuffle Their Genotypes? Curr. Biol. 2006, 16, R696–R704. [Google Scholar] [CrossRef]
- Vogler, D.W.; Kalisz, S. Sex among the Flowers: The Distribution of Plant Mating Systems. Evolution 2001, 55, 202–204. [Google Scholar]
- Salas-Arcos, L.; Lara, C.; Ornelas, J.F. Reproductive Biology and Nectar Secretion Dynamics of Penstemon gentianoides (Plantaginaceae): A Perennial Herb with a Mixed Pollination System? PeerJ 2017, 5, e3636. [Google Scholar] [CrossRef]
- Barrett, S.C.H. Evolution of mating system: Outcrossing versus selfing. In The Princeton Guide to Evolution; Losos, J., Ed.; Princeton University Press: Princeton, NJ, USA, 2014; pp. 356–362. [Google Scholar]
- Lande, R.; Schemske, D.W. The evolution of self-fertilization and inbreeding depression. Genetic models. Evolution 1985, 39, 24–40. [Google Scholar] [PubMed]
- Webb, C.J.; Lloyd, D.G. The Avoidance of Interference between the Presentation of Pollen and Stigmas in Angiosperms II. Herkogamy. N. Z. J. Bot. 1986, 24, 163–178. [Google Scholar] [CrossRef]
- Faegri, K.; Vander, P.L. The Principles of Pollination Ecology; Pergamon Press: Oxford, UK, 1979. [Google Scholar]
- Lee, P.; Patel, R.; Conlan, R.; Wainwright, S.; Hipkin, C. Comparison of Genetic Diversities in Native and Alien Populations of Hoary Mustard (Hirschfeldia incana [L.] Lagreze-Fossat). Int. J. Plant Sci. 2004, 165, 833–843. [Google Scholar] [CrossRef]
- Qiao, Q.; Zhang, C.Q.; Milne, R. Population Genetics and Breeding System of Tupistra Pingbianensis (Liliaceae), a Naturally Rare Plant Endemic to SW China. J. Syst. Evol. 2010, 48, 47–57. [Google Scholar] [CrossRef]
- Freitas, L.; Sazima, M. Floral Biology and Mechanisms of Spontaneous Self-pollination in Five Neotropical Species of Gentianaceae. Bot. J. Linn. Soc. 2009, 160, 357–368. [Google Scholar] [CrossRef]
- Duan, Y.W.; Dafni, A.; Hou, Q.Z.; He, Y.P.; Liu, J.Q. Delayed Selfing in an Alpine Biennial Gentianopsis Paludosa (Gentianaceae) in the Qinghai-Tibetan Plateau. J. Integr. Plant Biol. 2010, 52, 593–599. [Google Scholar] [CrossRef]
- Williams, C.F.; Ruvinsky, J.; Scott, P.E.; Hews, D.K. Pollination, Breeding System, and Genetic Structure in Two Sympatric delphinium (Ranunculaceae) Species. Am. J. Bot. 2001, 88, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.P.M.; Fracasso, C.M.; Luciene dos Santos, M.; Romero, R.; Sazima, M.; Oliveira, P.E. Reproductive Biology and Species Geographical Distribution in the Melastomataceae: A Survey Based on New World Taxa. Ann. Bot. 2012, 110, 667–679.55. [Google Scholar] [CrossRef]
- Williams, C.F. Effects of Floral Display Size and Biparental Inbreeding on Outcrossing Rates in Delphinium barbeyi (Ranunculaceae). Am. J. Bot. 2007, 94, 1696–1705. [Google Scholar] [CrossRef]
- Shih, C.; Fu, G.X. Compositae (3) anthemideae. In Flora Reipublicae Popularis Sinicae, Angiospermae, Dicotyledoneae; Lin, Y., Shih, C., Eds.; Science Press: Beijing, China, 1983; pp. 144–145. [Google Scholar]
- Ming-chang, C. Spatial Variability of Topsoil Organic Matter and Total Nitrogen in Linfen Basin, Shanxi and Its Influencing Factors. Chin. J. Soil Sci. 2010, 41, 839–844. [Google Scholar]
- Peng, J.; Liu, Y.Z.; Wang, T. A Tree-Ring Record of 1920’s-1940’s Droughts and Mechanism Analyses in Henan Province. Shengtai Xuebao/Acta Ecol. Sin. 2014, 34, 3509–3518. [Google Scholar]
- Gao, L.W. Characteristics of Vegetation Fluctuation as Well as Consequent Impact on Climate since Late Pleistocene in Handan Area, Hebei. Acta Sedimentol. Sin. 2010, 28, 1206–1212. [Google Scholar]
- Inouye, D. The Terminology of Floral Larceny. Ecology 1980, 61, 1251–1253. [Google Scholar] [CrossRef]
- Oriani, A.; Scatena, V.L. Reproductive Biology of Abolboda pulchella and A. poarchon (Xyridaceae: Poales). Ann. Bot. 2011, 107, 611–619. [Google Scholar] [CrossRef]
- Osborn, M.M.; Kevan, P.G.; Lane, M.A. Pollination Biology of Opuntia polyacantha and Opuntia phaeacantha (Cactaceae) in Southern Colorado. Plant Syst. Evol. 1988, 159, 85–94. [Google Scholar] [CrossRef]
- Smitha, G.R.; Thondaiman, V. Reproductive Biology and Breeding System of Saraca asoca (Roxb.) De Wilde: A Vulnerable Medicinal Plant. Springerplus 2016, 5, 2025. [Google Scholar] [CrossRef] [PubMed]


| Capitulum Diameter (mm) | Ligules Number | Ligules Length (mm) | Ligules Width (mm) | Florets Number | Diameter of All Florets (mm) | |
|---|---|---|---|---|---|---|
| O. taihangensis | 47.09 ± 1.67 a | 29.70 ± 2.64 a | 21.50 ± 1.08 a | 5.46 ± 0.67 a | 302.52 ± 65.31 a | 14.11 ± 0.38 a |
| O. longilobus | 38.70 ± 0.85 b | 23.20 ± 1.74 b | 16.36 ± 1.20 a | 4.99 ± 0.45 a | 285.04 ± 60.27 b | 12.18 ± 0.77 a |
| 1d | 2d | 3d | 4d | 5d | 6d | 7d | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| O. taihangensis | 93.69 ± 0.01% | 91.93 ± 0.09% | 90.75 ± 0.27% | 80.4 ± 0.47% | 60.24 ± 2.29% | 53.89 ± 1.08% | 40.73 ± 1.54% | |||
| O. longilobus | 88.69 ± 0.09% | 86.22 ± 0.25% | 81.76 ± 0.17% | 68.52 ± 0.85% | 52.90 ± 0.33% | 45.31 ± 0.39% | 39.44 ± 0.15% | |||
| 8:00 | 9:00 | 10:00 | 11:00 | 12:00 | 13:00 | 14:00 | 15:00 | 16:00 | 17:00 | |
| O. taihangensis | 70.0 ± 0.05% | 83.5 ± 0.14% | 88.7 ± 0.22% | 90.3 ± 0.19% | 96.0 ± 0.32% | 97.2 ± 1.60% | 89.3 ± 0.15% | 83.3 ± 0.20% | 80.9 ± 3.11% | 76.1 ± 0.29% |
| O. longilobus | 68.7 ± 0.09% | 78.9 ± 1.39% | 82.2 ± 0.19% | 88.4 ± 0.28% | 94.9 ± 0.17% | 96.4 ± 0.11% | 87 ± 1.03% | 83.5 ± 0.03% | 82.2 ± 0.03% | 73.7 ± 1.81% |
| 1d | 2d | 3d | 4d | 5d | 6d | 7d | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| O. taihangensis | + | ++ | +++ | +++ | ++ | + | - | |||
| O. longilobus | + | ++ | ++ | +++ | ++ | + | - | |||
| 8:00 | 9:00 | 10:00 | 11:00 | 12:00 | 13:00 | 14:00 | 15:00 | 16:00 | 17:00 | |
| O. taihangensis | ++ | ++ | ++ | +++ | +++ | +++ | ++ | ++ | ++ | ++ |
| O. longilobus | ++ | ++ | ++ | ++ | ++ | +++ | ++ | ++ | ++ | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Lan, Y.; Ye, H.; Feng, X.; Qie, Q.; Liu, L.; Chai, M. Reproductive Biology and Breeding Systems of Two Opisthopappus Endemic and Endangered Species on the Taihang Mountains. Plants 2023, 12, 1954. https://doi.org/10.3390/plants12101954
Wang Y, Lan Y, Ye H, Feng X, Qie Q, Liu L, Chai M. Reproductive Biology and Breeding Systems of Two Opisthopappus Endemic and Endangered Species on the Taihang Mountains. Plants. 2023; 12(10):1954. https://doi.org/10.3390/plants12101954
Chicago/Turabian StyleWang, Yiling, Yafei Lan, Hang Ye, Xiaolong Feng, Qiyang Qie, Li Liu, and Min Chai. 2023. "Reproductive Biology and Breeding Systems of Two Opisthopappus Endemic and Endangered Species on the Taihang Mountains" Plants 12, no. 10: 1954. https://doi.org/10.3390/plants12101954
APA StyleWang, Y., Lan, Y., Ye, H., Feng, X., Qie, Q., Liu, L., & Chai, M. (2023). Reproductive Biology and Breeding Systems of Two Opisthopappus Endemic and Endangered Species on the Taihang Mountains. Plants, 12(10), 1954. https://doi.org/10.3390/plants12101954








