Priorities for Bark Anatomical Research: Study Venues and Open Questions
Abstract
:1. Bark Structure and Development
2. Bark Dilatation
3. Hormonal Control of Bark Structure
4. Architectural Types of Bark: Relationships between Macroscopic Appearance and Anatomical Structure
5. Phloem
6. Bark Ecology
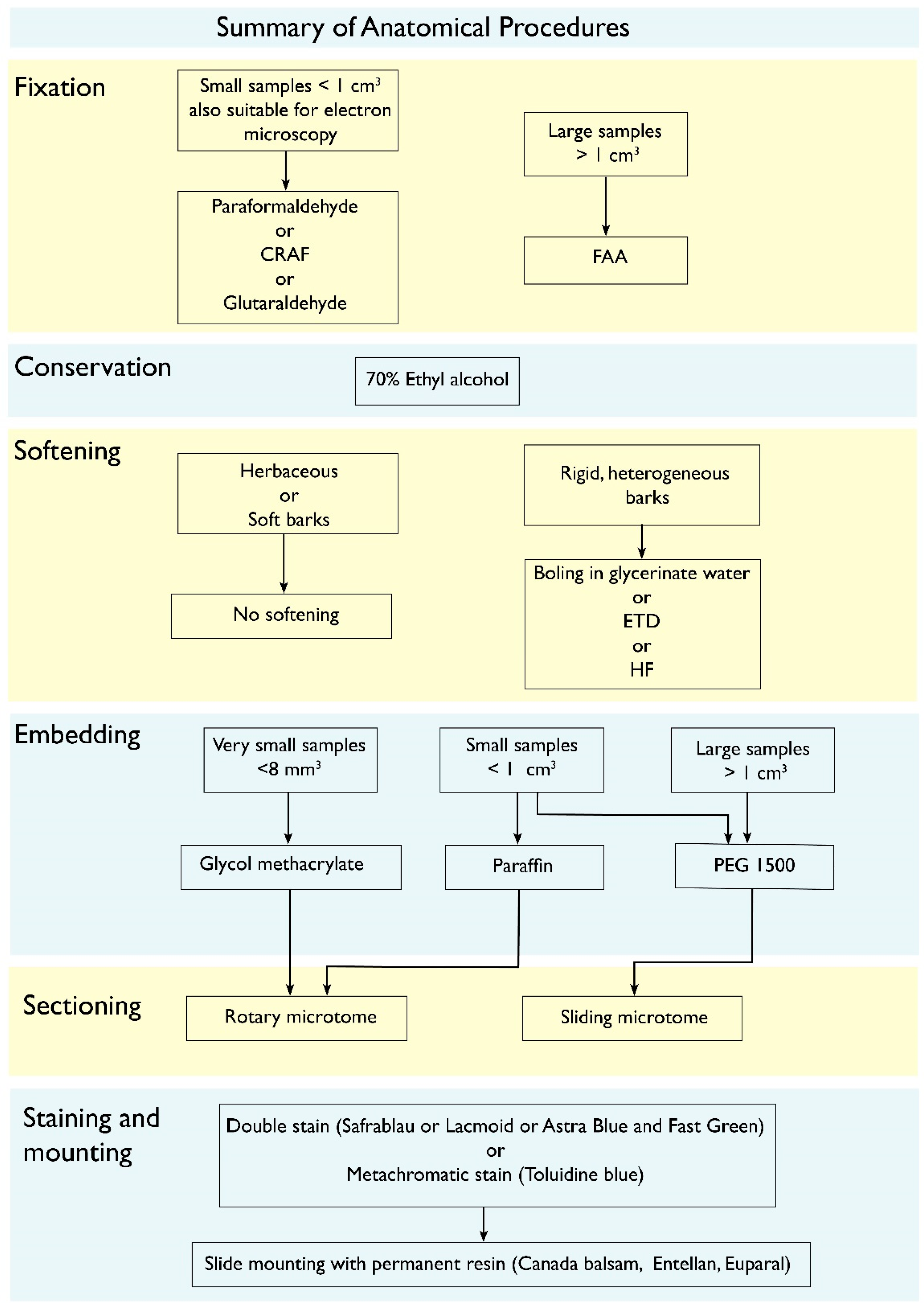
7. Overcoming the Methodological Limitation
8. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
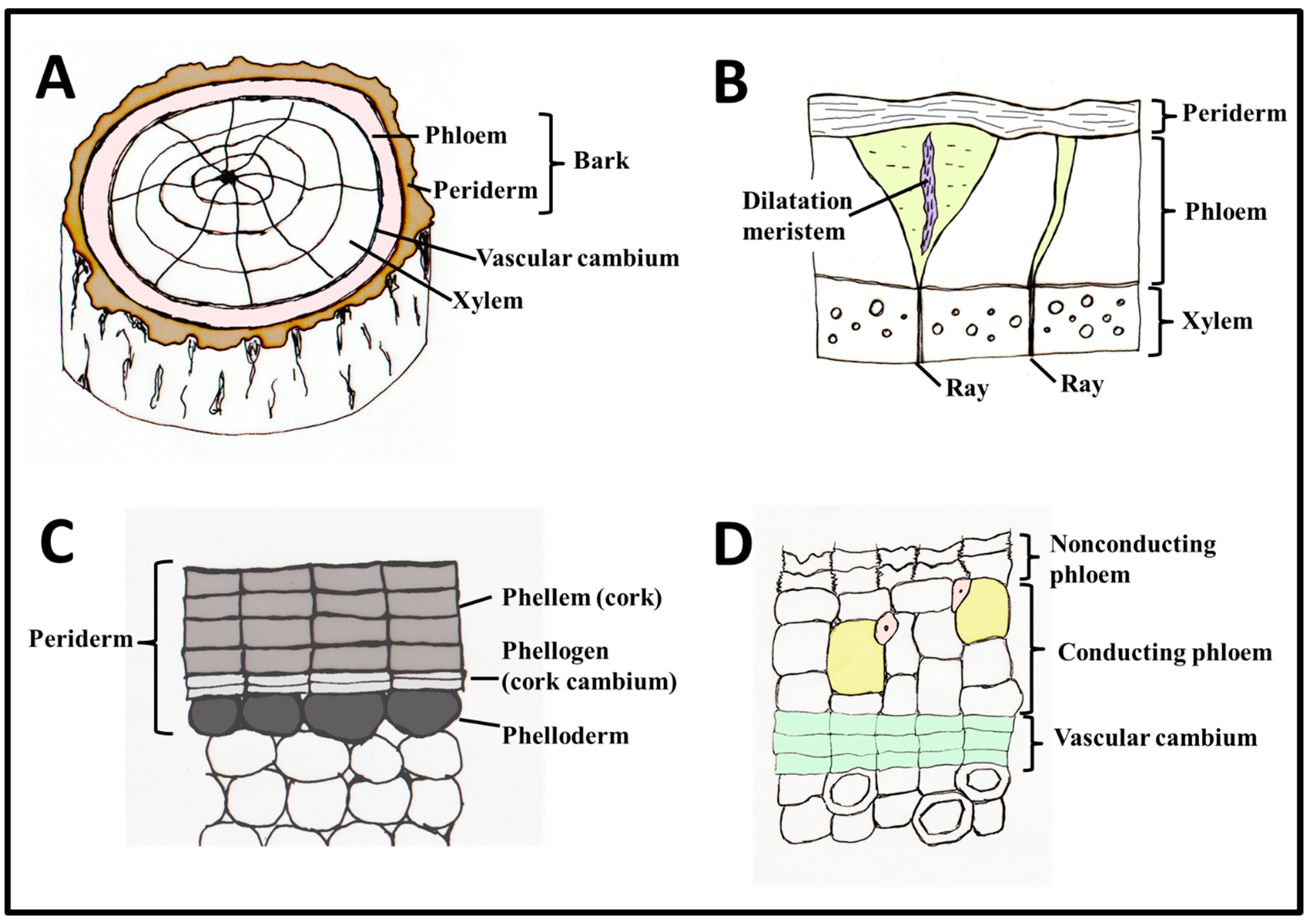
Glossary (After Angyalossy et al. [17] (also see Figure 1)
References
- Fahn, A. Plant Anatomy, 4th ed.; Pergamon Press: Oxford, UK, 1990. [Google Scholar]
- Lev-Yadun, S. Patterns of dilatation growth in Ficus pumila and Ficus sycomorus. Aliso 1996, 14, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Rosner, S.; Morris, H. Breathing life into trees: The physiological and biomechanical functions of lenticels. IAWA J. 2022, 43, 234–262. [Google Scholar] [CrossRef]
- Holloway, P.J. Some variations in the composition of suberin from the cork layers of higher plants. Phytochemistry 1983, 22, 495–502. [Google Scholar] [CrossRef]
- Pereira, H. Variability of the chemical composition of cork. Bioresources 2013, 8, 2246–2256. [Google Scholar] [CrossRef]
- Pinto, P.C.R.O.; Sousa, A.F.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A.; Eckerman, C.; Holmbom, B. Quercus suber and Betula pendula outer barks as renewable sources of oleochemicals: A comparative study. Ind. Crops Prod. 2009, 29, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Pereira, H. Structure and chemical composition of cork from Calotropis procera. IAWA J. 1988, 9, 53–58. [Google Scholar] [CrossRef]
- Esau, K. Anatomy and cytology of Vitis phloem. Hilgardia 1965, 37, 17–72. [Google Scholar] [CrossRef] [Green Version]
- Lev-Yadun, S.; Aloni, R. Polar patterns of periderm ontogeny, their relationship to leaves and buds, and the control of cork formation. IAWA Bull. 1990, 11, 289–300. [Google Scholar] [CrossRef]
- Trockenbrodt, M. Qualitative structural changes during bark development in Quercus robur, Ulmus glabra, Populus tremula and Betula pendula. IAWA Bull. 1991, 12, 5–22. [Google Scholar] [CrossRef]
- Cardoso, S.; Quilhó, T.; Pereira, H. Influence of cambial age on the bark structure of Douglas-fir. Wood Sci. Technol. 2019, 53, 191–210. [Google Scholar] [CrossRef]
- Quilhó, T.; Pereira, H.; Richter, H.G. Within–tree variation in phloem cell dimensions and proportions in Eucalyptus globulus. IAWA J. 2000, 21, 31–40. [Google Scholar] [CrossRef]
- Arzee, T.; Lipshschitz, N.; Waisel, Y. The origin and development of the phellogen in Robinia pseudacacia L. New Phytol. 1968, 67, 87–93. [Google Scholar] [CrossRef]
- Roth, I. Structural Patterns of Tropical Barks; Gebrüder Borntraeger: Berlin, Germany, 1981. [Google Scholar]
- Nejapa, R.; Pace, M.R. Wood and bark anatomy of the charismatic Wisteria vines (Leguminosae). IAWA J. 2023, 44, 1–13. [Google Scholar] [CrossRef]
- Fink, S. Pathological and Regenerative Plant Anatomy; Gebrüder Borntraeger: Berlin, Germany, 1999. [Google Scholar]
- Angyalossy, V.; Pace, M.R.; Evert, R.F.; Marcati, C.R.; Oskolski, A.A.; Terrazas, T.; Kotina, E.; Lens, F.; Mazzoni-Viveiros, S.C.; Angeles, G.; et al. IAWA List of Microscopic Bark Features. IAWA J. 2016, 37, 517–615. [Google Scholar] [CrossRef] [Green Version]
- Amano, E. Cordia trichotoma, Boraginaceae: Caracterização e Sazonalidade na Formação Do Xilema e Do Floema. Master’s Thesis, University of São Paulo, São Paulo, Brazil, 2002. [Google Scholar]
- Evert, R.F. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body—Their Structure, Function, and Development, 3rd ed.; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Lev-Yadun, S.; Aloni, R. Experimental induction of dilatation meristem in Melia azedarach. Ann. Bot. 1992, 70, 379–386. [Google Scholar] [CrossRef]
- Sachs, T. The control of patterned differentiation of vascular tissues. Adv. Bot. Res. 1981, 9, 151–262. [Google Scholar]
- Gorshkova, T.; Brutch, N.; Chabbert, B.; Deyholos, M.; Hayashi, T.; Lev-Yadun, S.; Mellerowicz, E.J.; Morvan, C.; Neutelings, G.; Pilate, G. Plant fiber formation: State of the art, recent and expected progress, and open questions. Crit. Rev. Plant Sci. 2012, 31, 201–228. [Google Scholar] [CrossRef]
- Lev-Yadun, S.; Liphschitz, N. Sites of first phellogen initiation in conifers. IAWA Bull. 1989, 10, 43–52. [Google Scholar] [CrossRef]
- Lev-Yadun, S.; Aloni, R. Bark structure and mode of canopy regeneration in trees of Melia azedarach L. Trees Struct. Funct. 1993, 7, 144–147. [Google Scholar] [CrossRef]
- Wetmore, R.H. Organization and significance of lenticels in dicotyledons I. Lenticels in relation to aggregate and compound storage rays in woody stems. Lenticels and roots. Bot. Gaz. 1926, 82, 71–88. [Google Scholar] [CrossRef]
- Wetmore, R.H. Organization and significance of lenticels in dicotyledons II. Lenticels in relation to diffuse storage rays of woody stems. Bot. Gaz. 1926, 82, 113–131. [Google Scholar] [CrossRef]
- Lev-Yadun, S.; Aloni, R. Differentiation of the ray system in woody plants. Bot. Rev. 1995, 61, 45–88. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Krekling, T.; Christianses, E. Application of Methyl Jasmonate on Picea abies (Pinaceae) stems induces defense-related responses in phloem and xylem. Am. J. Bot. 2002, 89, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Hudgins, J.W.; Franceschi, V.R. Methyl jasmonate-induced ethylene production is responsible for conifer phloem defense responses and reprogramming of stem cambial zone for traumatic resin duct formation. Plant Physiol. 2004, 135, 2134–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudgins, J.W.; Christiansen, E.; Franceschi, V.R. Methyl jasmonate induces changes mimicking anatomical defenses in diverse members of the Pinaceae. Tree Physiol. 2003, 23, 361–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, S.T.; Sobral, D.; Costa, B.; Perdiguero, P.; Chaves, I.; Costa, A.; Miguel, C.M. Phellem versus xylem: Genome-wide transcriptomic analysis reveals novel regulators of cork formation in cork oak. Tree Physiol. 2020, 40, 129–141. [Google Scholar] [CrossRef]
- Vulavala, V.K.R.; Fogelman, E.; Faigenboim, A.; Shoseyov, O.; Ginzberg, I. The transcriptome of potato tuber phellogen reveals cellular functions of cork cambium and genes involved in periderm formation and maturation. Sci. Rep. 2019, 9, 10216. [Google Scholar] [CrossRef] [Green Version]
- Wunderling, A.; Ripper, D.; Barra-Jimenez, A.; Mahn, S.; Sajak, K.; Targem, M.B.; Ragni, L. A molecular framework to study periderm formation in Arabidopsis. New Phytol. 2018, 219, 216–229. [Google Scholar] [CrossRef]
- Chattaway, M.M. The anatomy of bark. V. Eucalyptus species with stringy bark. Aust. J. Bot. 1955, 3, 165–169. [Google Scholar] [CrossRef]
- Chattaway, M.M. The anatomy of bark. VI. Peppermints, boxes, ironbarks, and other eucalypts with cracked and furrowed barks. Aust. J. Bot. 1955, 3, 170–176. [Google Scholar] [CrossRef]
- Whitmore, T.C. Studies in systematic bark morphology I Bark morphology in Dipterocarpaceae. New Phytol. 1962, 61, 191–207. [Google Scholar] [CrossRef]
- Whitmore, T.C. Studies in systematic bark morphology II General features of bark construction in Dipterocarpaceae. New Phytol. 1962, 61, 208–220. [Google Scholar] [CrossRef]
- Whitmore, T.C. Studies in systematic bark morphology III Bark taxonomy in Dipterocarpaeae. Gardens’ Bull. 1962, 19, 321–372. [Google Scholar]
- Kotina, E.L.; Oskolski, A.A.; Tilney, P.M.; van Wyk, B.-E. Bark anatomy of Adansonia digitata (Malvaceae). Adansonia 2003, 39, 31–40. [Google Scholar] [CrossRef]
- Kotina, E.L.; Tilney, P.M.; van Wyk, A.E.; Oskolski, A.A.; van Wyk, B.-E. “Hairy” bark in Lannea schweinfurthii (Anacardiaceae): Hyperhydric-like tissue formed under arid conditions. IAWA J. 2018, 39, 221–233. [Google Scholar] [CrossRef]
- Trockenbrodt, M. Survey and discussion of the terminology used in bark anatomy. IAWA Bull. 1990, 11, 141–166. [Google Scholar] [CrossRef]
- Junikka, L. Survey of English macroscopic bark terminology. IAWA J. 1994, 15, 3–45. [Google Scholar] [CrossRef]
- Burrows, G.E.; Chisnall, K.L. Buds buried in bark: The reason why Quercus suber (cork oak) is an excellent post-fire epicormic resprouter. Trees 2016, 30, 241–254. [Google Scholar] [CrossRef]
- Frankiewicz, K.E.; Chau, J.H.; Oskolski, A.A. Wood and bark of Buddleja: Uniseriate phellem, and systematic and ecological patterns. IAWA J. 2020, 42, 3–30. [Google Scholar] [CrossRef]
- Schweingruber, F.H.; Landolt, W. The Xylem Database. Swiss Federal Institute for Forest, Snow and Landscape Research. Available online: https://www.wsl.ch/dendropro/xylemdb/ (accessed on 20 November 2022).
- Petit, G.; Crivellaro, A. Comparative axial widening of phloem and xylem conduits in small woody plants. Trees 2014, 28, 915–921. [Google Scholar] [CrossRef]
- Jyske, T.; Hölttä, T. Comparison of phloem and xylem hydraulic architecture in Picea abies stems. New Phytol. 2015, 205, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Kiorapostolou, N.; Petit, G. Similarities and differences in the balances between leaf, xylem and phloem structures in Fraxinus ornus along an environmental gradient. Tree Physiol. 2018, 39, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Balzano, A.; De Micco, V.; Čufar, K.; De Luis, M.; Gričar, J. Intra-seasonal trends in phloem traits in Pinus spp. from drought-prone environments. IAWA J. 2020, 41, 219–235. [Google Scholar] [CrossRef]
- Gričar, J.; Jevšenak, J.; Hafner, P.; Prislan, P.; Ferlan, M.; Lavrič, M.; Vodnik, D.; Eler, K. Climatic regulation of leaf and cambial phenology in Quercus pubescens: Their interlinkage and impact on xylem and phloem conduits. Sci. Total Environ. 2022, 802, 149968. [Google Scholar] [CrossRef] [PubMed]
- Gričar, J.; Prislan, P.; De Luis, M.; Novak, K.; Longares, L.A.; Martinez del Castillo, E.; Čufar, K. Lack of annual periodicity in cambial production of phloem in trees from Mediterranean areas. IAWA J. 2016, 37, 332–348. [Google Scholar] [CrossRef] [Green Version]
- Gričar, J.; Prislan, P. Seasonal changes in the width and structure of non-collapsed phloem affect the assessment of its potential conducting efficiency. IAWA J. 2022, 43, 219–233. [Google Scholar] [CrossRef]
- Dannoura, M.; Epron, D.; Desalme, D.; Massonnet, C.; Tsuji, S.; Plain, C.; Priault, P.; Gérant, D. The impact of prolonged drought on phloem anatomy and phloem transport in young beech trees. Tree Physiol. 2019, 39, 201–210. [Google Scholar] [CrossRef]
- Gričar, J.; Hafner, P.; Lavrič, M.; Ferlan, M.; Ogrinc, N.; Krajnc, B.; Eler, K.; Vodnik, D. Post-fire effects on development of leaves and secondary vascular tissues in Quercus pubescens. Tree Physiol. 2020, 40, 796–809. [Google Scholar] [CrossRef]
- Kiorapostolou, N.; Camarero, J.J.; Carrer, M.; Sterck, F.; Brigita, B.; Sangüesa-Barreda, G.; Petit, G. Scots pine trees react to drought by increasing xylem and phloem conductivities. Tree Physiol. 2020, 40, 774–781. [Google Scholar] [CrossRef]
- Mullendore, D.L.; Windt, C.W.; Van As, H.; Knoblauch, M. Sieve tube geometry in relation to phloem flow. Plant Cell 2010, 22, 579–593. [Google Scholar] [CrossRef] [Green Version]
- Lachaud, S.; Catesson, A.M.; Bonnemain, J.L. Structure and functions of the vascular cambium. Comptes rendus de l’Academie des sciences. Ser. III Sci. De La Vie 1999, 322, 633–650. [Google Scholar]
- Larson, P.R. The Vascular Cambium: Development and Structure; Springer: Berlin, Germany, 1994. [Google Scholar]
- Rosner, S.; Baier, P.; Kikuta, S.B. Osmotic potential of Norway spruce [Picea abies (L.) Karst.] secondary phloem in relation to anatomy. Trees 2001, 15, 472–482. [Google Scholar] [CrossRef]
- Paine, C.E.T.; Stahl, C.; Courtois, E.A.; Patiño, S.; Sarmiento, C.; Baraloto, C. Functional explanations for variation in bark thickness in tropical rain forest trees. Funct. Ecol. 2010, 24, 1202–1210. [Google Scholar] [CrossRef]
- Romero, C. Bark structure and functional ecology. In Advances in Economic Botany. Bark: Use, Management, and Commerce in Africa; Cunningham, A.B., Campbell, B.M., Luckert, M.K., Eds.; The New York Botanical Garden Press: New York, NY, USA, 2014; pp. 5–25. [Google Scholar]
- Rosell, J.A. Bark in woody plants: Understanding the diversity of a multifunctional structure. Integr. Comp. Biol. 2019, 59, 535–547. [Google Scholar] [CrossRef]
- Lawes, M.J.; Adie, H.; Russell-Smith, J.; Murphy, B.; Midgley, J.J. How do small savanna trees avoid stem mortality by fire? The roles of stem diameter, height and bark thickness. Ecosphere 2011, 2, 42. [Google Scholar] [CrossRef]
- Graves, S.J.; Rifai, S.W.; Putz, F.E. Outer bark thickness decreases more with height on stems of fire-resistant than fire-sensitive Floridian oaks (Quercus spp.; Fagaceae). Am. J. Bot. 2014, 101, 2183–2188. [Google Scholar] [CrossRef] [Green Version]
- Savage, J.A.; Clearwater, M.J.; Haines, D.F.; Klein, T.; Mencuccini, M.; Sevanto, S.; Turgeon, R.; Zhang, C. Allocation, stress tolerance and carbon transport in plants: How does phloem physiology affect plant ecology? Plant Cell Environ. 2016, 39, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, L.M. Anatomy, chemistry and physiology of bark. Int. Rev. For. Res. 1964, 1, 203–277. [Google Scholar]
- Rosell, J.A.; Olson, M.E. The evolution of bark mechanics and storage across habitats in a clade of tropical trees. Am. J. Bot. 2014, 101, 764–777. [Google Scholar] [CrossRef] [Green Version]
- Loram-Lourenco, L.; Farnese, F.D.S.; Sousa, L.F.D.; Alves, R.D.F.B.; Andrade, M.C.P.D.; Almeida, S.E.D.S.; Moura, L.M.D.F.; Costa, A.C.; Silva, F.G.; Galmes, J.; et al. A structure shaped by fire, but also water: Ecological consequences of the variability in bark properties across 31 species from the Brazilian Cerrado. Front. Plant Sci. 2020, 10, 1718. [Google Scholar] [CrossRef] [PubMed]
- Rosell, J.A.; Piper, F.; Jiménez-Vera, C.; Vergílio, P.; Marcati, C.; Castorena, M.; Olson, M. Inner bark as a crucial tissue for non-structural carbohydrate storage across three tropical woody plant communities. Plant Cell Environ. 2021, 44, 156–170. [Google Scholar] [CrossRef]
- Niklas, K.J. The mechanical role of bark. Am. J. Bot. 1999, 86, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Lehnebach, R.; Alméras, T.; Clair, B. How does bark contribution to postural control change during tree ontogeny? A study of six Amazonian tree species. J. Exp. Bot. 2020, 71, 2641–2649. [Google Scholar] [CrossRef] [PubMed]
- Pfanz, H. Bark photosynthesis. Trees 2008, 22, 137–138. [Google Scholar] [CrossRef]
- Rosell, J.A.; Castorena, M.; Laws, C.; Westoby, M. Bark ecology of twigs vs main stems: Functional traits across 85 species of angiosperms. Oecologia 2015, 178, 1033–1043. [Google Scholar] [CrossRef]
- Lev-Yadun, S. Why is the bark of common temperate Betula and Populus species white? Int. J. Plant Sci. 2019, 180, 632–642. [Google Scholar] [CrossRef]
- Ireland, H.M.; Ruxton, G.D. White bark in birch species as a warning signal for bark-stripping mammals. Plant Ecol. Divers. 2022, 15, 93–109. [Google Scholar] [CrossRef]
- Harvey, R.B. Relation of the color of bark to the temperature of the cambium in winter. Ecology 1923, 4, 391–394. [Google Scholar] [CrossRef]
- Rosell, J.A.; Gleason, S.M.; Méndez-Alonzo, R.; Chang, Y.; Westoby, M. Bark functional ecology: Evidence for tradeoffs, functional coordination, and environment producing bark diversity. New Phytol. 2014, 201, 486–497. [Google Scholar] [CrossRef]
- Dossa, G.G.O.; Paudel, E.; Cao, K.; Schaefer, D.; Harrison, R.D. Factors controlling bark decomposition and its role in wood decomposition in five tropical tree species. Sci. Rep. 2016, 6, 34153. [Google Scholar] [CrossRef] [Green Version]
- Poorter, L.; McNeil, A.; Hurtado, V.H.; Prins, H.; Putz, J. Bark traits and life history strategies of tropical dry- and moist forest trees. Funct. Ecol. 2014, 28, 232–242. [Google Scholar] [CrossRef] [Green Version]
- Van Bel, A.J.E. Xylem-phloem exchange via the rays: The undervalued route of transport. J. Exp. Bot. 1990, 41, 631–644. [Google Scholar] [CrossRef]
- Pfautsch, S.; Renard, J.; Tjoelker, M.G.; Salih, A. Phloem as capacitor: Radial transfer of water into xylem of tree stems occurs via symplastic transport in ray parenchyma. Plant Physiol. 2015, 167, 963–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrenberg, S.; Mitton, J.B. Smooth bark surfaces can defend trees against insect attack: Resurrecting a 'slippery' hypothesis. Funct. Ecol. 2014, 28, 837–845. [Google Scholar] [CrossRef]
- Pace, M.R. Optimal preparation of tissue sections for light- microscopic analysis of phloem anatomy. In Phloem; Methods in Molecular Biology; Liesche, J., Ed.; Springer Protocols: Berlin/Heidelberg, Germany; Humana Press: New York, NY, USA, 2019. [Google Scholar]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill: New York, NY, USA, 1940; p. 523. [Google Scholar]
- Carlquist, S. The use of ethylenediamine in softening hard plant structures for paraffin sectioning. Stain. Technol. 1982, 57, 311–317. [Google Scholar] [CrossRef]
- Kukachka, B.F. Sectioning refractory woods for anatomical studies. In Forest Service Research Note FPL-0236; USDA: Washington, DC, USA, 1977; pp. 1–9. [Google Scholar]
- Rupp, P. Polyglykol als Einbettungsmedium zum Schneiden botanischer Präparate. Mikrokosmos 1964, 53, 123–128. [Google Scholar]
- Mozzi, G.; Romero, E.; Martínez-Quezada, D.M.; Hultine, K.R.; Crivellaro, A. PEG infiltration: An alternative method to obtain thin sections of cacti tissues. IAWA J. 2021, 42, 204–208. [Google Scholar] [CrossRef]
- Barbosa, A.C.F.; Pace, M.R.; Witovisk, L.; Angyalossy, V. A new method to obtain good anatomical slides of heterogeneous plant parts. IAWA J. 2010, 31, 373–383. [Google Scholar] [CrossRef]
- Pace, M.R.; Alcantara, S.; Lohmann, L.G.; Angyalossy, V. Secondary phloem diversity and evolution in Bignonieae (Bignoniaceae). Ann. Bot. 2015, 116, 33–358. [Google Scholar] [CrossRef] [Green Version]
- Kraus, J.E.; Arduin, M. Manual Básico de Métodos em Morfologia Vegetal; EDUR: Rio de Janeiro, Brazil, 1997. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shtein, I.; Gričar, J.; Lev-Yadun, S.; Oskolski, A.; Pace, M.R.; Rosell, J.A.; Crivellaro, A. Priorities for Bark Anatomical Research: Study Venues and Open Questions. Plants 2023, 12, 1985. https://doi.org/10.3390/plants12101985
Shtein I, Gričar J, Lev-Yadun S, Oskolski A, Pace MR, Rosell JA, Crivellaro A. Priorities for Bark Anatomical Research: Study Venues and Open Questions. Plants. 2023; 12(10):1985. https://doi.org/10.3390/plants12101985
Chicago/Turabian StyleShtein, Ilana, Jožica Gričar, Simcha Lev-Yadun, Alexei Oskolski, Marcelo R. Pace, Julieta A. Rosell, and Alan Crivellaro. 2023. "Priorities for Bark Anatomical Research: Study Venues and Open Questions" Plants 12, no. 10: 1985. https://doi.org/10.3390/plants12101985
APA StyleShtein, I., Gričar, J., Lev-Yadun, S., Oskolski, A., Pace, M. R., Rosell, J. A., & Crivellaro, A. (2023). Priorities for Bark Anatomical Research: Study Venues and Open Questions. Plants, 12(10), 1985. https://doi.org/10.3390/plants12101985







