Comparison of Phytochemical Composition and Untargeted Metabolomic Analysis of an Extract from Cnidoscolus aconitifolius (Mill.) I. I. Johnst and Porophyllum ruderale (Jacq.) Cass. and Biological Cytotoxic and Antiproliferative Activity In Vitro
Abstract
1. Introduction
2. Results
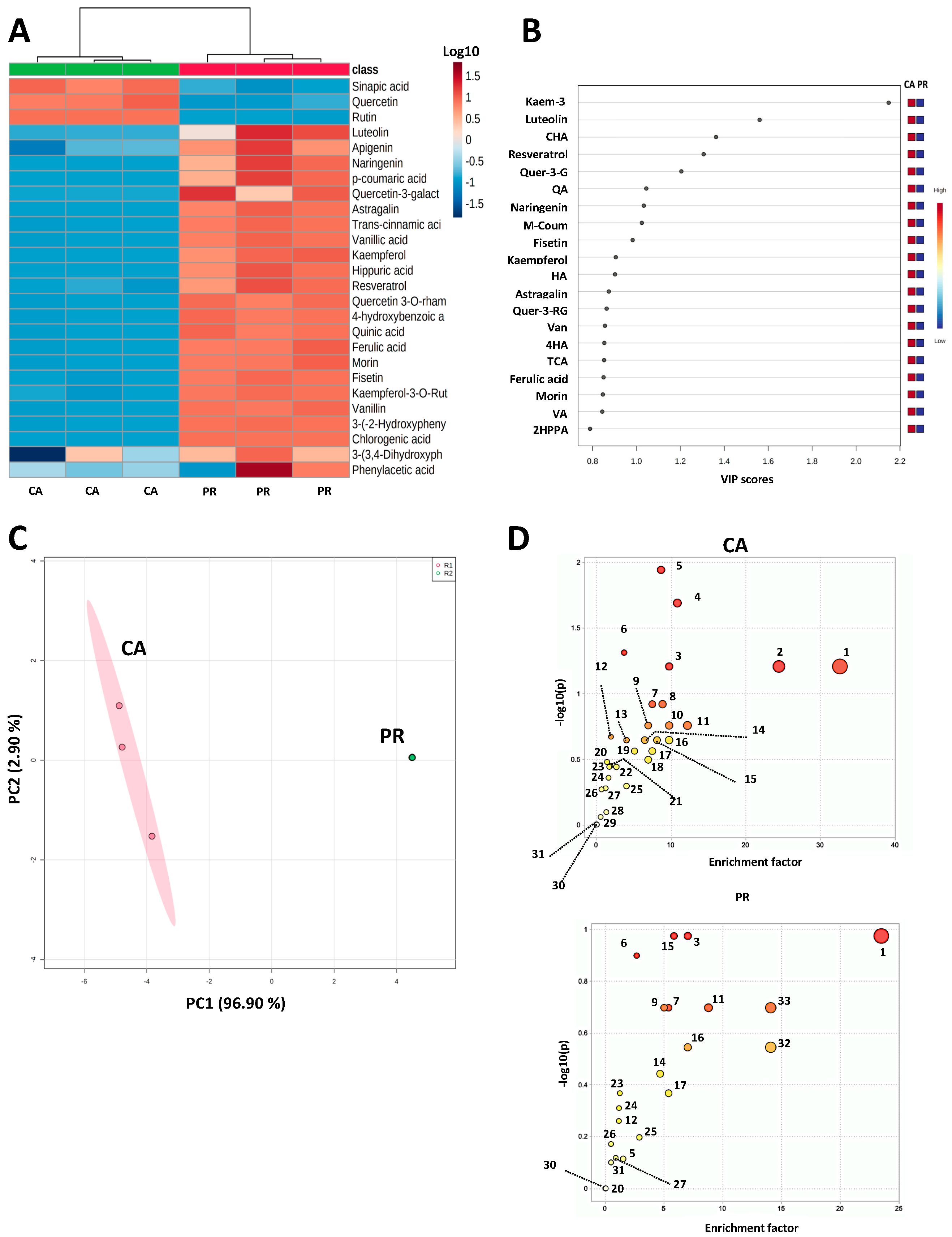
2.1. Polyphenol Profile and Untargeted Metabolomics from CA and PR Samples
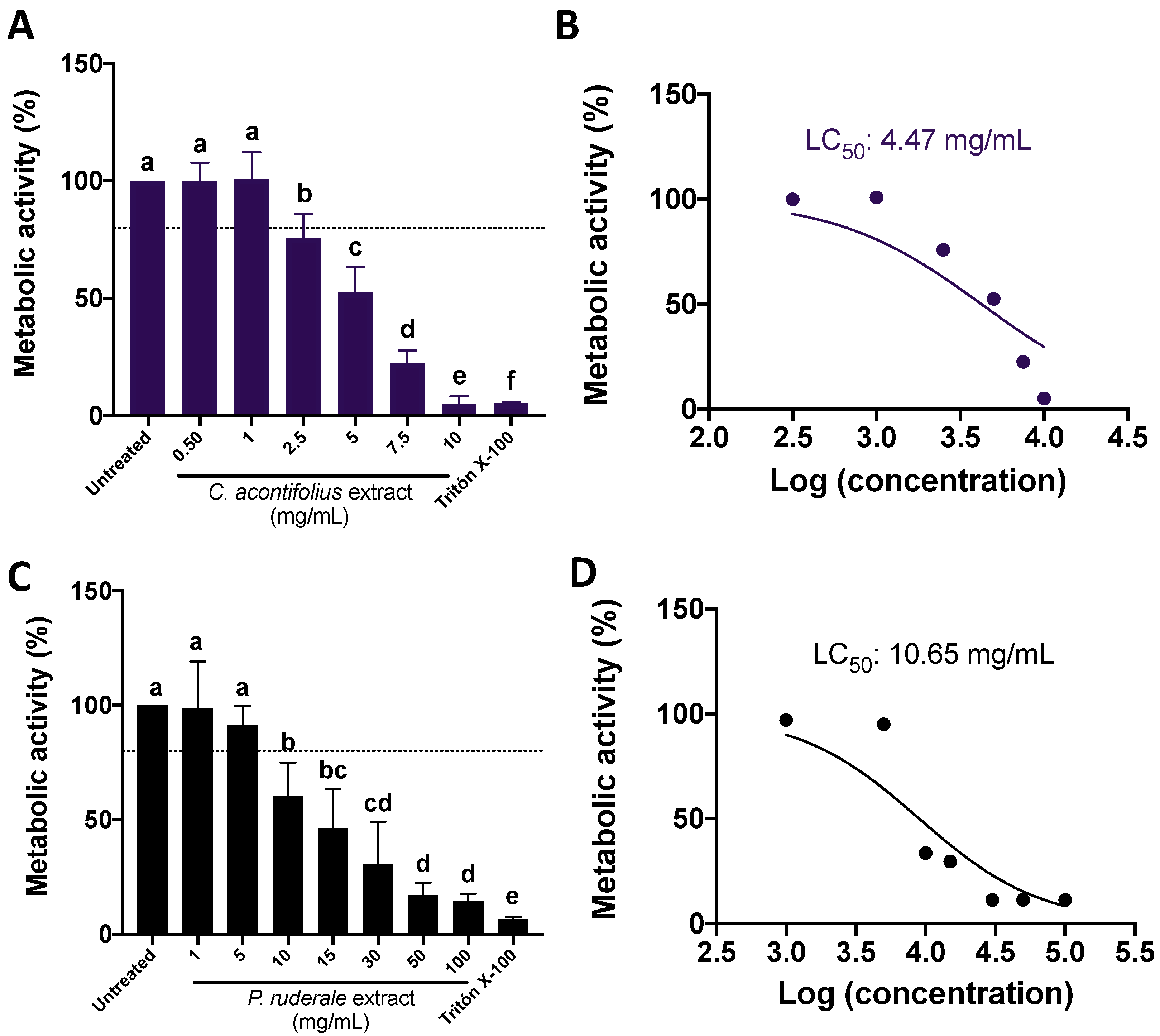
2.2. CA and PR Extracts Decreased SW480 Metabolic Activity in a Dose–Response Manner
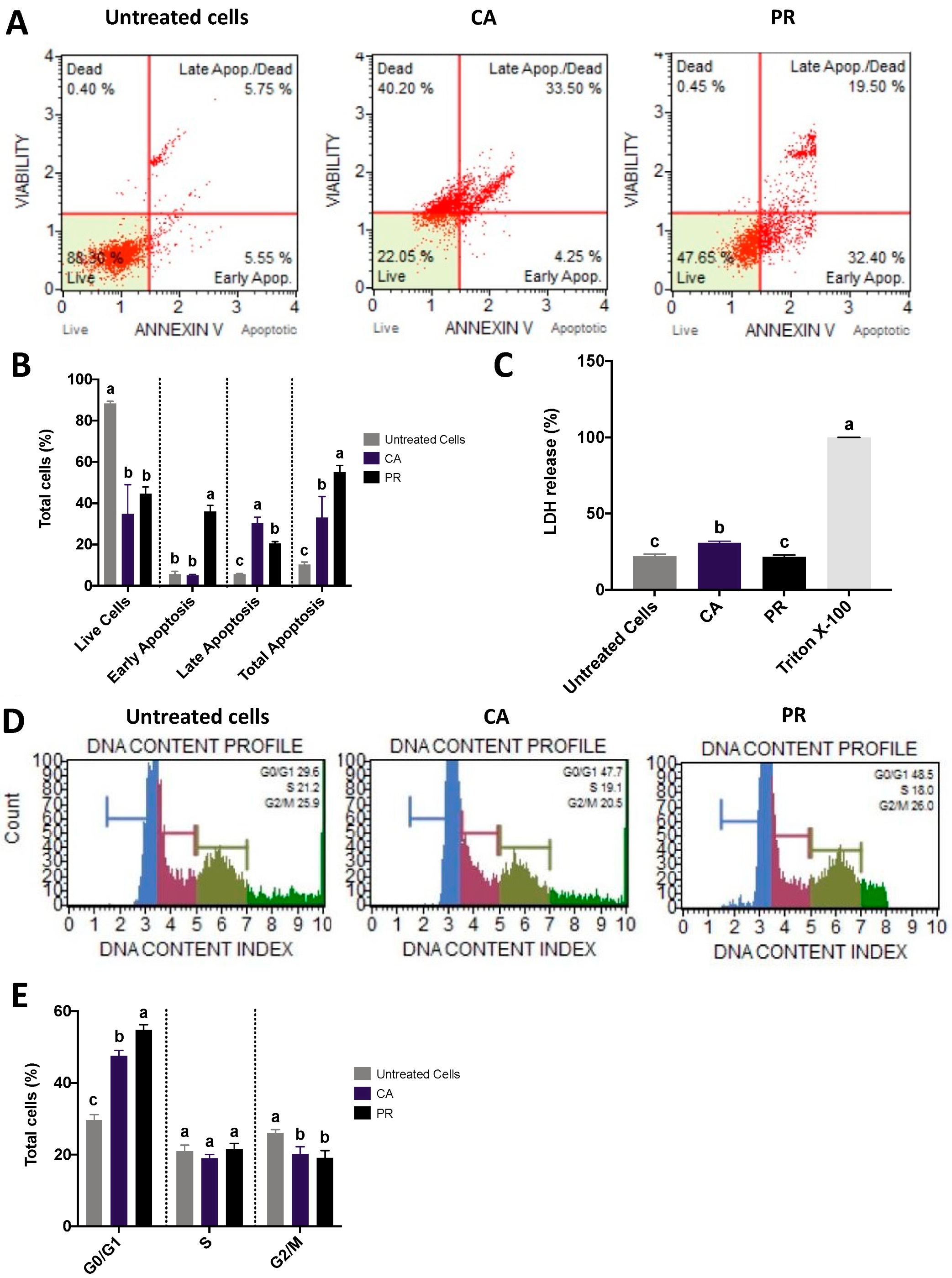
2.3. CA and PR Extracts Induced SW480 Apoptosis and Arrested Cell Cycle at G0/G1 Stage
2.4. Impact of CA and PR Extracts on Apc and Kras Gene Expression
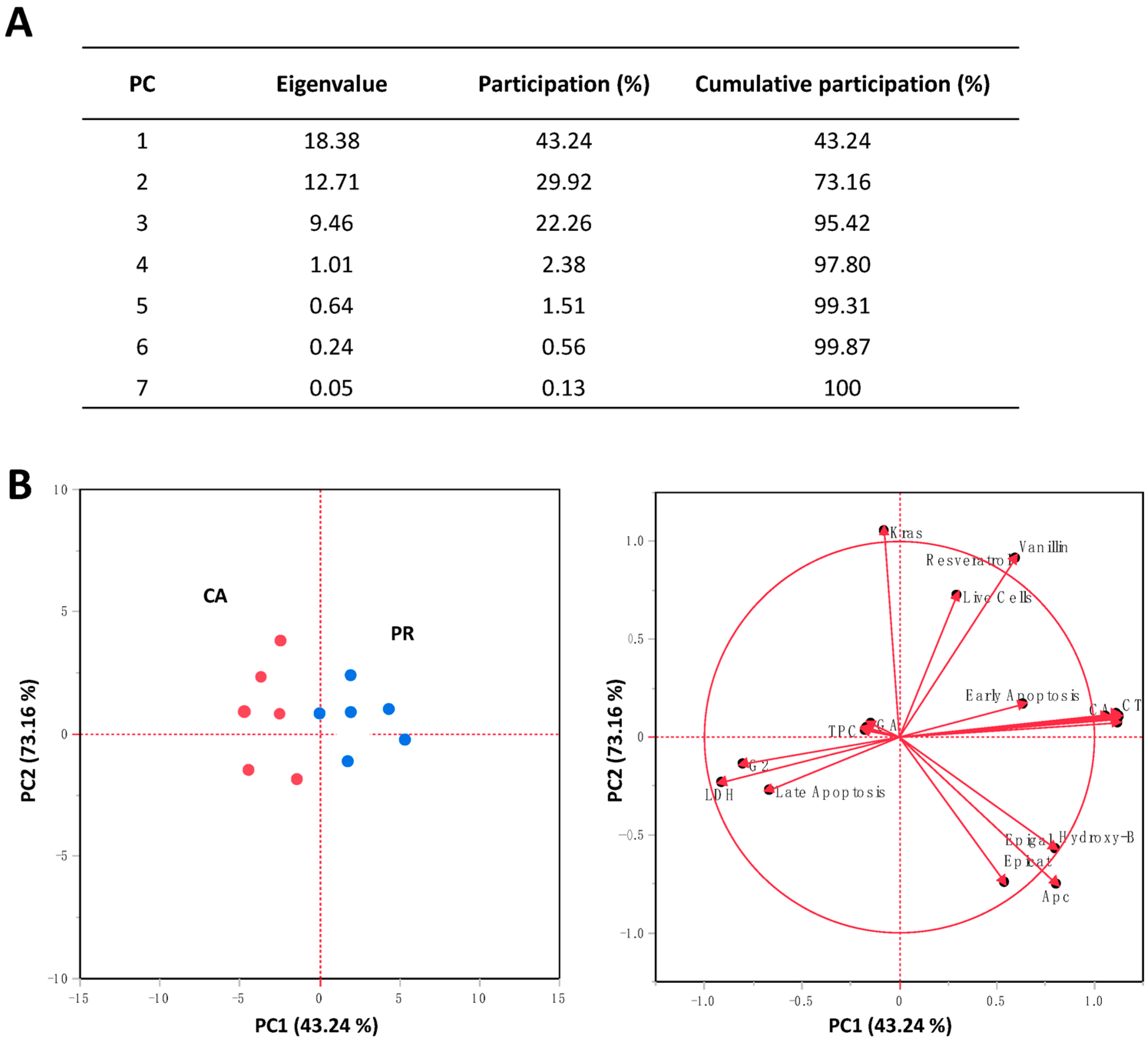
2.5. Principal Components Analysis (PCA) for the Evaluated Responses in SW480 Cells
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Extracts Preparation, Identification, and Quantification of Phenolic Compounds
4.2.1. Extracts Preparation
4.2.2. Quantification of Total Phenolic Compounds, Total Flavonoids, and Condensed Tannins
4.2.3. Identification of Phenolic Compounds by UPLC-DAD-QTof/MS-ESI
4.2.4. Identification and Quantification of Free Phenolic Compounds by HPLC-DAD
4.3. Untargeted Metabolomic Analysis
4.4. Cell Culture Assays
4.4.1. Determination of Cell’s Metabolic Activity by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
4.4.2. Determination of Lactate Dehydrogenase (LDH) Release
4.4.3. Apoptosis Assessment by Flow Cytometry
4.4.4. Cell Cycle Analysis by Flow Cytometry
4.4.5. Evaluation of Apc and Kras Gene Expression by qPCR
4.5. In Silico Assessment of the Interaction between Phenolic Compounds and Target Proteins
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mateos-Maces, L.; Chávez-Servia, J.L.; Vera-Guzmán, A.M.; Aquino-Bolaños, E.N.; Alba-Jiménez, J.E.; Villagómez-González, B.B. Edible Leafy Plants from Mexico as Sources of Antioxidant Compounds, and Their Nutritional, Nutraceutical and Antimicrobial Potential: A Review. Antioxidants 2020, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Mapes, C.; Basurto, F. Biodiversity and Edible Plants of Mexico. In Ethnobotany of Mexico; Lira, R., Casas, A., Eds.; Springer: New York, NY, USA, 2016; pp. 83–131. ISBN 978-1-4614-6668-0. [Google Scholar]
- Fukalova-Fukalova, T.; García-Martínez, M.D.; Raigón, M.D. Nutritional Composition, Bioactive Compounds, and Volatiles Profile Characterization of Two Edible Undervalued Plants: Portulaca oleracea L. and Porophyllum ruderale (Jacq.) Cass. Plants 2022, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Moura, L.F.W.; da Silva-Neto, J.X.; Pereira-Lopes, T.D.; Rathinaraj-Benjamin, S.; Rodrigues-Brito, F.C.; Alves-Magalhães, F.E.; Pereira-Tramontina-Florean, E.O.; de Bezerra-de Sousa, D.O.; Florindo-Guedes, M.I. Ethnobotanic, Phytochemical Uses and Ethnopharmacological Profile of Genus Cnidoscolus Spp. (Euphorbiaceae): A Comprehensive Overview. Biomed. Pharmacother. 2019, 109, 1670–1679. [Google Scholar] [CrossRef]
- Jiménez-Arellanes, M.A.; García-Martínez, I.; Rojas-Tomé, S. Potencial Biológico de Especies Medicinales Del Género Cnidoscolus (Euphorbiacea). Rev. Mex. Cienc. Fram. 2015, 45, 1–6. [Google Scholar]
- Vázquez-Atanacio, M.J.; Bautista-ávila, M.; Velázquez-González, C.; Castañeda-Ovando, A.; González-Cortazar, M.; Sosa-Gutiérrez, C.G.; Ojeda-Ramírez, D. Porophyllum Genus Compounds and Pharmacological Activities: A Review. Sci. Pharm. 2021, 89, 7. [Google Scholar] [CrossRef]
- Hernandez-Carrillo, M. Estudio Del Pápaloquelite (Porophyllum ruderale) Como Alimento Funcional. Facultad de Química. 2014. Available online: https://ru.dgb.unam.mx/handle/DGB_UNAM/TES01000710090 (accessed on 2 July 2022).
- Cheng, Y.; Ling, Z.; Li, L. The Intestinal Microbiota and Colorectal Cancer. Front. Immunol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Kuri-García, A.; Godínez-Santillán, R.I.; Mejía, C.; Ferriz-Martínez, R.A.; García-Solís, P.; Enríquez-Vázquez, A.; García-Gasca, T.; Guzmán-Maldonado, S.H.; Chávez-Servín, J.L. Preventive Effect of an Infusion of the Aqueous Extract of Chaya Leaves (Cnidoscolus aconitifolius) in an Aberrant Crypt Foci Rat Model Induced by Azoxymethane and Dextran Sulfate Sodium. J. Med. Food 2019, 22, 851–860. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef]
- De, S.; Paul, S.; Manna, A.; Majumder, C.; Pal, K.; Casarcia, N.; Mondal, A.; Banerjee, S.; Nelson, V.K.; Ghosh, S.; et al. Phenolic Phytochemicals for Prevention and Treatment of Colorectal Cancer: A Critical Evaluation of In Vivo Studies. Cancers 2023, 15, 993. [Google Scholar] [CrossRef]
- Arango-Varela, S.S.; Luzardo-Ocampo, I.; Maldonado-Celis, M.E. Andean Berry (Vaccinium meridionale Swartz) Juice, in Combination with Aspirin, Displayed Antiproliferative and pro-Apoptotic Mechanisms in Vitro While Exhibiting Protective Effects against AOM-Induced Colorectal Cancer in Vivo. Food Res. Int. 2022, 157, 111244. [Google Scholar] [CrossRef]
- László, L.; Kurilla, A.; Takács, T.; Kudlik, G.; Koprivanacz, K.; Buday, L.; Vas, V. Recent Updates on the Significance of KRAS Mutations in Colorectal Cancer Biology. Cells 2021, 10, 667. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, M.; Zhao, R.; Wang, D.; Ma, Y.; Ai, L. Plant Natural Products: Promising Resources for Cancer Chemoprevention. Molecules 2021, 26, 933. [Google Scholar] [CrossRef] [PubMed]
- Rendón-Sandoval, F.J.; Casas, A.; Moreno-Calles, A.I.; Torres-García, I.; García-Frapolli, E. Traditional Agroforestry Systems and Conservation of Native Plant Diversity of Seasonally Dry Tropical Forests. Sustainability 2020, 12, 4600. [Google Scholar] [CrossRef]
- Santiago-Saenz, Y.O.; Hernández-Fuentes, A.D.; Monroy-Torres, R.; Cariño-Cortés, R.; Jiménez-Alvarado, R. Physicochemical, Nutritional and Antioxidant Characterization of Three Vegetables (Amaranthus hybridus L., Chenopodium berlandieri L., Portulaca oleracea L.) as Potential Sources of Phytochemicals and Bioactive Compounds. J. Food Meas. Charact. 2018, 12, 2855–2864. [Google Scholar] [CrossRef]
- Manzanero-Medina, G.I.; Vásquez-Dávila, M.A.; Lustre-Sánchez, H.; Pérez-Herrera, A. Ethnobotany of Food Plants (Quelites) Sold in Two Traditional Markets of Oaxaca, Mexico. S. Afr. J. Bot. 2020, 130, 215–223. [Google Scholar] [CrossRef]
- Kuri-Garcia, A.; Chávez-Servín, J.L.; Guzmán-Mandonado, S. Phenolic Profile and Antioxidant Capacity of Cnidoscolus Chayamansa and Cnidoscolus Aconitifolius: A Review. J. Med. Plants Res. 2017, 11, 713–727. [Google Scholar] [CrossRef]
- Babalola, J.O.; Alabi, O.O. Effect of Processing Methods on Nutritional Composition, Phytochemicals, and Anti-Nutrient Properties of Chaya Leaf (Cnidoscolus aconitifolius). Afr. J. Food Sci. 2015, 9, 560–565. [Google Scholar] [CrossRef]
- Jiménez-Aguilar, D.M.; Grusak, M.A. Evaluation of Minerals, Phytochemical Compounds and Antioxidant Activity of Mexican, Central American, and African Green Leafy Vegetables. Plant Foods Hum. Nutr. 2015, 70, 357–364. [Google Scholar] [CrossRef]
- Pawłowska, K.A.; Baracz, T.; Skowrońska, W.; Piwowarski, J.P.; Majdan, M.; Malarz, J.; Stojakowska, A.; Zidorn, C.; Granica, S. The Contribution of Phenolics to the Anti-Inflammatory Potential of the Extract from Bolivian Coriander (Porophyllum ruderale Subsp. Ruderale). Food Chem. 2022, 371, 131116. [Google Scholar] [CrossRef]
- Kuti, J.O.; Konuru, H.B. Antioxidant Capacity and Phenolic Content in Leaf Extracts of Tree Spinach (Cnidoscolus Spp.). J. Agric. Food Chem. 2004, 52, 117–121. [Google Scholar] [CrossRef]
- Numa, S.; Rodríguez, L.; Rodríguez, D.; Coy-Barrera, E. Susceptibility of Tetranychus Urticae Koch to an Ethanol Extract of Cnidoscolus Aconitifolius Leaves under Laboratory Conditions. Springerplus 2015, 4, 338. [Google Scholar] [CrossRef] [PubMed]
- de Athayde, A.E.; Richetti, E.; Wolff, J.; Lusa, M.G.; Biavatti, M.W. “Arnicas” from Brazil: Comparative Analysis among Ten Species. Rev. Bras. Farmacogn. 2019, 29, 401–424. [Google Scholar] [CrossRef]
- de Athayde, A.E.; de Araujo, C.E.S.; Sandjo, L.P.; Biavatti, M.W. Metabolomic Analysis among Ten Traditional “Arnica” (Asteraceae) from Brazil. J. Ethnopharmacol. 2021, 265, 113149. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Andrade-Cetto, A. Ethnobotanical Study of the Medicinal Plants from Tlanchinol, Hidalgo, México. J. Ethnopharmacol. 2009, 122, 163–171. [Google Scholar] [CrossRef]
- Ikpefan, E.O.; Ayinde, B.A.; Mudassir, A.; Farooq, A.D. Comparative In Vitro Assessment of the Methanol Extracts of the Leaf, Stem, and Root Barks of Cnidoscolus Aconitifolius on Lung and Breast Cancer Cell Lines. Turkish J. Pharm. Sci. 2019, 16, 375–379. [Google Scholar] [CrossRef]
- Khalighi-Sigaroodi, F.; Ahvazi, M.; Yazdani, D.; Kashefi, M. Cytotoxicity and Antioxidant Activity of 23 Plant Species of Leguminosae Family. J. Med. Plants 2012, 11, 41–53. [Google Scholar]
- Canga, I.; Vita, P.; Oliveira, A.I.; Castro, M.á.; Pinho, C. In Vitro Cytotoxic Activity of African Plants: A Review. Molecules 2022, 27, 4989. [Google Scholar] [CrossRef]
- Babaei-Ghaghelestany, A.; Alebrahim, M.T.; Farzaneh, S.; Mehrabi, M. The Anticancer and Antibacterial Properties of Aqueous and Methanol Extracts of Weeds. J. Agric. Food Res. 2022, 10, 100433. [Google Scholar] [CrossRef]
- Godínez-Santillán, R.I.; Chávez-Servín, J.L.; García-Gasca, T.; Guzmán-Maldonado, S.H. Caracterización Fenólica y Capacidad Antioxidante de Extractos Alcohólicos de Hojas Crudas y Hervidas de Cnidoscolus Aconitifolius (Euphorbiaceae). Acta Bot. Mex. 2019, 126, 1–15. [Google Scholar] [CrossRef]
- Heredia Vieira, S.C.; Sartori Schwerz, G.; Oliveira Moraes Gaspar, C.; Feitosa Malzac, C.; Alencar Fernandes, F.H.; Marcondes Ibrahim, I.; Trennepohl Souza, M.E.; Caspers, T.; Matias, R.; Kato da Silva, B.A.; et al. Effect of Porophyllum ruderale (Jacq.) Cass. in the Liver of the B16-F10 Murine Melanoma Model and Antioxidant Potential. Ensaios Ciência C Biológicas Agrárias Saúde 2021, 25, 309–314. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Loarca-Piña, G.; Gonzalez de Mejia, E. Gallic and Butyric Acids Modulated NLRP3 Inflammasome Markers in a Co-Culture Model of Intestinal Inflammation. Food Chem. Toxicol. 2020, 146, 111835. [Google Scholar] [CrossRef]
- Shang, L.-H.; Yu, Y.; Che, D.-H.; Pan, B.; Jin, S.; Zou, X.-L. Luffa Echinata Roxb. Induced Apoptosis in Human Colon Cancer Cell (SW-480) in the Caspase-Dependent Manner and through a Mitochondrial Apoptosis Pathway. Pharmacogn. Mag. 2016, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Caicedo-Lopez, L.H.; Cuellar-Nuñez, M.L.; Luzardo-Ocampo, I.; Campos-Vega, R.; Lóarca-Piña, G. Colonic Metabolites from Digested Moringa Oleifera Leaves Induced HT-29 Cell Death via Apoptosis, Necrosis, and Autophagy. Int. J. Food Sci. Nutr. 2021, 72, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Pu, S.; Lin, C.; He, L.; Zhao, H.; Yang, C.; Guo, Z.; Xu, S.; Zhou, Z. Curcumin Selectively Induces Colon Cancer Cell Apoptosis and S Cell Cycle Arrest by Regulates Rb/E2F/P53 Pathway. J. Mol. Struct. 2022, 1263, 133180. [Google Scholar] [CrossRef]
- Perumal, P.; Annamalai, P.; Thayman, M.; Rajan, S.; Raman, L.; Ramasubbu, S. Ethyl Acetate Extract from Marine Sponge Hyattella Cribriformis Exhibit Potent Anticancer Activity by Promoting Tubulin Polymerization as Evidenced Mitotic Arrest and Induction of Apoptosis. Pharmacogn. Mag. 2015, 11, 345. [Google Scholar] [CrossRef]
- Sangfelt, O.; Erickson, S.; Castro, J.; Heiden, T.; Gustafsson, A.; Einhorn, S.; Grandér, D. Molecular Mechanisms Underlying Interferon-α-Induced G0/G1 Arrest: CKI-Mediated Regulation of G1 Cdk-Complexes and Activation of Pocket Proteins. Oncogene 1999, 18, 2798–2810. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An Overview on the Role of Dietary Phenolics for the Treatment of Cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef]
- Soto, K.M.; Luzardo-Ocampo, I.; López-Romero, J.M.; Mendoza, S.; Loarca-Piña, G.; Rivera-Muñoz, E.M.; Manzano-Ramírez, A. Gold Nanoparticles Synthesized with Common Mullein (Verbascum Thapsus) and Castor Bean (Ricinus Communis) Ethanolic Extracts Displayed Antiproliferative Effects and Induced Caspase 3 Activity in Human HT29 and SW480 Cancer Cells. Pharmaceutics 2022, 14, 2069. [Google Scholar] [CrossRef]
- Rahim, N.F.C.; Hussin, Y.; Aziz, M.N.M.; Mohamad, N.E.; Yeap, S.K.; Masarudin, M.J.; Abdullah, R.; Akhtar, M.N.; Alitheen, N.B. Cytotoxicity and Apoptosis Effects of Curcumin Analogue (2E,6E)-2,6-Bis(2,3-Dimethoxybenzylidine) Cyclohexanone (DMCH) on Human Colon Cancer Cells HT29 and SW620 In Vitro. Molecules 2021, 26, 1261. [Google Scholar] [CrossRef]
- Ghodousi-Dehnavi, E.; Hosseini, R.H.; Arjmand, M.; Nasri, S.; Zamani, Z. A Metabolomic Investigation of Eugenol on Colorectal Cancer Cell Line HT-29 by Modifying the Expression of APC, P53, and KRAS Genes. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.-J.; Ro, E.J.; Choi, K.-Y. Interaction between Wnt/β-Catenin and RAS-ERK Pathways and an Anti-Cancer Strategy via Degradations of β-Catenin and RAS by Targeting the Wnt/β-Catenin Pathway. NPJ Precis. Oncol. 2018, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Khan, F.; Qari, H.A.; Oves, M. Rutin (Bioflavonoid) as Cell Signaling Pathway Modulator: Prospects in Treatment and Chemoprevention. Pharmaceuticals 2021, 14, 1069. [Google Scholar] [CrossRef]
- Cuellar-Nuñez, M.L.; Luzardo-Ocampo, I.; Campos-Vega, R.; Gallegos-Corona, M.A.; González de Mejía, E.; Loarca-Piña, G. Physicochemical and Nutraceutical Properties of Moringa (Moringa oleifera) Leaves and Their Effects in an in Vivo AOM/DSS-Induced Colorectal Carcinogenesis Model. Food Res. Int. 2018, 105, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Academic Press-Elsevier: North Andover, MA, USA, 1999; Volume 299, pp. 152–178. ISBN 9780121822002. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Feregrino-Pérez, A.A.; Berumen, L.C.; García-Alcocer, G.; Guevara-Gonzalez, R.G.R.G.; Ramos-Gomez, M.; Reynoso-Camacho, R.R.; Acosta-Gallegos, J.A.; Loarca-Piña, G. Composition and Chemopreventive Effect of Polysaccharides from Common Beans (Phaseolus vulgaris L.) on Azoxymethane-Induced Colon Cancer. J. Agric. Food Chem. 2008, 56, 8737–8744. [Google Scholar] [CrossRef]
- Sánchez-Recillas, E.; Campos-Vega, R.; Pérez-Ramírez, I.F.; Luzardo-Ocampo, I.; Cuéllar-Núñez, M.L.; Vergara-Castañeda, H.A. Garambullo (Myrtillocactus geometrizans): Effect of in Vitro Gastrointestinal Digestion on the Bioaccessibility and Antioxidant Capacity of Phytochemicals. Food Funct. 2022, 13, 4699–4713. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Comprehensive Characterization of Phenolic and Other Polar Compounds in the Seed and Seed Coat of Avocado by HPLC-DAD-ESI-QTOF-MS. Food Res. Int. 2018, 105, 752–763. [Google Scholar] [CrossRef]
- Gertsman, I.; Barshop, B.A. Promises and Pitfalls of Untargeted Metabolomics. J. Inherit. Metab. Dis. 2018, 41, 355–366. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS Spectra Processing, Multi-Omics Integration and Covariate Adjustment of Global Metabolomics Data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]

| Phenolic Compounds | CA | PR |
|---|---|---|
| TPC (mg GAE/100 g LE) | 5109.00 ± 1034.00 * | 2645.50 ± 10.70 * |
| TFC (mg CE/100 g LE) | 309.50 ± 29.00 * | 475.40 ± 26.10 * |
| CT (mg CE/100 g LE) | 2.40 ± 0.10 * | 3.00 ± 0.10 * |
| Compound Name | RT (min) | CA (mg eq./g DW) | PR (mg. eq./g. DW) |
|---|---|---|---|
| Hydroxycinnamic acids and derivatives | |||
| Chlorogenic acid | 10.58 | 6.51 ± 0.01 * | 0.22 ± 0.01 * |
| Sinapic acid | 11.67 | 3.87 ± 0.02 * | 2.82 ± 0.06 * |
| Caffeic acid | 11.82 | 14.46 ± 0.27 * | 1.45 ± 0.01 * |
| p-Coumaric acid | 14.42 | 6.36 ± 0.03 * | 1.26 ± 0.13 * |
| Ferulic acid | 15.30 | 5.17 ± 0.05 * | 2.78 ± 0.02 * |
| Hydroxybenzoic acids and derivatives and benzaldehydes | |||
| Gallic acid | 7.49 | 2.04 ± 0.01 * | 0.71 ± 0.02 * |
| Hydroxybenzoic acid | 12.35 | 0.60 ± 0.01 | 0.11 ± 0.01 |
| Benzenoids | |||
| Hydroxyphenylacetic acid | 9.89 | 1.05 ± 0.07 * | 0.11 ± 0.02 * |
| Flavonols | |||
| Rutin | 12.90 | 1.22 ± 0.18 * | 2.83 ± 0.14 * |
| Quercetin | 18.44 | 0.20 ± 0.01 | 0.14 ± 0.01 |
| Flavones | |||
| (+)-catechin | 10.85 | 0.45 ± 0.01 * | 14.01 ± 0.04 * |
| Epicatechin | 11.55 | 1.06 ± 0.1 * | 57.74 ± 0.29 * |
| Epigallocatechin gallate | 11.80 | 3.29 ± 0.01 * | 14.37 ± 0.04 * |
| Other compounds | |||
| Resveratrol | 17.46 | 3.62 ± 0.31 * | 0.02 ± 0.01 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Madriz, Á.F.; Luzardo-Ocampo, I.; Moreno-Celis, U.; Roldán-Padrón, O.; Chávez-Servín, J.L.; Vergara-Castañeda, H.A.; Martínez-Pacheco, M.; Mejía, C.; García-Gasca, T.; Kuri-García, A. Comparison of Phytochemical Composition and Untargeted Metabolomic Analysis of an Extract from Cnidoscolus aconitifolius (Mill.) I. I. Johnst and Porophyllum ruderale (Jacq.) Cass. and Biological Cytotoxic and Antiproliferative Activity In Vitro. Plants 2023, 12, 1987. https://doi.org/10.3390/plants12101987
Vargas-Madriz ÁF, Luzardo-Ocampo I, Moreno-Celis U, Roldán-Padrón O, Chávez-Servín JL, Vergara-Castañeda HA, Martínez-Pacheco M, Mejía C, García-Gasca T, Kuri-García A. Comparison of Phytochemical Composition and Untargeted Metabolomic Analysis of an Extract from Cnidoscolus aconitifolius (Mill.) I. I. Johnst and Porophyllum ruderale (Jacq.) Cass. and Biological Cytotoxic and Antiproliferative Activity In Vitro. Plants. 2023; 12(10):1987. https://doi.org/10.3390/plants12101987
Chicago/Turabian StyleVargas-Madriz, Ángel Félix, Ivan Luzardo-Ocampo, Ulisses Moreno-Celis, Octavio Roldán-Padrón, Jorge Luis Chávez-Servín, Haydé A. Vergara-Castañeda, Mónica Martínez-Pacheco, Carmen Mejía, Teresa García-Gasca, and Aarón Kuri-García. 2023. "Comparison of Phytochemical Composition and Untargeted Metabolomic Analysis of an Extract from Cnidoscolus aconitifolius (Mill.) I. I. Johnst and Porophyllum ruderale (Jacq.) Cass. and Biological Cytotoxic and Antiproliferative Activity In Vitro" Plants 12, no. 10: 1987. https://doi.org/10.3390/plants12101987
APA StyleVargas-Madriz, Á. F., Luzardo-Ocampo, I., Moreno-Celis, U., Roldán-Padrón, O., Chávez-Servín, J. L., Vergara-Castañeda, H. A., Martínez-Pacheco, M., Mejía, C., García-Gasca, T., & Kuri-García, A. (2023). Comparison of Phytochemical Composition and Untargeted Metabolomic Analysis of an Extract from Cnidoscolus aconitifolius (Mill.) I. I. Johnst and Porophyllum ruderale (Jacq.) Cass. and Biological Cytotoxic and Antiproliferative Activity In Vitro. Plants, 12(10), 1987. https://doi.org/10.3390/plants12101987










