Agronomic Evaluation and Chemical Characterization of Lavandula latifolia Medik. under the Semiarid Conditions of the Spanish Southeast
Abstract
:1. Introduction
2. Results
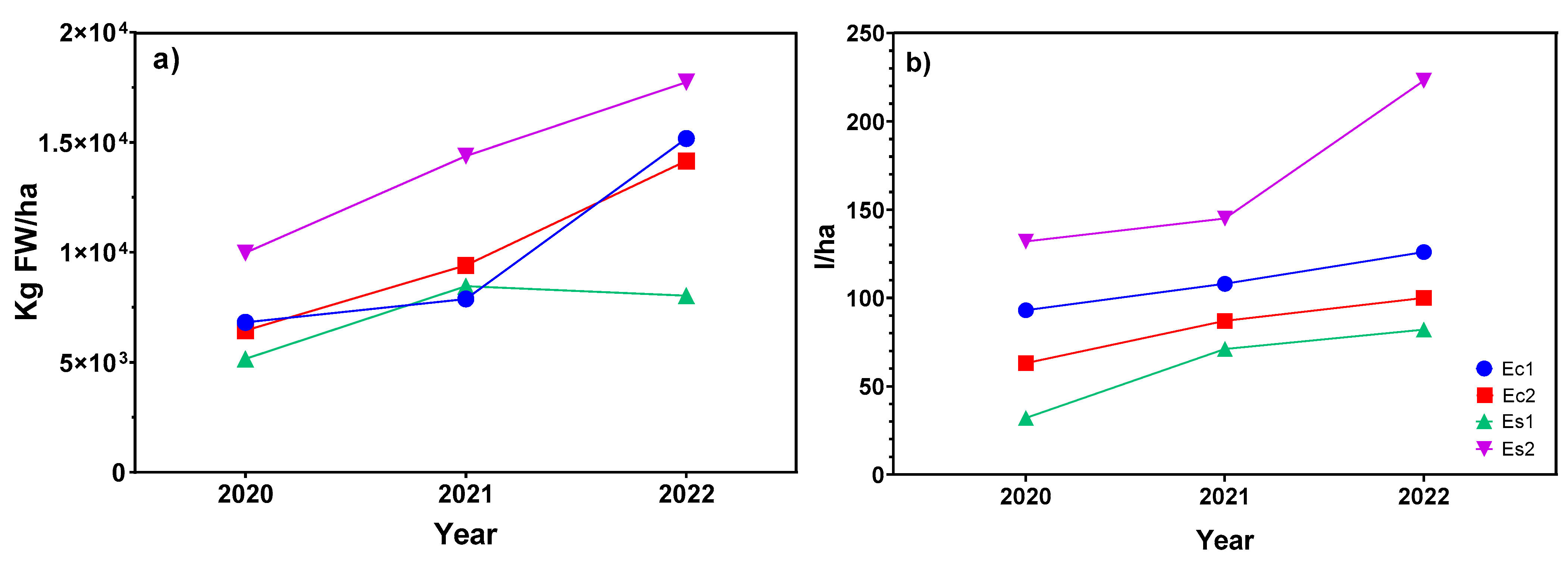
2.1. Phytomass Production and Essential Oil Yield
2.2. Essential Oil Composition
2.3. Phenolic Profile
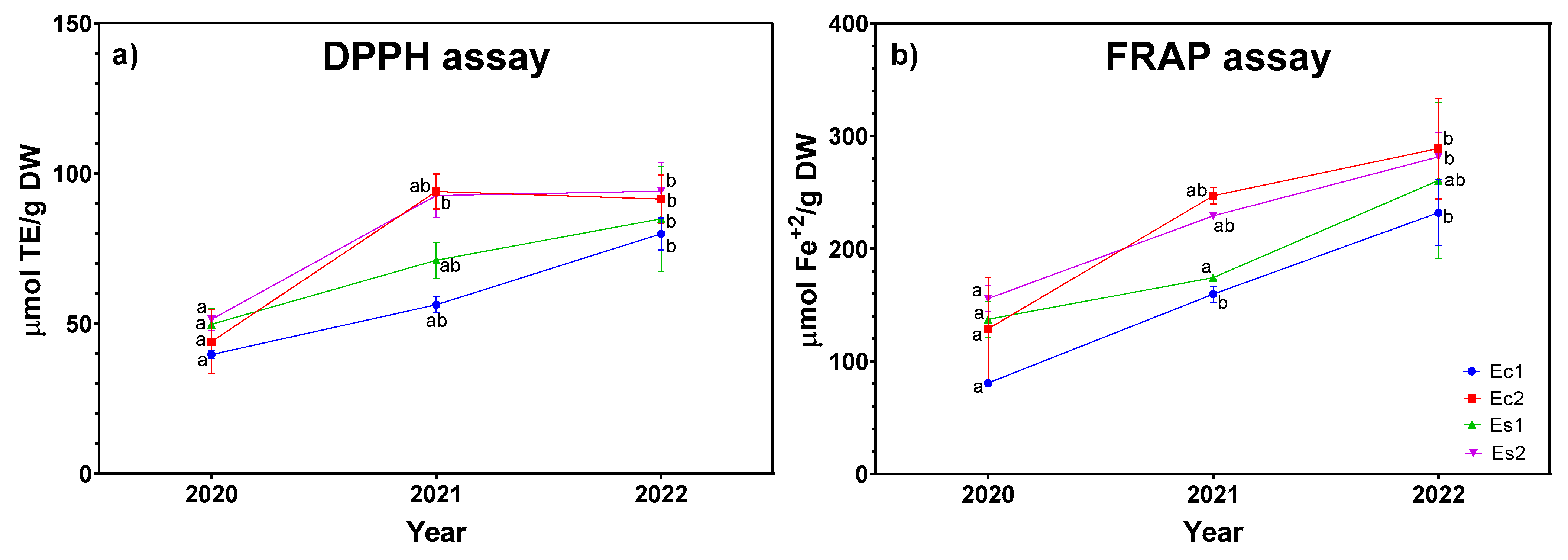
2.4. Antioxidant Activity
3. Discussion
4. Materials and Methods
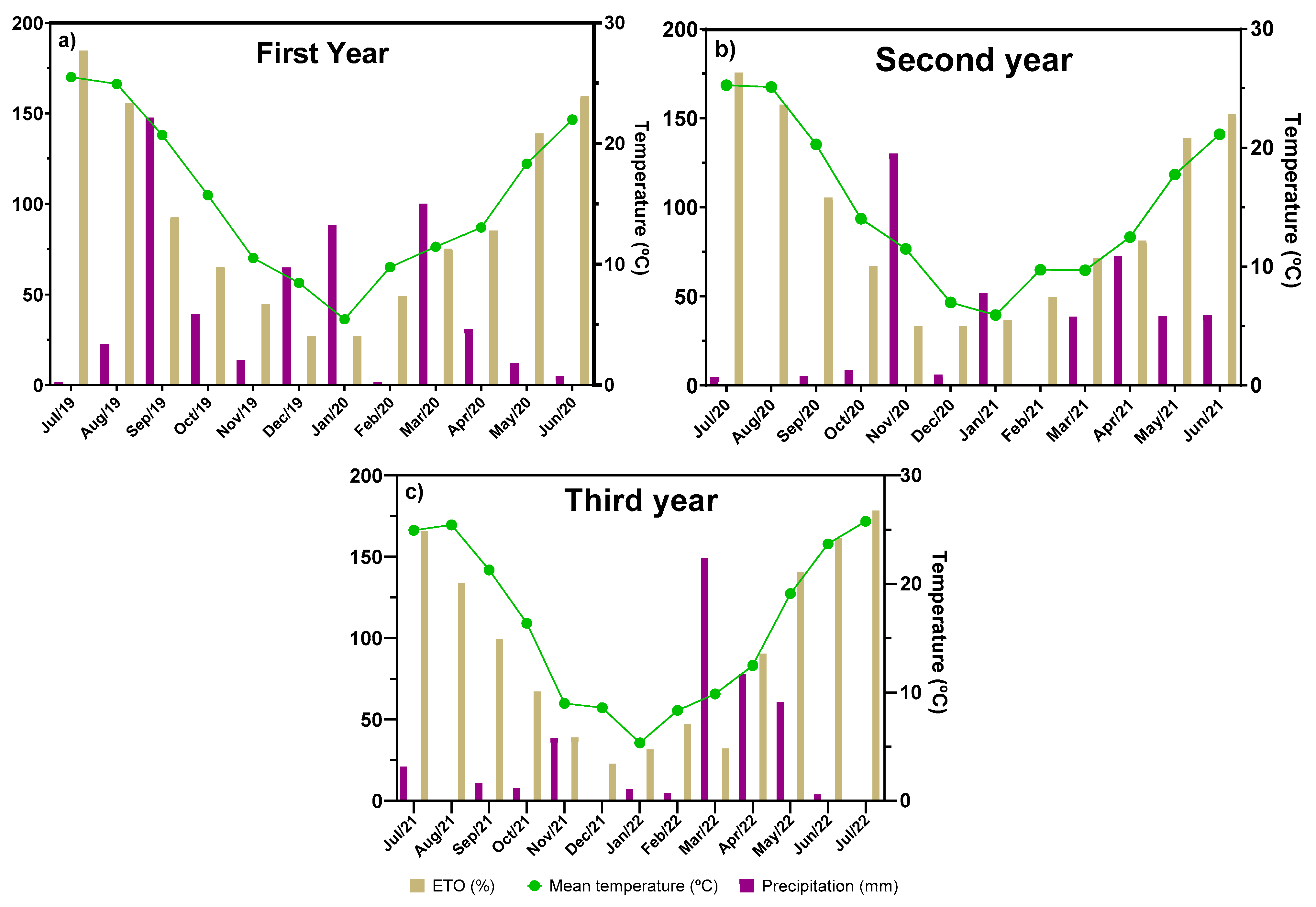
4.1. Crop Experimental Design and Plant Material
4.2. Essential Oil Extraction
4.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
4.4. Extraction of Phenolic Compounds
4.5. HPLC-DAD Analysis
4.6. Antioxidant Capacity (FRAP and DPPH Assays)
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamacque, L.; Charrier, G.; dos Santos Farnese, F.; Lemaire, B.; Améglio, T.; Herbette, S. Drought-Induced Mortality: Branch Diameter Variation Reveals a Point of No Recovery in Lavender Species. Plant Physiol. 2020, 183, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Aqeel, U.; Aftab, T.; Khan, M.M.A.; Naeem, M. Regulation of Essential Oil in Aromatic Plants under Changing Environment. J. Appl. Res. Med. Aromat. Plants 2023, 32, 100441. [Google Scholar] [CrossRef]
- Cáceres-Cevallos, G.J.; Albacete-Moreno, A.A.; Ferreres, F.; Gil-Izquierdo, Á.; Jordán, M.J. Evaluation of the Physiological Parameters in Lavandula latifolia Medik. under Water Deficit for Preselection of Elite Drought-Resistant Plants. Ind. Crops Prod. 2023, 199, 116742. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A Status Review on the Medicinal Properties of Essential Oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Gorgini Shabankareh, H.; Khorasaninejad, S.; Soltanloo, H.; Shariati, V. Physiological Response and Secondary Metabolites of Three Lavender Genotypes under Water Deficit. Sci. Rep. 2021, 11, 19164. [Google Scholar] [CrossRef] [PubMed]
- Lesage-Meessen, L.; Bou, M.; Sigoillot, J.C.; Faulds, C.B.; Lomascolo, A. Essential Oils and Distilled Straws of Lavender and Lavandin: A Review of Current Use and Potential Application in White Biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 3375–3385. [Google Scholar] [CrossRef]
- Salido, S.; Altarejos, J.; Nogueras, M.; Sánchez, A.; Luque, P. Chemical Composition and Seasonal Variations of Spike Lavender Oil from Southern Spain. J. Essent. Oil Res. 2004, 16, 206–210. [Google Scholar] [CrossRef]
- Fernández-Sestelo, M.; Carrillo, J. Environmental Effects on Yield and Composition of Essential Oil in Wild Populations of Spike Lavender (Lavandula latifolia Medik.). Agriculture 2020, 10, 626. [Google Scholar] [CrossRef]
- Mendoza-Poudereux, I.; Kutzner, E.; Huber, C.; Segura, J.; Arrillaga, I.; Eisenreich, W. Dynamics of Monoterpene Formation in Spike Lavender Plants. Metabolites 2017, 7, 65. [Google Scholar] [CrossRef]
- Herraiz-Peñalver, D.; Cases, M.Á.; Varela, F.; Navarrete, P.; Sánchez-Vioque, R.; Usano-Alemany, J. Chemical Characterization of Lavandula latifolia Medik. Essential Oil from Spanish Wild Populations. Biochem. Syst. Ecol. 2013, 46, 59–68. [Google Scholar] [CrossRef]
- Chamkhi, I.; Benali, T.; Aanniz, T.; El Menyiy, N.; Guaouguaou, F.-E.; El Omari, N.; Zengin, G.; Bouyahya, A.; El-shazly, M. Plant-Microbial Interaction: The Mechanism and the Application of Microbial Elicitor Induced Secondary Metabolites Biosynthesis in Medicinal Plants. Plant Physiol. Biochem. 2021, 167, 269–295. [Google Scholar] [CrossRef] [PubMed]
- Angioni, A.; Barra, A.; Coroneo, V.; Dessi, S.; Cabras, P. Chemical Composition, Seasonal Variability, and Antifungal Activity of Lavandula stoechas L. ssp. stoechas Essential Oils from Stem/Leaves and Flowers. J. Agric. Food Chem. 2006, 54, 4364–4370. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, V.S.; Miron, A. Essential Oils of Lavandula Genus: A Systematic Review of Their Chemistry. Phytochem. Rev. 2017, 16, 761–799. [Google Scholar] [CrossRef]
- Farhat, M.B.; Jordán, M.J.; Chaouech-Hamada, R.; Landoulsi, A.; Sotomayor, J.A. Variations in Essential Oil, Phenolic Compounds, and Antioxidant Activity of Tunisian Cultivated Salvia officinalis L. J. Agric. Food Chem. 2009, 57, 10349–10356. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Tovar, I.; Novak, J.; Sponza, S.; Herrero, B.; Asensio-S-Manzanera, M.C. Variability in Essential Oil Composition of Wild Populations of Labiatae Species Collected in Spain. Ind. Crops Prod. 2016, 79, 18–28. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Iapichino, G.; Licata, M.; Virga, G.; Leto, C.; La Bella, S. Agronomic Evaluation and Chemical Characterization of Sicilian Salvia sclarea L. Accessions. Agronomy 2020, 10, 1114. [Google Scholar] [CrossRef]
- Jordán, M.J.; Lax, V.; Rota, M.C.; Lorán, S.; Sotomayor, J.A. Effect of the Phenological Stage on the Chemical Composition, and Antimicrobial and Antioxidant Properties of Rosmarinus officinalis L Essential Oil and Its Polyphenolic Extract. Ind. Crops Prod. 2013, 48, 144–152. [Google Scholar] [CrossRef]
- Jordán, M.J.; Lax, V.; Rota, M.C.; Lorán, S.; Sotomayor, J.A. Influence of the Bioclimatic Area on the Polyphenolic Composition, and Antioxidant and Bacteriostatic Activities of Rosmarinus officinalis. Nat. Prod. Commun. 2013, 8, 817–822. [Google Scholar] [CrossRef]
- Jordán, M.J.; Martínez, R.M.; Martínez, C.; Moñino, I.; Sotomayor, J.A. Polyphenolic Extract and Essential Oil Quality of Thymus zygis ssp. gracilis Shrubs Cultivated under Different Watering Levels. Ind. Crops Prod. 2009, 29, 145–153. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Seymour, E.; Kaufman, P.B.; Warber, S.; Bolling, S.; Chang, S.C. Antioxidant Capacity of Polyphenolic Extracts from Leaves of Crataegus laevigata and Crataegus monogyna (Hawthorn) Subjected to Drought and Cold Stress. J. Agric. Food Chem. 2003, 51, 3973–3976. [Google Scholar] [CrossRef]
- Kumarappan, C.T.; Thilagam, E.; Mandal, S.C. Antioxidant Activity of Polyphenolic Extracts of Ichnocarpus frutescens. Saudi J. Biol. Sci. 2012, 19, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Dobros, N.; Zawada, K.; Paradowska, K. Phytochemical Profile and Antioxidant Activity of Lavandula angustifolia and Lavandula x intermedia Cultivars Extracted with Different Methods. Antioxidants 2022, 11, 711. [Google Scholar] [CrossRef] [PubMed]
- Ben Farhat, M.; Jordán, M.J.; Chaouch-Hamada, R.; Landoulsi, A.; Sotomayor, J.A. Changes in Phenolic Profiling and Antioxidant Capacity of Salvia aegyptiaca L. by-Products during Three Phenological Stages. LWT—Food Sci. Technol. 2015, 63, 791–797. [Google Scholar] [CrossRef]
- Pourreza, N. Phenolic Compounds as Potential Antioxidant. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 149–150. [Google Scholar] [CrossRef]
- Farías, G.; Brutti, O.; Grau, R.; Di Leo Lira, P.; Retta, D.; van Baren, C.; Vento, S.; Bandoni, A.L. Morphological, Yielding and Quality Descriptors of Four Clones of Origanum Spp. (Lamiaceae) from the Argentine Littoral Region Germplasm Bank. Ind. Crops Prod. 2010, 32, 472–480. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Skaltsa, H.; Konstantopoulou, M. Medicinal and Aromatic Plants (MAPs): The Connection between Cultivation Practices and Biological Properties. Agronomy 2022, 12, 3108. [Google Scholar] [CrossRef]
- Sintaha, M.; Man, C.K.; Yung, W.S.; Duan, S.; Li, M.W.; Lam, H.M. Drought Stress Priming Improved the Drought Tolerance of Soybean. Plants 2022, 11, 2954. [Google Scholar] [CrossRef] [PubMed]
- Najar, B.; Pistelli, L.; Ferri, B.; Angelini, L.G.; Tavarini, S. Crop Yield and Essential Oil Composition of Two Thymus vulgaris Chemotypes along Three Years of Organic Cultivation in a Hilly Area of Central Italy. Molecules 2021, 26, 5109. [Google Scholar] [CrossRef]
- Muñoz-Bertomeu, J.; Arrillaga, I.; Segura, J. Essential Oil Variation within and among Natural Populations of Lavandula latifolia and Its Relation to Their Ecological Areas. Biochem. Syst. Ecol. 2007, 35, 479–488. [Google Scholar] [CrossRef]
- Méndez-Tovar, I.; Herrero, B.; Pérez-Magariño, S.; Pereira, J.A.; Asensio-S.-Manzanera, M.C. By-Product of Lavandula latifolia Essential Oil Distillation as Source of Antioxidants. J. Food Drug Anal. 2015, 23, 225–233. [Google Scholar] [CrossRef]
- Dobros, N.; Zawada, K.D.; Paradowska, K. Phytochemical Profiling, Antioxidant and Anti-Inflammatory Activity of Plants Belonging to the Lavandula Genus. Molecules 2023, 28, 256. [Google Scholar] [CrossRef] [PubMed]
- Nurzyńska-Wierdak, R. Phenolic Compounds from New Natural Sources—Plant Genotype and Ontogenetic Variation. Molecules 2023, 28, 1731. [Google Scholar] [CrossRef] [PubMed]
- Di Ferdinando, M.; Brunetti, C.; Agati, G.; Tattini, M. Multiple Functions of Polyphenols in Plants Inhabiting Unfavorable Mediterranean Areas. Environ. Exp. Bot. 2014, 103, 107–116. [Google Scholar] [CrossRef]
- Gunnaiah, R.; Kushalappa, A.C.; Duggavathi, R.; Fox, S.; Somers, D.J. Integrated Metabolo-Proteomic Approach to Decipher the Mechanisms by Which Wheat Qtl (Fhb1) Contributes to Resistance against Fusarium graminearum. PLoS ONE 2012, 7, e40695. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants in Plants: Location and Functional Significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Cáceres-Cevallos, G.; Martínez-Conesa, C.; Romero-Espinar, P.; Quílez, M.; García-Aledo, I.; Jordán, M.J. ESTABLECIMIENTO DE UN PROTOCOLO DE MICROPROPAGACIÓN DE DOS ECOTIPOS DE ESPLIEGO (Lavandula latifolia Medik.) DE INTERÉS EN LA INDUSTRIA ALIMENTARIA. In Proceedings of the XIV Reunión De La Sociedad Española De Cultivo In Vitro De Tejidos Vegetales Alimentaria; Barceló, A., Sánchez, P., Antón, T., Eds.; Semillero Laimund S.L.: Almería, Spain, 2021; p. 140. [Google Scholar]
- Sotomayor, J.A.; Martínez, R.M.; García, A.J.; Jordán, M.J. Thymus zygis Subsp. gracilis: Watering Level Effect on Phytomass Production and Essential Oil Quality. J. Agric. Food Chem. 2004, 52, 5418–5424. [Google Scholar] [CrossRef]
- Council of Europe, European Pharmacopoeia Commission and European Directorate for the Quality of Medicines & Healthcare. European Pharmacopoeia, 7th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) of the Council of Europe: Strasbourg, France, 2010. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
| Ec1 | Ec2 | Es1 | Es2 | |
|---|---|---|---|---|
| Essential oil yield (%) | 3.0 | 2.2 | 2.3 | 2.9 |
| Chemotype | linalool/1,8-cineol/camphor | |||
| Relative concentration (%) | 48/32/9 | 10/46/9 | 40/35/9 | 26/45/9 |
| Components Relative Concentration (%) | RI | Year | Ec1 | Ec2 | Es1 | Es2 |
|---|---|---|---|---|---|---|
| α-Pinene | 963 | 2020 | 0.9 ± 0.20 | 1.9 ± 0.22 | 2.1 ± 0.18 | 1.5 ± 0.20 |
| 2021 | 0.9 ± 0.06 | 1.7 ± 0.20 | 1.5 ± 0.37 | 1.5 ± 0.12 | ||
| 2022 | 1.0 ± 0.09 | 2.0 ± 0.20 | 1.4 ± 0.27 | 1.6 ± 0.10 | ||
| Camphene | 971 | 2020 | 0.2 ± 0.11 | 0.2 ± 0.03 a | 0.2 ± 0.04 a | 0.2 ± 0.02 a |
| 2021 | 0.3 ± 0.01 | 0.3 ± 0.02 b | 0.3 ± 0.06 ab | 0.3 ± 0.05 b | ||
| 2022 | 0.3 ± 0.11 | 0.4 ± 0.01 b | 0.3 ± 0.02 b | 0.4 ± 0.01 b | ||
| Sabinene | 987 | 2020 | 0.4 ± 0.02 a | 1.1 ± 0.12 | 0.7 ± 0.03 | 0.8 ± 0.05 |
| 2021 | 0.5 ± 0.02 a | 1.0 ± 0.04 | 0.6 ± 0.12 | 0.8 ± 0.04 | ||
| 2022 | 0.6 ± 0.02 b | 1.2 ± 0.05 | 0.7 ± 0.07 | 0.9 ± 0.09 | ||
| β-Pinene | 989 | 2020 | 1.4 ± 0.18 | 3.5 ± 0.34 b | 2.8 ± 0.19 b | 2.7 ± 0.26 |
| 2021 | 1.3 ± 0.07 | 2.8 ± 0.17 a | 1.9 ± 0.33 a | 2.3 ± 0.11 | ||
| 2022 | 1.6 ± 0.18 | 3.5 ± 0.26 b | 2.1 ± 0.26 a | 2.7 ± 0.14 | ||
| 1-Octen-3-ol | 991 | 2020 | 0.0 ± 0.01 | 0.1 ± 0.02 | 0.0 ± 0.01 | 0.0 ± 0.00 |
| 2021 | 0.0 ± 0.01 | 0.1 ± 0.00 | 0.0 ± 0.01 | 0.0 ± 0.01 | ||
| 2022 | 0.1 ± 0.01 | 0.1 ± 0.05 | 0.1 ± 0.05 | 0.0 ± 0.01 | ||
| Myrcene | 998 | 2020 | 0.3 ± 0.03 a | 0.7 ± 0.09 a | 0.5 ± 0.01 | 0.5 ± 0.06 a |
| 2021 | 0.4 ± 0.02 a | 0.7 ± 0.06 a | 0.4 ± 0.07 | 0.6 ± 0.0 ab | ||
| 2022 | 0.5 ± 0.03 b | 0.9 ± 0.03 b | 0.5 ± 0.04 | 0.6 ± 0.00 b | ||
| α-Terpinene | 1017 | 2020 | 0.1 ± 0.06 | 0.2 ± 0.06 | 0.1 ± 0.00 a | 0.2 ± 0.06 |
| 2021 | 0.1 ± 0.01 | 0.2 ± 0.05 | 0.1 ± 0.02 a | 0.2 ± 0.03 | ||
| 2022 | 0.2 ± 0.06 | 0.2 ± 0.05 | 0.2 ± 0.02 b | 0.3 ± 0.01 | ||
| p-Cymene | 1024 | 2020 | 0.1 ± 0.03 | 0.1 ± 0.02 | 0.1 ± 0.00 | 0.1 ± 0.04 |
| 2021 | 0.1 ± 0.01 | 0.1 ± 0.00 | 0.1 ± 0.02 | 0.1 ± 0.01 | ||
| 2022 | 0.1 ± 0.03 | 0.1 ± 0.01 | 0.1 ± 0.03 | 0.1 ± 0.01 | ||
| Limonene | 1027 | 2020 | 0.5 ± 0.27 | 1.3 ± 0.08 b | 0.8 ± 0.16 | 0.7 ± 0.15 |
| 2021 | 0.5 ± 0.06 | 1.0 ± 0.05 a | 0.6 ± 0.14 | 0.7 ± 0.06 | ||
| 2022 | 0.8 ± 0.27 | 1.5 ± 0.05 c | 0.8 ± 0.04 | 0.7 ± 0.08 | ||
| 1,8-Cineole | 1030 | 2020 | 31.8 ± 2.52 | 62.1 ± 0.90 c | 43.2 ± 0.10 c | 49.5 ± 2.85 b |
| 2021 | 27.1 ± 1.00 | 45.5 ± 0.97 a | 31.7 ± 0.82 a | 39.9 ± 1.31 a | ||
| 2022 | 30.0 ± 3.01 | 53.3 ± 1.08 b | 36.3 ± 2.01 b | 45.7 ± 3.86 b | ||
| (E)-β-Ocimene | 1035 | 2020 | 0.2 ± 0.08 | 0.2 ± 0.02 a | 0.2 ± 0.03 | 0.2 ± 0.03 |
| 2021 | 0.3 ± 0.01 | 0.3 ± 0.03 b | 0.2 ± 0.01 | 0.2 ± 0.02 | ||
| 2022 | 0.2 ± 0.07 | 0.2 ± 0.02 a | 0.2 ± 0.04 | 0.1 ± 0.01 | ||
| γ-Terpinene | 1053 | 2020 | 0.2 ± 0.04 | 0.5 ± 0.02 a | 0.2 ± 0.00 a | 0.3 ± 0.09 |
| 2021 | 0.2 ± 0.02 | 0.4 ± 0.03 b | 0.2 ± 0.03 a | 0.3 ± 0.05 | ||
| 2022 | 0.2 ± 0.04 | 0.5 ± 0.02 a | 0.3 ± 0.02 b | 0.4 ± 0.02 | ||
| (Z)-Sabinenehydrate | 1061 | 2020 | 0.1 ± 0.06 a | 0.3 ± 0.04 | 0.3 ± 0.13 | 0.3 ± 0.10 |
| 2021 | 0.3 ± 0.06 b | 0.5 ± 0.12 | 0.4 ± 0.01 | 0.4 ± 0.12 | ||
| 2022 | 0.3 ± 0.06 b | 0.5 ± 0.10 | 0.2 ± 0.06 | 0.2 ± 0.12 | ||
| α-Terpinolene | 1080 | 2020 | 0.1 ± 0.08 | 0.1 ± 0.01 a | 0.1 ± 0.00 a | 0.1 ± 0.02 a |
| 2021 | 0.2 ± 0.01 | 0.2 ± 0.02 b | 0.2 ± 0.03 b | 0.2 ± 0.03 b | ||
| 2022 | 0.2 ± 0.08 | 0.3 ± 0.02 b | 0.2 ± 0.01 b | 0.2 ± 0.14 b | ||
| Linalool | 1094 | 2020 | 47.5 ± 0.98 | 8.7 ± 0.82 a | 34.8 ± 0.32 a | 26.5 ± 4.32 |
| 2021 | 47.3 ± 0.66 | 18.7 ± 0.76 c | 41.3 ± 3.14 b | 30.5 ± 0.49 | ||
| 2022 | 43.5 ± 0.98 | 11.0 ± 1.08 b | 36.1 ± 0.96 a | 25.8 ± 3.49 | ||
| α-Campholenal | 1118 | 2020 | 0.1 ± 0.01 | 0.1 ± 0.02 | 0.1 ± 0.01 a | 0.1 ± 0.01 |
| 2021 | 0.1 ± 0.02 | 0.1 ± 0.04 | 0.2 ± 0.00 b | 0.1 ± 0.06 | ||
| 2022 | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.02 b | 0.1 ± 0.01 | ||
| Pinocarveol | 1130 | 2020 | 0.1 ± 0.07 | 0.2 ± 0.04 | 0.2 ± 0.03 | 0.2 ± 0.05 |
| 2021 | 0.1 ± 0.03 | 0.2 ± 0.02 | 0.2 ± 0.01 | 0.1 ± 0.05 | ||
| 2022 | 0.1 ± 0.01 | 0.1 ± 0.04 | 0.0 ± 0.01 | 0.0 ± 0.01 | ||
| Camphor | 1136 | 2020 | 9.2 ± 1.43 | 7.0 ± 0.57 a | 5.9 ± 1.48 | 7.7 ± 0.15 a |
| 2021 | 9.5 ± 0.30 | 10.2 ± 0.42 c | 8.6 ± 0.98 | 10.4 ± 1.18 b | ||
| 2022 | 8.7 ± 0.45 | 9.2 ± 0.09 b | 9.1 ± 1.30 | 9.5 ± 0.30 b | ||
| (E)-Pinocarveol acetate | 1138 | 2020 | 0.1 ± 0.01 | 0.2 ± 0.04 b | 0.2 ± 0.03 | 0.2 ± 0.02 |
| 2021 | 0.1 ± 0.01 | 0.2 ± 0.02 b | 0.3 ± 0.03 | 0.1 ± 0.02 | ||
| 2022 | 0.0 ± 0.01 | 0.1 ± 0.04 a | 0.3 ± 0.01 | 0.0 ± 0.01 | ||
| Pinocarvone | 1156 | 2020 | 0.1 ± 0.01 | 0.1 ± 0.04 b | 0.1 ± 0.02 a | 0.1 ± 0.03 |
| 2021 | 0.1 ± 0.01 | 0.1 ± 0.02 b | 0.2 ± 0.01 b | 0.1 ± 0.01 | ||
| 2022 | 0.0 ± 0.01 | 0.0 ± 0.01 a | 0.0 ± 0.01 a | 0.0 ± 0.01 | ||
| Borneol | 1159 | 2020 | 0.7 ± 0.15 | 0.4 ± 0.09 a | 0.4 ± 0.07 a | 0.5 ± 0.03 a |
| 2021 | 0.8 ± 0.06 | 0.8 ± 0.03 b | 0.8 ± 0.11 b | 0.8 ± 0.06 b | ||
| 2022 | 0.9 ± 0.19 | 0.8 ± 0.01 b | 0.8 ± 0.14 b | 0.8 ± 0.07 b | ||
| γ-Terpineol | 1161 | 2020 | 0.6 ± 0.03 | 1.2 ± 0.05 b | 0.7 ± 0.02 a | 1.0 ± 0.10 a |
| 2021 | 0.6 ± 0.02 | 1.1 ± 0.02 a | 0.7 ± 0.00 a | 0.9 ± 0.01 a | ||
| 2022 | 0.7 ± 0.08 | 1.3 ± 0.04 c | 0.9 ± 0.04 b | 1.1 ± 0.03 b | ||
| Terpinen-4-ol | 1172 | 2020 | 0.6 ± 0.07 | 1.2 ± 0.03 b | 0.7 ± 0.00 | 0.9 ± 0.21 |
| 2021 | 0.5 ± 0.05 | 1.0 ± 0.08 a | 0.6 ± 0.03 | 0.9 ± 0.10 | ||
| 2022 | 0.6 ± 0.07 | 1.1 ± 0.04 b | 0.8 ± 0.08 | 1.1 ± 0.06 | ||
| α-Terpineol | 1187 | 2020 | 1.6 ± 0.16 | 3.6 ± 0.10 b | 2.2 ± 0.15 | 2.7 ± 0.11 |
| 2021 | 1.6 ± 0.07 | 3.2 ± 0.05 a | 1.9 ± 0.14 | 2.6 ± 0.04 | ||
| 2022 | 1.7 ± 0.16 | 3.5 ± 0.10 b | 2.1 ± 0.05 | 2.7 ± 0.14 | ||
| Myrtenal | 1192 | 2020 | 0.2 ± 0.02 | 0.3 ± 0.12 | 0.2 ± 0.03 | 0.2 ± 0.05 a |
| 2021 | 0.2 ± 0.02 | 0.3 ± 0.03 | 0.3 ± 0.01 | 0.2 ± 0.02 a | ||
| 2022 | 0.2 ± 0.02 | 0.3 ± 0.02 | 0.3 ± 0.03 | 0.3 ± 0.02 b | ||
| Myrtenol | 1193 | 2020 | 0.1 ± 0.01 | 0.0 ± 0.01 | 0.0 ± 0.01 | 0.0 ± 0.01 |
| 2021 | 0.1 ± 0.01 | 0.1 ± 0.06 | 0.1 ± 0.04 | 0.1 ± 0.01 | ||
| 2022 | 0.1 ± 0.01 | 0.2 ± 0.02 | 0.1 ± 0.02 | 0.1 ± 0.01 | ||
| β-Caryophyllene | 1423 | 2020 | 0.1 ± 0.01 a | 1.3 ± 0.18 b | 0.2 ± 0.00 a | 0.3 ± 0.08 a |
| 2021 | 0.4 ± 0.06 c | 0.2 ± 0.01 a | 0.5 ± 0.07 b | 0.5 ± 0.08 b | ||
| 2022 | 0.3 ± 0.02 b | 1.5 ± 0.13 b | 0.4 ± 0.01 b | 0.3 ± 0.05 a | ||
| β-Farnesene | 1468 | 2020 | 0.1 ± 0.06 | 0.0 ± 0.01 a | n.d. | 0.0 ± 0.00 |
| 2021 | 0.1 ± 0.02 | 0.2 ± 0.06 b | n.d. | 0.1 ± 0.03 | ||
| 2022 | 0.1 ± 0.02 | 0.0 ± 0.01 a | n.d. | 0.0 ± 0.00 | ||
| Germacrene D | 1485 | 2020 | 0.1 ± 0.04 a | 0.1 ± 0.04 a | 0.1 ± 0.06 | 0.2 ± 0.03 a |
| 2021 | 0.4 ± 0.05 c | 0.4 ± 0.40 c | 0.5 ± 0.07 | 0.4 ± 0.07 b | ||
| 2022 | 0.3 ± 0.01 b | 0.5 ± 0.07 b | 0.2 ± 0.05 | 0.2 ± 0.05 a | ||
| (Z)-α-Bisabolene | 1555 | 2020 | 1.3 ± 0.23 | 0.0 ± 0.01 a | 1.6 ± 0.76 | 1.0 ± 0.04 |
| 2021 | 1.9 ± 0.35 | 0.2 ± 0.06 b | 2.0 ± 0.04 | 1.2 ± 0.28 | ||
| 2022 | 2.1 ± 0.31 | 0.0 ± 0.01 a | 1.9 ± 0.44 | 0.7 ± 0.26 | ||
| Caryophyllene oxide | 1591 | 2020 | 0.4 ± 0.07 | 0.9 ± 0.12 ab | 0.1 ± 0.01 a | 0.3 ± 0.14 |
| 2021 | 0.4 ± 0.03 | 1.2 ± 0.14 b | 0.4 ± 0.07 b | 0.5 ± 0.07 | ||
| 2022 | 0.4 ± 0.04 | 0.7 ± 0.23 a | 0.1 ± 0.03 a | 0.4 ± 0.06 | ||
| Viridiflorol | 2020 | 0.2 ± 0.02 a | 0.9 ± 0.10 | 0.3 ± 0.18 | 0.4 ± 0.17 | |
| 2021 | 0.9 ± 0.10 b | 0.9 ± 0.13 | 0.7 ± 0.08 | 0.6 ± 0.13 | ||
| 2022 | 0.9 ± 0.11 b | 0.8 ± 0.07 | 0.6 ± 0.13 | 0.5 ± 0.14 |
| Phenolic Compounds mg *g of Dry Weight (DW)−1 | Year | Ec1 | Ec2 | Es1 | Es2 |
|---|---|---|---|---|---|
| 2020 | 0.3 ± 0.05 a | 0.6 ± 0.03 a | 0.7 ± 0.19 a | 0.6 ± 0.19 a | |
| Salvianic acid | 2021 | 1.2 ± 0.48 b | 0.6 ± 0.22 a | 0.7 ± 0.18 a | 1.8 ± 0.56 b |
| 2022 | 3.4 ± 0.51 c | 3.7 ± 0.06 b | 3.9 ± 1.29 b | 5.4 ± 0.76 c | |
| 2020 | 1.5 ± 0.30 | 1.7 ± 0.09 a | 1.3 ± 0.16 a | 1.0 ± 0.23 a | |
| Rosmarinic acid derivative | 2021 | 2.8 ± 0.75 | 4.0 ± 0.82 b | 2.9 ± 0.58 b | 4.5 ± 1.23 b |
| 2022 | 2.9 ± 0.91 | 3.7 ± 0.34 b | 3.3 ± 0.28 b | 3.7 ± 1.21 b | |
| 2020 | 0.04 ± 0.02 a | 0.1 ± 0.02 | 0.1 ± 0.01 | 0.1 ± 0.02 a | |
| Caffeic acid | 2021 | 0.1 ± 0.02 b | 0.1 ± 0.02 | 0.1 ± 0.01 | 0.2 ± 0.01 b |
| 2022 | 0.1 ± 0.02 b | 0.1 ± 0.03 | 0.1 ± 0.01 | 0.1 ± 0.01 a | |
| 2020 | 0.3 ± 0.08 a | 0.7 ± 0.02 a | 0.7 ± 0.14 a | 0.4 ± 0.10 a | |
| p-Coumaric acid glycoside | 2021 | 1.4 ± 0.32 b | 1.5 ± 0.27 b | 0.8 ± 0.12 a | 1.4 ± 0.39 b |
| 2022 | 1.5 ± 0.27 b | 1.7 ± 0.23 b | 1.6 ± 0.16 b | 1.6 ± 0.35 b | |
| 2020 | 0.3 ± 0.09 | 0.4 ± 0.08 a | 0.3 ± 0.04 a | 0.2 ± 0.07 a | |
| Ferulic acid hexoside | 2021 | 0.3 ± 0.08 | 0.7 ± 0.13 b | 0.5 ± 0.09 b | 0.5 ± 0.06 b |
| 2022 | 0.4 ± 0.07 | 0.9 ± 0.12 b | 0.6 ± 0.08 c | 0.6 ± 0.06 b | |
| 2020 | 0.3 ± 0.11 a | 0.3 ± 0.03 a | 0.3 ± 0.13 a | 0.3 ± 0.07 a | |
| Luteolin-7-O-glucoside | 2021 | 0.5 ± 0.11 a | 0.4 ± 0.12 a | 0.4 ± 0.05 a | 0.5 ± 0.06 b |
| 2022 | 0.8 ± 0.09 b | 0.8 ± 0.14 b | 0.7 ± 0.15 b | 0.8 ± 0.02 c | |
| 2020 | 0.9 ± 0.26 a | 1.1 ± 0.03 a | 1.0 ± 0.15 a | 0.8 ± 0.15 a | |
| Luteolin-7-O-glucuronide | 2021 | 1.3 ± 0.25 ab | 1.4 ± 0.37 a | 1.1 ± 0.23 a | 1.4 ± 0.33 b |
| 2022 | 1.9 ± 0.47 b | 2.5 ± 0.33 b | 2.2 ± 0.32 b | 2.3 ± 0.12 c | |
| 2020 | 0.3 ± 0.09 a | 0.4 ± 0.01 | 0.4 ± 0.08 | 0.3 ± 0.03 | |
| Apigenin-7-O-glucoside | 2021 | 0.6 ± 0.10 b | 0.5 ± 0.14 | 0.4 ± 0.02 | 0.5 ± 0.07 |
| 2022 | 0.6 ± 0.10 b | 0.7 ± 0.11 | 0.6 ± 0.04 | 0.5 ± 0.05 | |
| 2020 | 0.6 ± 0.17 | 0.7 ± 0.00 ab | 0.4 ± 0.21 a | 1.2 ± 0.43 b | |
| o-Coumaric acid | 2021 | 0.7 ± 0.18 | 1.2 ± 0.37 b | 2.8 ± 0.48 b | 1.1 ± 0.53 a |
| 2022 | 0.4 ± 0.19 | 0.3 ± 0.07 a | 0.3 ± 0.04 a | 0.4 ± 0.04 ba | |
| 2020 | 0.3 ± 0.15 a | 0.6 ± 0.01 a | 0.9 ± 0.31 | 0.6 ± 0.12 a | |
| Rosmarinic acid | 2021 | 1.5 ± 0.09 c | 1.5 ± 0.08 b | 1.4 ± 0.01 | 1.4 ± 0.03 b |
| 2022 | 0.9 ± 0.16 b | 1.5 ± 0.42 b | 1.3 ± 0.30 | 1.3 ± 0.20 b | |
| 2020 | 1.0 ± 0.26 | 1.1 ± 0.09 a | 0.7 ± 0.15 a | 0.7 ± 0.26 a | |
| Salvianolic acid A | 2021 | 1.6 ± 0.62 | 2.1 ± 0.26 b | 1.2 ± 0.39 a | 1.9 ± 0.33 b |
| 2022 | 1.9 ± 0.22 | 3.0 ± 0.92 c | 2.5 ± 0.48 b | 2.6 ± 0.16 c | |
| 2020 | 0.4 ± 0.06 | 0.4 ± 0.01 b | 0.4 ± 0.17 b | 0.5 ± 0.14 b | |
| Luteolin | 2021 | 0.3 ± 0.10 | 0.4 ± 0.09 b | 1.6 ± 0.13 c | 0.3 ± 0.09 b |
| 2022 | 0.2 ± 0.11 | 0.1 ± 0.02 a | 0.1 ± 0.01 a | 0.1 ± 0.01 a | |
| 2020 | 0.1 ± 0.01 | 0.1 ± 0.02 | 0.1 ± 0.01 | 0.1 ± 0.01 | |
| Salvianolic acid C | 2021 | 0.1 ± 0.01 | 0.1 ± 0.03 | 0.1 ± 0.01 | 0.1 ± 0.01 |
| 2022 | 0.1 ± 0.01 | 0.1 ± 0.02 | 0.1 ± 0.02 | 0.2 ± 0.02 | |
| 2020 | 0.1 ± 0.01 b | 0.1 ± 0.01 b | 0.1 ± 0.05 ab | 0.1 ± 0.03 b | |
| Apigenin | 2021 | 0.1 ± 0.03 ab | 0.1 ± 0.02 b | 0.1 ± 0.04 b | 0.1 ± 0.04 b |
| 2022 | 0.04 ± 0.0 a | 0.03 ± 0.00 a | 0.02 ± 0.00 a | 0.02 ± 0.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cáceres-Cevallos, G.J.; Quílez, M.; Ortiz de Elguea-Culebras, G.; Melero-Bravo, E.; Sánchez-Vioque, R.; Jordán, M.J. Agronomic Evaluation and Chemical Characterization of Lavandula latifolia Medik. under the Semiarid Conditions of the Spanish Southeast. Plants 2023, 12, 1986. https://doi.org/10.3390/plants12101986
Cáceres-Cevallos GJ, Quílez M, Ortiz de Elguea-Culebras G, Melero-Bravo E, Sánchez-Vioque R, Jordán MJ. Agronomic Evaluation and Chemical Characterization of Lavandula latifolia Medik. under the Semiarid Conditions of the Spanish Southeast. Plants. 2023; 12(10):1986. https://doi.org/10.3390/plants12101986
Chicago/Turabian StyleCáceres-Cevallos, Gustavo J., María Quílez, Gonzalo Ortiz de Elguea-Culebras, Enrique Melero-Bravo, Raúl Sánchez-Vioque, and María J. Jordán. 2023. "Agronomic Evaluation and Chemical Characterization of Lavandula latifolia Medik. under the Semiarid Conditions of the Spanish Southeast" Plants 12, no. 10: 1986. https://doi.org/10.3390/plants12101986







