GC/MS Profiling, Antibacterial, Anti-Quorum Sensing, and Antibiofilm Properties of Anethum graveolens L. Essential Oil: Molecular Docking Study and In-Silico ADME Profiling
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of A. graveolens EO
2.2. Antibacterial Potential of A. graveolens EO and Limonene
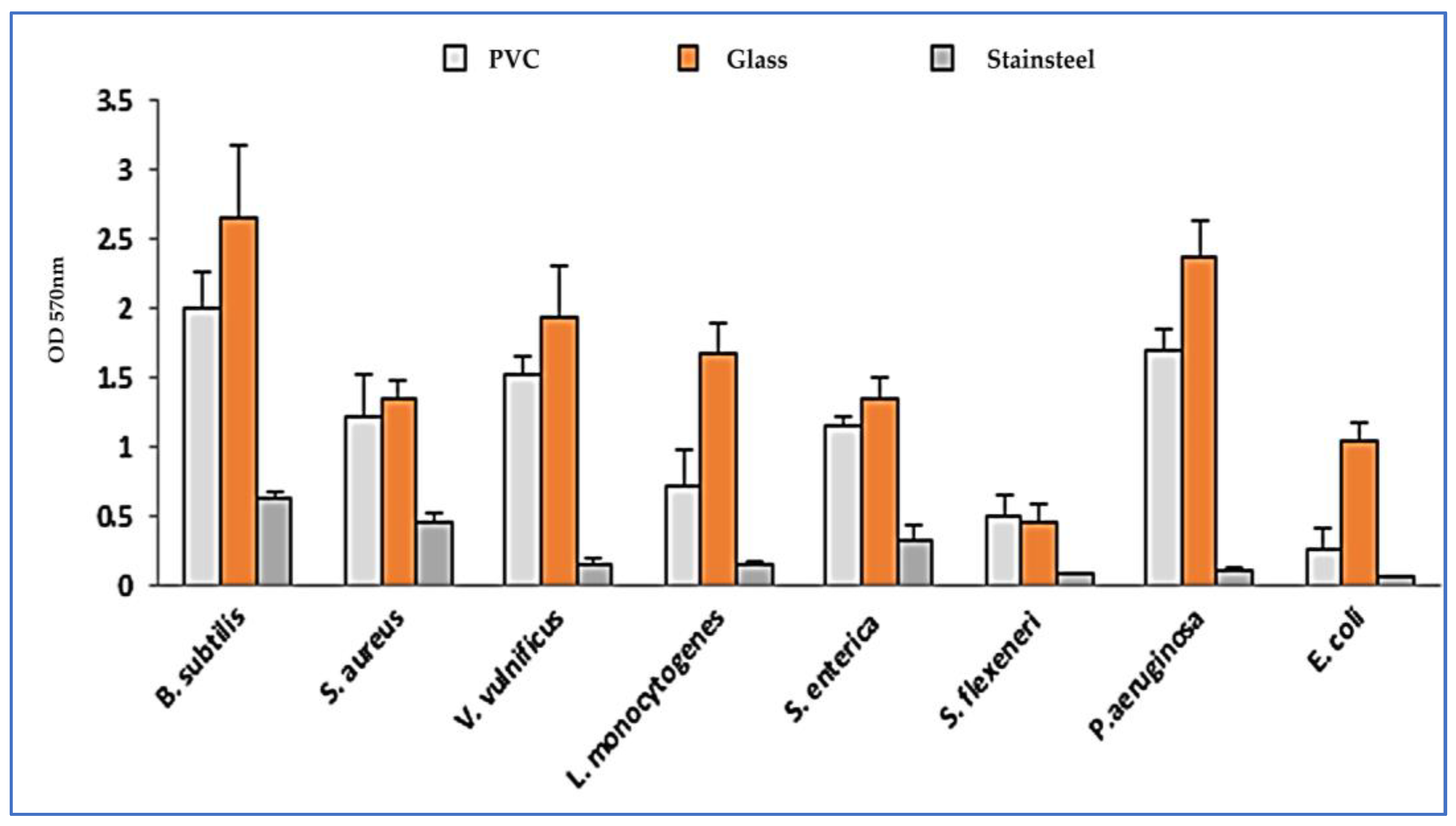
2.3. Adhesive Properties of Bacterial Strains
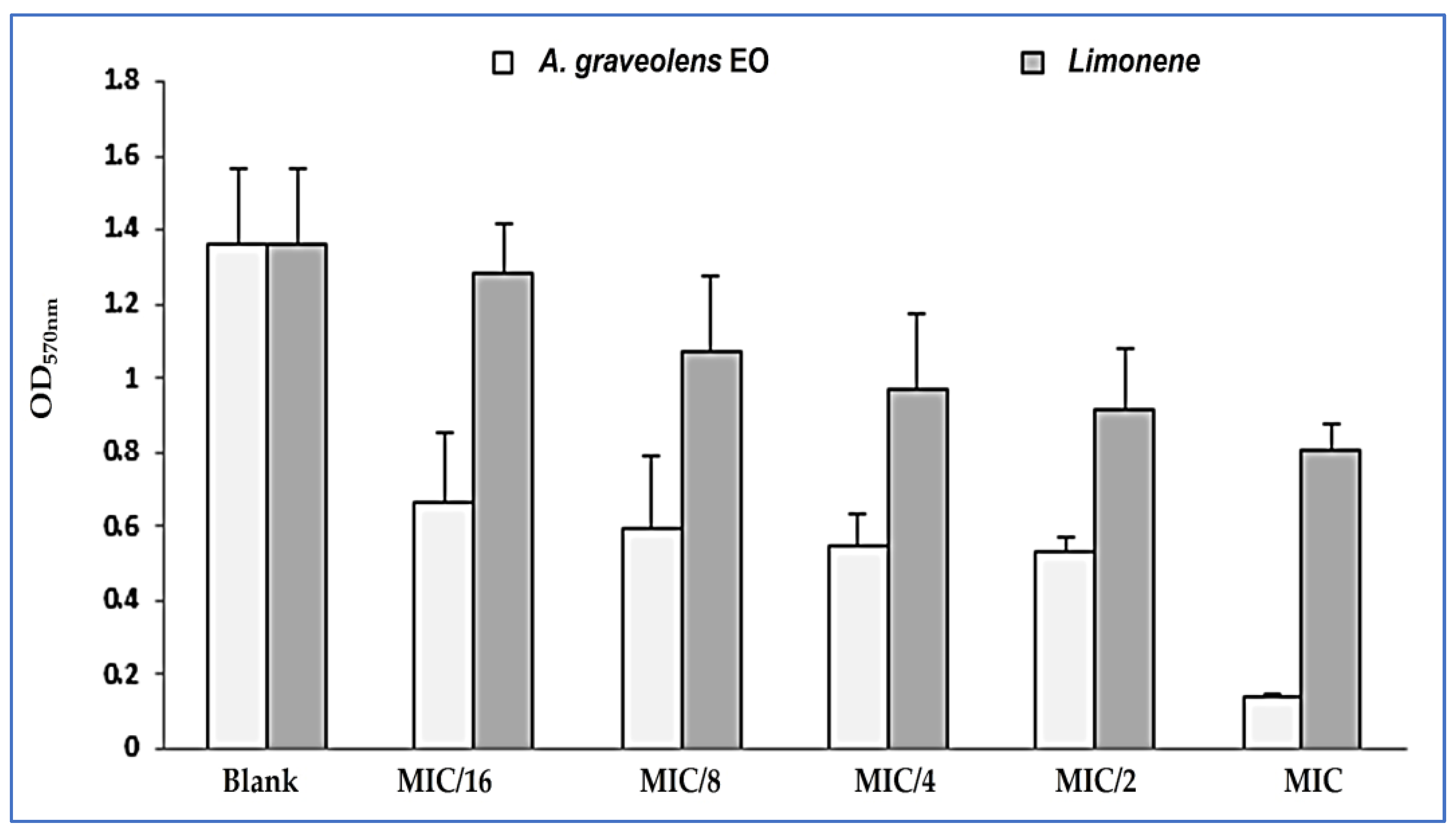
2.4. Anti-Adhesion and Anti-Biofilm Formation Activities
2.5. Anti-Quorum Sensing Activities of the Tested Agents
2.5.1. Anti-Swarming Activity
2.5.2. Violacein Inhibition Assay
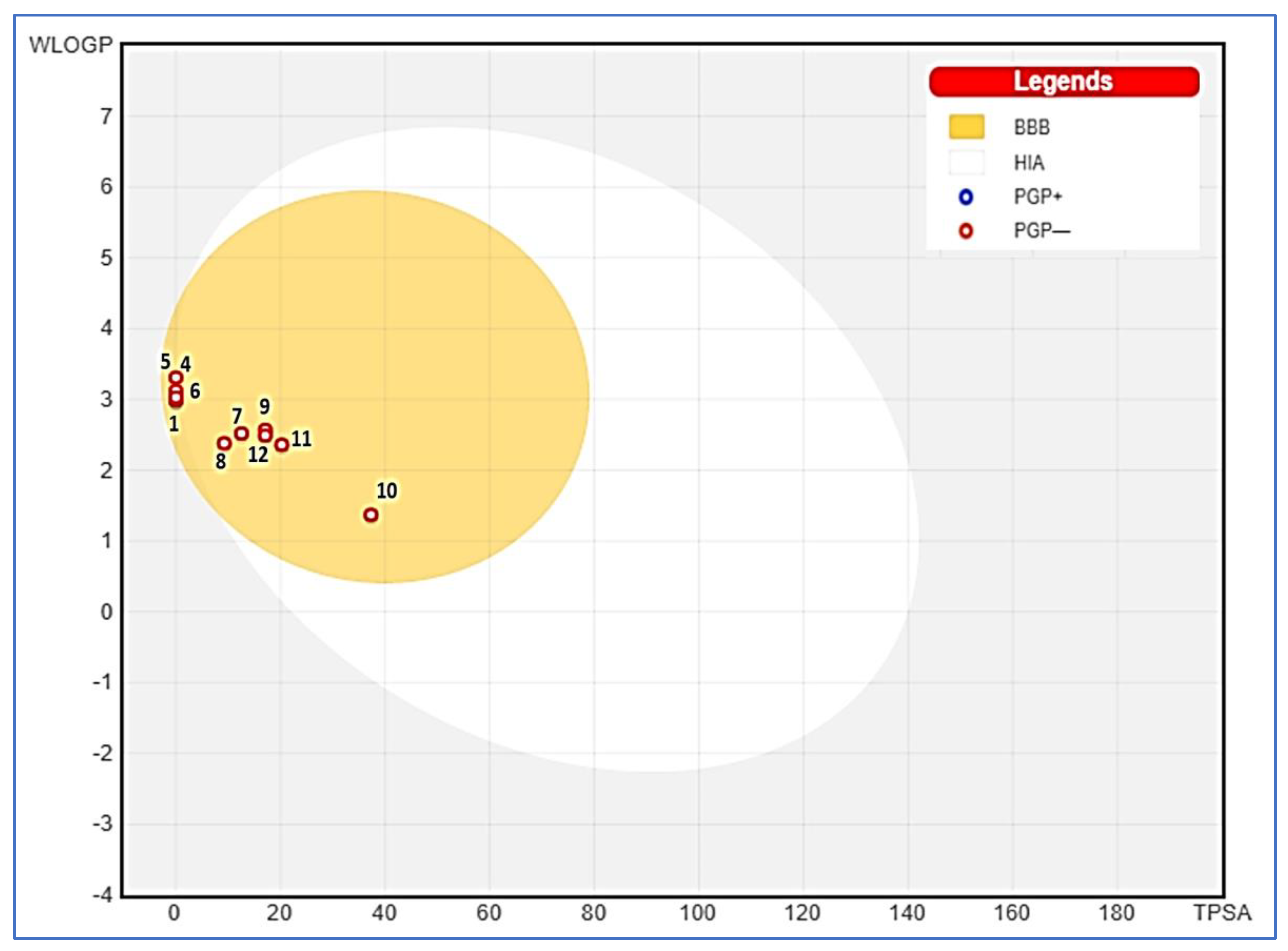
2.6. Draggability and Pharmacokinetic Properties of A. graveolens Main Compounds
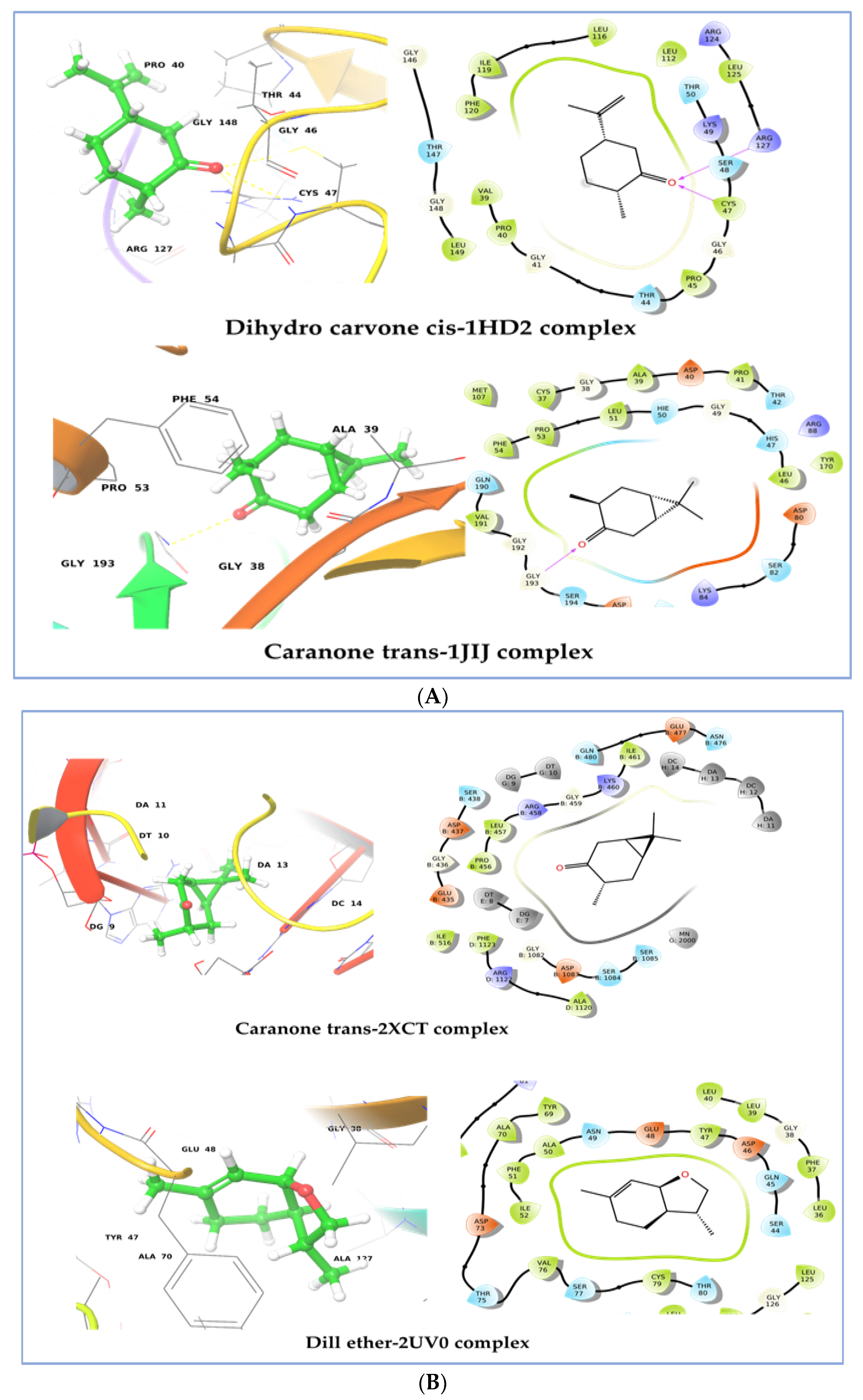
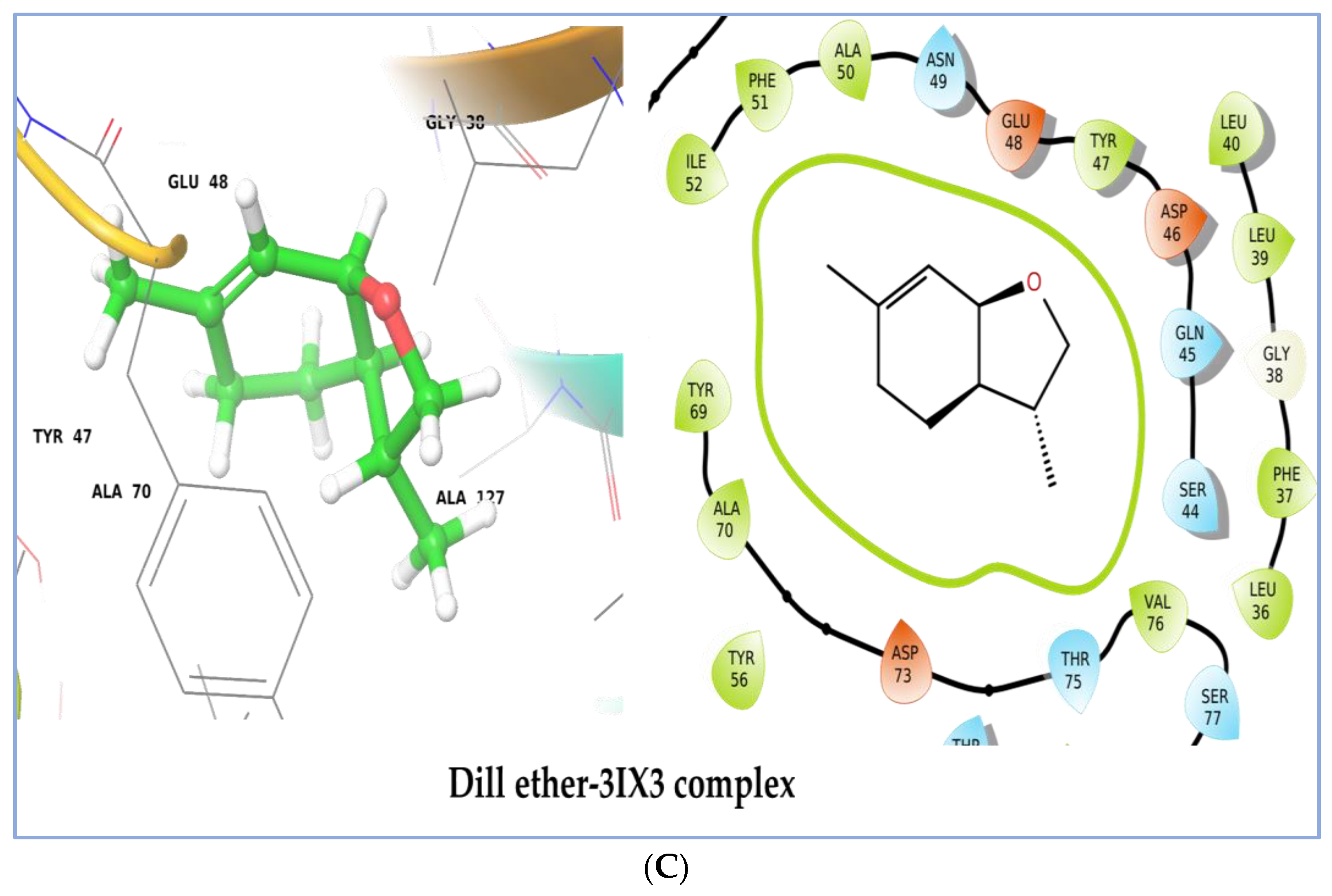
2.7. Molecular Docking Study
3. Discussion
4. Materials and Methods
4.1. Chemical Composition Analyses
4.2. Disk Diffusion Test
4.3. Microdilution Assay
4.4. Adhesive Potentiality
4.5. Biofilm Formation Capacity on Abiotic Surfaces
4.6. Anti-Biofilm Activities
4.6.1. Biofilm Inhibition
4.6.2. Biofilm Eradication
4.7. Anti-Quorum Sensing Activity
4.8. Anti-Swarming Activity
4.9. ADMET Profile
4.10. Molecular Docking Study
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Aminzare, M.; Hashemi, M.; Azar, H.H.; Hejazi, J. The use of herbal extracts and essential oils as a potential antimicrobial in meat and meat products; a review. J. Hum. Environ. Health Promot. 2016, 1, 63–74. [Google Scholar] [CrossRef]
- Al-Haidari, R.A.; Shaaban, M.I.; Ibrahim, S.R.M.; Mohamed, G.A. Anti-quorum sensing activity of some medicinal plants. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A.; et al. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Mseddi, K.; Alimi, F.; Noumi, E.; Veettil, V.N.; Deshpande, S.; Adnan, M.; Hamdi, A.; Elkahoui, S.; Alghamdi, A.; Kadri, A.; et al. Thymus musilii Velen. as a promising source of potent bioactive compounds with its pharmacological properties: In vitro and in silico analysis. Arab. J. Chem. 2020, 13, 6782–6801. [Google Scholar] [CrossRef]
- Chopra, I.; Hodgson, J.; Metcalf, B.; Poste, G. The search for antibacterial agents effective against bacteria resistant to multiple antibiotics. Antimicrob. Agents Chemother. 1997, 4, 497–503. [Google Scholar] [CrossRef]
- Rojas, J.J.; Ochoa, V.J.; Ocampo, S.A.; Munoz, J.F. Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: A possible alternative in the treatment of non-nosocomial infections. BMC Complement Altern. Med. 2006, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007, 117, 112–119. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sokmen, M.; Polissiou, M.; Sokmen, A. In vitro antimicrobial and antioxidant activities of the essential oils and various extracts of Thymus eigii M. Zohary et P.H. Davis. J. Agric. Food Chem. 2004, 52, 1132–1137. [Google Scholar] [CrossRef]
- Wren, R.C. Potter’s New Cyclopedia of Botanical Drugs and Preparations. Walden, S., Ed.; The C.W. Daniel Company Ltd.: Saffron Walden, UK, 1988. [Google Scholar]
- Leung, A.Y.; Foster, S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics; John Wiley and Sons: New York, NY, USA, 1996. [Google Scholar]
- Yazdanparast, R.; Bahramikia, S. Evaluation of the effect of Anethum graveolens L. crude extracts on serum lipids and lipoproteins profiles in hypercholesterolaemic rats. DARU J. Pharm. Sci. 2008, 16, 88–94. [Google Scholar]
- Lagos, Organization of African Unity, Scientific Technical and Research Commission. African Pharmacopoeia; Lagos, Organization of African Unity, Scientific Technical and Research Commission: Addis Ababa, Ethiopia, 1985; Volume 1. [Google Scholar]
- Zargari, A. Medicinal Plants, 6th ed.; Tehran University Press: Tehran, Iran, 1996; Volume II, pp. 528–531. [Google Scholar]
- Ishikawa, T.M.; Kudo, M.; Kitajima, J. Water-soluble constituents of dill. Chem. Pharm. Bull. 2002, 55, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.J.; Arora, D.S. Bioactive potential of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi belonging to the family Umbelliferae-Current status. J. Med. Plants Res. 2010, 4, 87–94. [Google Scholar]
- Leung, A.Y.; Foster, S. Encyclopedia of Common Natural Ingredients (Used in Food, Drugs, and Cosmetics), 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; p. 649. [Google Scholar]
- Hosseinzadeh, H.; Karimi, G.R.; Ameri, M. Effects of Anethum graveolens L. Seed extracts on experimental gastric irritation models in mice. BMC Pharmacol. 2002, 2, 21. [Google Scholar]
- Vishaldeep, K.; Ramandeep, K.; Urvashi, B. A review on dill essential oil and its chief compounds as natural biocide. Flavour Fragr. J. 2021, 36, 412–431. [Google Scholar]
- Rădulescu, V.; Popescu, M.L.; Ilieş, D.C. Chemical composition of the volatile oil from different plant parts of Anethum graveolens L. (Umbelliferae) cultivated in Romania. Farmacia 2010, 58, 594–600. [Google Scholar]
- Stanojević, L.P.; Radulović, N.S.; Djokić, T.M.; Stanković, B.M.; Ilić, D.P.; Cakić, M.D.; Nikolić, V.D. The yield, composition and hydrodistillation kinetics of the essential oil of dill seeds (Anethii fructus) obtained by different hydrodistillation techniques. Indus. Crops Prod. 2015, 65, 429–436. [Google Scholar] [CrossRef]
- Jump up- Saiman, L. Microbiology of early CF lung disease. Paedia. Resp. Rev. 2004, 5 (Suppl. A), S367–S369. [Google Scholar]
- Dhama, k.; Tiwari, R.; Chakraborty, S.; Saminathan, M.; Kumar, A.; Karthik, K.; Wani, Y.; Amarpal, S.V.S.; Rahal, A. Evidence Based Antibacterial Potentials of Medicinal Plants and Herbs Countering Bacterial Pathogens Especially in the Era of Emerging Drug Resistance: An Integrated Update. Int. J. Pharmacol. 2014, 10, 1–43. [Google Scholar] [CrossRef]
- Meng, X.Y.; Zhang, H.X.; Mezei, M.; Cui, M. Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput. Aided Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L. The many roles of computation in drug discovery. Science 2004, 303, 1813–1818. [Google Scholar] [CrossRef]
- Bajorath, J. Integration of virtual and high-throughput screening. Nat. Rev. Drug Discov. 2002, 1, 882–894. [Google Scholar] [CrossRef]
- Haddaji, F.; Papetti, A.; Noumi, E.; Colombo, R.; Deshpande, S.; Aouadi, K.; Adnan, M.; Kadri, A.; Selmi, B.; Snoussi, M.; et al. Bioactivities and in silico study of Pergularia tomentosa L. phytochemicals as potent antimicrobial agents targeting type IIA topoisomerase, TyrRS, and Sap1 virulence proteins. Environ. Sci. Pollut. Res. Int. 2021, 28, 25349–25367. [Google Scholar] [CrossRef]
- Noumi, E.; Snoussi, M.; Anouar, E.H.; Alreshidi, M.; Veettil, V.N.; Elkahoui, S.; Adnan, M.; Patel, M.; Kadri, A.; Aouadi, K.; et al. HR-LCMS-based metabolite profiling, antioxidant, and anticancer properties of Teucrium polium L. methanolic extract: Computational and in vitro study. Antioxidants 2020, 9, 1089. [Google Scholar] [CrossRef] [PubMed]
- Badraoui, R.; Saeed, M.; Bouali, N.; Hamadou, W.S.; Elkahoui, S.; Alam, M.J.; Siddiqui, A.J.; Adnan, M.; Saoudi, M.; Rebai, T. Expression Profiling of Selected Immune Genes and Trabecular Microarchitecture in Breast Cancer Skeletal Metastases Model: Effect of α-Tocopherol Acetate Supplementation. Calcif. Tissue Int. 2022, 110, 475–488. [Google Scholar] [CrossRef]
- Gatsing, D.; Tchakoute, V.; Ngamga, D.; Kuiate, J.R.; Tamokou, J.D.D.; Nji-Nkah, B.F.; Tchouanguep, F.M.; Fodouop, S.P.C. In vitro antibacterial activity of Crinum purpurascens Herb. leaf extract against the Salmonella species causing typhoid fever and its toxicological evaluation. Iran J. Med. Sci. 2009, 34, 126–136. [Google Scholar]
- Moroh, J.L.; Bahi, C.; Dje, K.; Loukou, Y.G.; Guide Guina, F. Etude de l’activité antibactérienne de l’extrait acétatique de Morinda morindoides (Baker) Milne-Redheat (Rubiaceae) sur la croissance in vitro des souches d’Escherichia coli. Bull. Soc. R Sci. Liege 2008, 77, 44–61. [Google Scholar]
- Al-Ma’adhedi, S.H.F. Phytochemical Screening, Estimation of Some Heavy Metals Concentrations, and Specific Extraction of Bioactive Components from Iraqi Anethum graveolens L. Seeds and Studying their Antibacterial Activity. Anbar J. Vet. Sci. 2012, 5, 30. [Google Scholar]
- Singh, G.; Maurya, S.; de Lampasona, C.C. Chemical Constituents, Antimicrobial Investigations, and Antioxidative Potentials of Anethum graveolens L. Essential Oil and Acetone Extract. J. Food Sci. 2006, 70, 208–215. [Google Scholar] [CrossRef]
- Singh, G.; Kapoor, I.P.S.; Pandey, S.K. Studies on essential oils. Antibacterial activity of volatile oils of some spices. Phytother. Res. 2001, 6, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Rifatuz-Zaman, M.S.; Akhtar, M.S.; Khan, M.S. In vitro antibacterial screening of Anethum graveolens L. Fruit, Cichorium intybus L. leaf, Plantago ovata L. seed husk and Polygonum viviparum L. root extracts against Helicobacter pylori. Int. J. Pharmacol. 2006, 2, 674–677. [Google Scholar]
- Jana, S.; Shekhawat, G.S. Phytochemical Analysis and Antibacterial Screening of in vivo and in vitro Extracts of Indian Medicinal Herb: Anethum graveolens. Res. J. Med. Plant. 2010, 4, 206–212. [Google Scholar] [CrossRef]
- Johnson, S. How to Distill Peppermint Oil. J. Ess. Oil. Bear. Plants 2014, 16, 506–512. [Google Scholar]
- Viuda-Martos, M.; Ruíz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Chemical composition of the essential oils obtained from some spices widely used in Mediterranean region. Acta Chim. Slov. 2007, 54, 921–926. [Google Scholar]
- Karpiński, T.M. Essential Oils of Lamiaceae Family Plants as Antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef]
- Jianu, C.; Misca, C.; Pop, G.; Rusu, L.C.; Ardelean, L.; Gruia, A.T. Chemical composition and antimicrobial activity of essential oils obtained from dill (Anethum graveolens L.) grown in western Romania. Rev. Chim. 2012, 63, 641–645. [Google Scholar]
- Kaur, G.J.; Arora, D.S. In vitro antibacterial activity of three plants belonging to the family Umbelliferae. Int. J. Antimicrob. Agents. 2008, 31, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.J.; Arora, D.S. Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Complement. Altern. Med. 2009, 9, 30. [Google Scholar] [CrossRef]
- Milanov, D.; Lazić, S.; Vidić, B.; Petronijević, J.; Bugarski, D.; Šugeljev, Z. Slime production and biofilm forming ability by Staphylococcus aureus bovine mastitis isolates. Acta Vet. 2010, 60, 217–226. [Google Scholar] [CrossRef]
- Samaržija, D.; Damjanović, S.; Pogačić, T. Staphylococcus aureus u siru. Mljekarstvo 2007, 57, 31–48. [Google Scholar]
- Markov, K.; Frece, J.; Čvek, D.; Delaš, F. Listeria monocytogenes i drugi kontaminanti u svježem siru i vrhnju domaće proizvodnje s područja grada Zagreba. Mljekarstvo 2009, 59, 225–231. [Google Scholar]
- Delaquis, P.J.; Stanich, K.; Girard, B.; Mazza, G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int. J. Food Microbiol. 2002, 74, 101–109. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R.; da Fonseca, M.M.R. Carvone: Why and how should one bother to produce this terpene. Food Chem. 2006, 95, 413–422. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils-A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Saleh-E-In, M.M.; Sultana, N.; Rahim, M.M.; Ahsan, M.A.; Bhuiyan, M.N.; Hossain, M.N.; Rahman, M.M.; Kumar Roy, S.; Islam, M.R. Chemical composition and pharmacological significance of Anethum Sowa L. Root. BMC Complement Altern Med. 2017, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Bauer, W.D.; Teplitski, M. Can plants manipulate bacterial quorum sensing? Aust. J. Plant Physiol. 2001, 28, 913–921. [Google Scholar] [CrossRef]
- Mohammadi Pelarti, S.; Karimi Zarehshuran, L.; Babaeekhou, L.; Ghane, M. Antibacterial, anti-biofilm and anti-quorum sensing activities of Artemisia dracunculus essential oil (EO): A study against Salmonella enterica serovar Typhimurium and Staphylococcus aureus. Arch. Microbiol. 2021, 203, 1529–1537. [Google Scholar] [CrossRef]
- Zala, A.R.; Dhanji, P.R.; Iqrar, A.; Haruny, P.; Premlata, K. Synthesis, characterization, molecular dynamic simulation, and biological assessment of cinnamates linked to imidazole/benzimidazole as a CYP51 inhibitor. J. Biomol. Struct. Dyn. 2023, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Noumi, E.; Ahmad, I.; Bouali, N.; Patel, H.; Ghannay, S.; ALrashidi, A.A.; Snoussi, M. Thymus musilii Velen. Methanolic Extract: In Vitro and In Silico Screening of Its Antimicrobial, Antioxidant, Anti-Quorum Sensing, Antibiofilm, and Anticancer Activities. Life 2023, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Alsagaby, S.A.; Iqbal, D.; Ahmad, I.; Patel, H.; Mir, S.A.; Madkhali, Y.A.; Oyouni, A.; Hawsawi, Y.M.; Alhumaydhi, F.A.; Alshehri, B.; et al. In silico investigations identified Butyl Xanalterate to competently target CK2α (CSNK2A1) for therapy of chronic lymphocytic leukemia. Sci. Rep. 2022, 12, 17648. [Google Scholar] [CrossRef]
- Snoussi, M.; Ahmad, I.; Aljohani, A.M.A.; Patel, H.; Abdulhakeem, M.A.; Alhazmi, Y.S.; Tepe, B.; Adnan, M.; Siddiqui, A.J.; Sarikurkcu, C.; et al. Phytochemical Analysis, Antioxidant, and Antimicrobial Activities of Ducrosia flabellifolia: A Combined Experimental and Computational Approaches. Antioxidants 2022, 11, 2174. [Google Scholar] [CrossRef]
- Bottomley, M.J.; Muraglia, E.; Bazzo, R.; Carfì, A. Molecular insights into quorum sensing in the human pathogen Pseudomonas aeruginosa from the structure of the virulence regulator LasR bound to its autoinducer. J. Biol. Chem. 2007, 282, 13592–13600. [Google Scholar] [CrossRef] [PubMed]
- McCready, A.R.; Paczkowski, J.E.; Henke, B.R.; Bassler, B.L. Structural determinants driving homoserine lactone ligand selection in the Pseudomonas aeruginosa LasR quorum-sensing receptor. Proc. Natl. Acad. Sci. USA 2019, 116, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Noumi, E.; Merghni, A.; Alreshidi, M.; Haddad, O.; Akmadar, G.; De Martino, L.; Mastouri, M.; Ceylan, O.; Snoussi, M.; Al-Sieni, A.; et al. Chromobacterium violaceum and Pseudomonas aeruginosa PAO1: Models for Evaluating Anti-Quorum Sensing Activity of Melaleuca alternifolia Essential Oil and Its Main Component Terpinen-4-ol. Molecules 2018, 23, 2672. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.; Agnese, A.M.; Cabrera, J.L. The essential oil of Senecio graveolens (Compositae): Chemical composition and antimicrobial activity tests. J. Ethnopharmacol. 1999, 66, 91–96. [Google Scholar] [CrossRef]
- ALrashidi, A.A.; Noumi, E.; Snoussi, M.; Feo, V. Chemical Composition, Antibacterial and Anti-Quorum Sensing Activities of Pimenta dioica L. Essential Oil and Its Major Compound (Eugenol) against Foodborne Pathogenic Bacteria. Plants 2022, 11, 540. [Google Scholar] [CrossRef]
- Touati, A.; Achour, W.; Abbassi, M.S.; Ben Hassen, A. Detection of ica genes and slime production in a collection of Staphylococcus epidermidis strains from catheter-related infections in neutropenic patients. Pathol. Biol. 2007, 55, 277–282. [Google Scholar] [CrossRef]
- Davenport, D.S.; Massanari, R.M.; Pfaller, M.A.; Bale, M.J.; Streed, S.A.; Hierholzer, W.J. Usefulness of a test for slime production as a marker for clinically significant infections with coagulase-negative staphylococci. J. Infect. Dis. 1986, 153, 332–339. [Google Scholar] [CrossRef]
- Mack, D.; Bartscht, K.; Fischer, C.; Rohde, H.; de Grahl, C.; Dobinsky, S. Genetic and biochemical analysis of Staphylococcus epidermidis biofilm accumulation. Methods Enzymol. 2001, 336, 215–239. [Google Scholar]
- Merghni, A.; Noumi, E.; Hadded, O.; Dridi, N.; Panwar, H.; Ceylan, O.; Mastouri, M.; Snoussi, M. Assessment of the antibiofilm and antiquorum sensing activities of Eucalyptus globulus essential oil and its main component 1,8-cineole against methicillin-resistant Staphylococcus aureus strains. Microb. Pathog. 2018, 118, 74–80. [Google Scholar] [CrossRef]
- Saising, J.; Dube, L.; Ziebandt, A.K.; Voravuthikunchai, S.P.; Nega, M.; Gotz, F. Activity of Gallidermin on Staphylococcus aureus and Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 2012, 56, 5804–5810. [Google Scholar] [CrossRef]
- Qais, F.A.; Ahmad, I. Anti-quorum sensing and biofilm inhibitory effect of some medicinal plants against gram-negative bacterial pathogens: In vitro and in silico investigations. Heliyon 2022, 8, e11113. [Google Scholar] [CrossRef]
- Weiser, J.; Henke, H.A.; Hector, N.; Both, A.; Christner, M.; Büttner, H.; Kaplan, J.B.; Rohde, H. Sub-inhibitory tigecycline concentrations induce extracellular matrix binding protein Embp dependent Staphylococcus epidermidis biofilm formation and immune evasion. Int. J. Med. Microbiol. 2016, 306, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Packiavathy, I.A.; Priya, S.; Pandian, S.K.; Ravi, A.V. Inhibition of biofilm development of uropathogens by curcumin–An anti-quorum sensing agent from Curcuma longa. Food Chem. 2014, 148, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Kadri, A.; Aouadi, K. In vitro antimicrobial and α-glucosidase inhibitory potential of enantiopure cycloalkylglycine derivatives: Insights into their in silico pharmacokinetic, druglikeness, and medicinal chemistry properties. J. App. Pharm. Sci. 2020, 10, 107–115. [Google Scholar]
- Othman, I.M.M.; Gad-Elkareem, M.A.M.; Anouar, E.H.; Aouadi, K.; Kadri, A.; Snoussi, M. Design, synthesis ADMET and molecular docking of new imidazo [4,5-b] pyridine-5-thione derivatives as potential tyrosyl-tRNA synthetase inhibitors. Bioorg. Chem. 2020, 102, 104105. [Google Scholar] [CrossRef]
- Othman, I.M.; Gad-Elkareem, M.A.; Snoussi, M.; Aouadi, K.; Kadri, A. Novel fused pyridine derivatives containing pyrimidine moiety as prospective tyrosyl-tRNA synthetase inhibitors: Design, synthesis, pharmacokinetics and molecular docking studies. J. Mol. Struct. 2020, 1219, 128651. [Google Scholar] [CrossRef]
- Ghannay, S.; Aouadi, K.; Kadri, A.; Snoussi, M. GC-MS Profiling, Vibriocidal, Antioxidant, Antibiofilm, and Anti-Quorum Sensing Properties of Carum carvi L. Essential Oil: In Vitro and In Silico Approaches. Plants 2022, 11, 1072. [Google Scholar] [CrossRef]
- Ghannay, S.; Aouadi, K.; Kadri, A.; Snoussi, M. In Vitro and In Silico Screening of Anti-Vibrio spp., Antibiofilm, Antioxidant and Anti-Quorum Sensing Activities of Cuminum cyminum L. Volatile Oil. Plants 2022, 11, 2236. [Google Scholar] [CrossRef]
- Kikiowo, B.; Ahmad, I.; Alade, A.A.; Ijatuyi, T.T.; Iwaloye, O.; Patel, H.M. Molecular dynamics simulation and pharmacokinetics studies of ombuin and quercetin against human pancreatic α-amylase. J. Biomol. Stru. Dyn. 2022, 16, 1–8. [Google Scholar] [CrossRef]
- Haque, M.A.; Hossain, M.S.; Ahmad, I.; Akbor, M.A.; Rahman, A.; Manir, M.S.; Patel, H.M.; Cho, K.M. Unveiling chlorpyrifos mineralizing and tomato plant-growth activities of Enterobacter sp. strain HSTU-ASh6 using biochemical tests, field experiments, genomics, and in silico analyses. Front. Microbiol. 2022, 13, 1060554. [Google Scholar] [CrossRef]
- Patel, K.B.; Mukherjee, S.; Bhatt, H.; Rajani, D.; Ahmad, I.; Patel, H.; Kumari, P. Synthesis, docking, and biological investigations of new coumarin-piperazine hybrids as potential antibacterial and anticancer agents. J. Mol. Struc. 2022, 1276, 134755. [Google Scholar] [CrossRef]
- Mathew, B.; Ravichandran, V.; Raghuraman, S.; Rangarajan, T.M.; Abdelgawad, M.A.; Ahmad, I.; Patel, H.M.; Kim, H. Two dimensional-QSAR and molecular dynamics studies of a selected class of aldoxime- and hydroxy-functionalized chalcones as monoamine oxidase-B inhibitors. J. Biomol. Struc. Dynamics. 2022, 21, 1–11. [Google Scholar]
- Halder, S.K.; Ahmad, I.; Shathi, J.F.; Mim, M.M.; Hassan, M.R.; Jewel, M.J.I.; Dey, P.; Islam, M.S.; Patel, H.; Morshed, M.R.; et al. A Comprehensive Study to Unleash the Putative Inhibitors of Serotype2 of Dengue Virus: Insights from an In Silico Structure-Based Drug Discovery. Mol. Biotech. 2022, 28, 1–14. [Google Scholar]
- Tivari, S.R.; Kokate, S.V.; Gayke, M.S.; Ahmad, I.; Patel, H.; Kumar, S.G.; Jadeja, Y.S. A Series of Dipeptide Derivatives Containing (S)-5-Oxo-pyrrolidine-2-carboxilic Acid Conjugates: Design, Solid-Phase Peptide Synthesis, in vitro Biological Evolution, and Molecular Docking Studies. Chem. Select. 2022, 7, e202203462. [Google Scholar] [CrossRef]
- Akintunde, J.K.; Akomolafe, V.O.; Taiwo, O.A.; Ahmad, I.; Patel, H.; Osifeso, A.; Ojo, O.A. Antihypertensive activity of Roasted cashew nut in mixed petroleum fractions-induced hypertension: An in vivo and in silico approaches. Heliyon 2022, 8, e12339. [Google Scholar] [CrossRef]
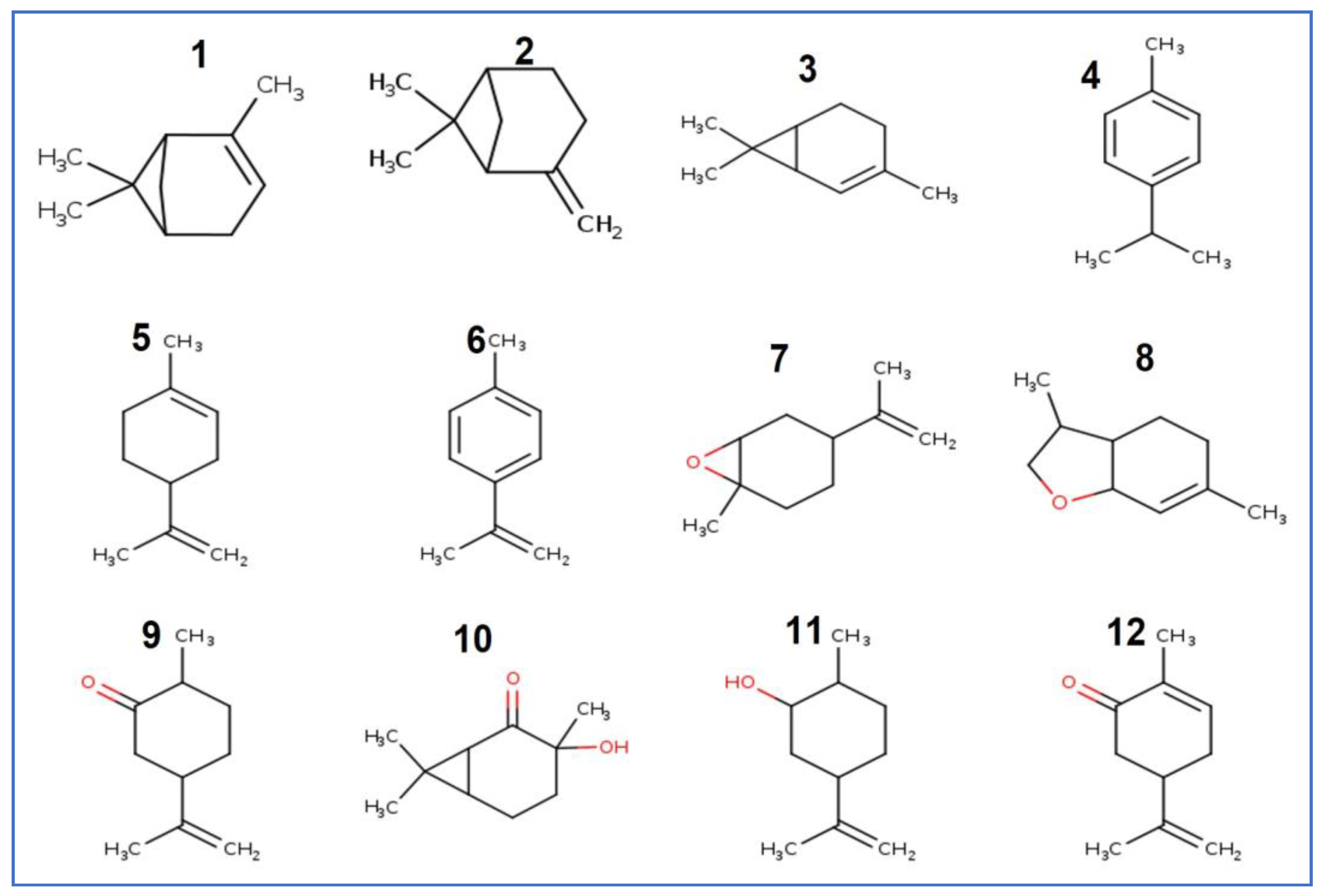




| N. | Compound | Percentage | Ki a | Ki b |
|---|---|---|---|---|
| 1 | α-pinene | 0.68 | 924 | 939 |
| 2 | β-pinene | 0.22 | 984 | 979 |
| 3 | δ-2-carene | 0.23 | 995 | 1002 |
| 4 | ρ- cymene | 0.26 | 1016 | 1026 |
| 5 | Limonene | 48.05 | 1017 | 1029 |
| 6 | Meta-cymene | 0.10 | 1082 | 1037 |
| 7 | Limonene oxide | 0.26 | 1039 | 1050 |
| 8 | Dill ether | 0.11 | 1179 | 1059 |
| 9 | Cis-Dihydro carvone | 3.5 | 1190 | 1070 |
| 10 | Caranone trans | 1.07 | 1197 | 1072 |
| 11 | Dihydro carveol (neoiso) | 0.13 | 1227 | 1090 |
| 12 | Carvone | 37.94 | 1244 | 1096 |
| Total (%) | 92.55 | |||
| Strains | A. graveolens EO | MBC/MIC Ratio | Limonene | MBC/MIC Ratio | ||||
|---|---|---|---|---|---|---|---|---|
| IZ ± SD (mm) | MIC (mg/mL) | MBC (mg/mL) | IZ ± SD (mm) | MIC (mg/mL) | MBC (mg/mL) | |||
| Listeria monocytogenes CECT 933 | 28.5 ± 0.71 | 0.048 | >50 | >4; Bacteriostatic | 11.66 ± 0.57 | 0.048 | 50 | >4; Bacteriostatic |
| Vibrio vulnificus CECT 529 | 22.5 ± 0.71 | 0.048 | >50 | >4; Bacteriostatic | 6 ± 0.1 | 0.048 | >50 | >4; Bacteriostatic |
| Shigella flexeneri CECT 4804 | 21.66 ± 0.57 | 0.097 | 12.5 | >4; Bacteriostatic | 8 ± 0.1 | 0.048 | >50 | >4; Bacteriostatic |
| Bacillus subtilis CIP 5265 | 27.66 ± 0.57 | 0.048 | 50 | >4; Bacteriostatic | 6 ± 0.1 | 0.048 | 50 | >4; Bacteriostatic |
| Salmonella enterica CECT 443 | 20 ± 1 | 0.048 | >50 | >4; Bacteriostatic | 17 ± 0.81 | 0.048 | >50 | >4; Bacteriostatic |
| Escherichia coli ATCC 35218 | 10 ± 0.1 | 0.048 | >50 | >4; Bacteriostatic | 7 ± 0.1 | 0.048 | 50 | >4; Bacteriostatic |
| Pseudomonas aeruginosa PAO1 | 6 ± 0.1 | 0.048 | 12.5 | >4; Bacteriostatic | 6 ± 0.1 | 0.048 | >50 | >4; Bacteriostatic |
| Staphylococcus aureus ATCC 6538 | 21 ± 1 | 0.048 | 50 | >4; Bacteriostatic | 10.33 ± 0.57 | 0.048 | >50 | >4; Bacteriostatic |
| Strains | Adhesion to Glass | Exopolysaccharide Production on CRA | Adhesion to Polystyrene | ||
|---|---|---|---|---|---|
| Colour | S+/S− | OD570 ± SD | Biofilm Production | ||
| S. aureus | ++ | Black | S+ | 1.36 ± 0.2 | High producer |
| L. monocytogenes | +++ | Red with black center | S+ | 0.19 ± 0.07 | Moderate producer |
| V. vulnificus | ++ | Red with black center | S+ | 0.13 ± 0.02 | Moderate producer |
| B. subtilis | + | Red bordeaux | S− | 0.12 ± 0.01 | Moderate producer |
| E. coli | ++ | Red with black center | S+ | 0.17 ± 0.03 | Moderate producer |
| S. flexeneri | +++ | Red with black center | S+ | 0.10 ± 0.01 | Moderate producer |
| S. enterica | + | Red bordeaux | S− | 0.15 ± 0.01 | Moderate producer |
| P. aeruginosa | ++ | Red bordeaux | S− | 0.42 ± 0.26 | Moderate producer |
| EO/Main Compound | Concentration | % Anti-Swarming Activity |
|---|---|---|
| A. graveolens | 50 µg/mL | 16.67 ± 0 |
| 75 µg/mL | 20.83 ± 1.17 | |
| 100 µg/mL | 33.33 ± 0 | |
| Limonene | 50 µg/mL | 10.4 ± 1.3 |
| 75 µg/mL | 19.22 ± 2.1 | |
| 100 µg/mL | 28.9 ± 0.9 |
| Concentration | % of Violacein Inhibition | |
|---|---|---|
| A. graveolens | Limonene | |
| MIC | 67.52 ± 1.1 | 18.68 ± 1.6 |
| MIC/2 | 57.91 ± 1.8 | 10.53 ± 1.1 |
| MIC/4 | 46.77 ± 2.3 | 9.12 ± 2.3 |
| MIC/8 | 33.56 ± 1 | 6.33 ± 1 |
| MIC/16 | 29.93 ± 1.7 | 1.33 ± 1.3 |
| MIC/32 | 23.66 ± 1.6 | 1.03 ± 0.9 |
| Entry | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physicochemical Properties | ||||||||||||
| Molecular weight (g/mol) | 136.23 | 136.23 | 136.23 | 136.23 | 136.23 | 132.20 | 152.23 | 152.23 | 152.23 | 168.23 | 154.25 | 150.22 |
| Num. heavy atoms | 10 | 10 | 10 | 10 | 10 | 10 | 11 | 11 | 11 | 12 | 11 | 11 |
| Num. arom. heavy atoms | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fraction Csp3 | 0.80 | 0.80 | 0.80 | 0.80 | 0.60 | 0.20 | 0.80 | 0.80 | 0.70 | 0.90 | 0.80 | 0.50 |
| Num. rotatable bonds | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 |
| Num. H-bond acceptors | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 1 | 1 |
| Num. H-bond donors | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Molar Refractivity | 45.22 | 45.22 | 45.22 | 45.22 | 47.12 | 46.31 | 46.60 | 46.57 | 47.80 | 47.10 | 48.76 | 47.32 |
| TPSA (Ų) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 12.53 | 9.23 | 17.07 | 37.30 | 20.23 | 17.07 |
| Consensus Log Po/w | 3.44 | 3.42 | 3.12 | 3.5 | 3.37 | 3.37 | 2.71 | 2.27 | 2.51 | 1.56 | 2.48 | 2.44 |
| Lipinski rules | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Bioavailability Score | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| Pharmacokinetics | ||||||||||||
| GI absorption | Low | Low | Low | Low | Low | Low | High | High | High | High | High | High |
| BBB permeant | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| P-gp substrate | No | No | No | No | No | No | No | No | No | No | No | No |
| CYP1A2 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No |
| CYP2C19 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No |
| CYP2C9 inhibitor | Yes | Yes | No | No | Yes | No | No | No | No | No | No | No |
| CYP2D6 inhibitor | No | No | No | Yes | No | Yes | No | No | No | No | No | No |
| CYP3A4 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No |
| Log Kp (cm/s) | −3.95 | −4.18 | −5.11 | −4.21 | −3.89 | −4.49 | −4. 6 | −5.88 | −5.21 | −6.41 | −4.96 | −5.29 |
| No. | Compound | 1HD2 | 1JIJ | 2XCT | 2UV0 | 3IX3 |
|---|---|---|---|---|---|---|
| 1 | α-pinene | −3.86 | −4.526 | −5.707 | −6.614 | −6.585 |
| 2 | β-pinene | −3.597 | −4.499 | −5.512 | −6.574 | −6.546 |
| 3 | δ-2-carene | −3.86 | −4.526 | −5.707 | −6.614 | −6.585 |
| 4 | ρ-cymene | −3.336 | −4.481 | −6.204 | −6.727 | −6.774 |
| 5 | Limonene | −3.032 | −3.521 | −5.525 | −6.153 | −5.874 |
| 6 | Meta-cymene | −3.537 | −4.592 | −6.238 | −6.875 | −7.051 |
| 7 | Limonene oxide | −3.309 | −4.323 | −5.525 | −6.547 | −5.917 |
| 8 | Dill ether | −3.99 | −4.716 | −6.282 | −6.976 | −7.077 |
| 9 | Cis-Dihydro carvone | −5.105 | −4.826 | −5.769 | −6.401 | −6.267 |
| 10 | Trans-caranone | −4.642 | −5.622 | −6.326 | −6.806 | −6.958 |
| 11 | Dihydro carveol (neoiso) | −4.332 | −4.916 | −5.769 | −6.771 | −6.739 |
| 12 | Carvone | −3.529 | −4.894 | −5.621 | −5.529 | −5.964 |
| Co-crystal inhibitor | −7.245 | −7.973 | −8.521 | −6.929 | −6.057 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noumi, E.; Ahmad, I.; Adnan, M.; Merghni, A.; Patel, H.; Haddaji, N.; Bouali, N.; Alabbosh, K.F.; Ghannay, S.; Aouadi, K.; et al. GC/MS Profiling, Antibacterial, Anti-Quorum Sensing, and Antibiofilm Properties of Anethum graveolens L. Essential Oil: Molecular Docking Study and In-Silico ADME Profiling. Plants 2023, 12, 1997. https://doi.org/10.3390/plants12101997
Noumi E, Ahmad I, Adnan M, Merghni A, Patel H, Haddaji N, Bouali N, Alabbosh KF, Ghannay S, Aouadi K, et al. GC/MS Profiling, Antibacterial, Anti-Quorum Sensing, and Antibiofilm Properties of Anethum graveolens L. Essential Oil: Molecular Docking Study and In-Silico ADME Profiling. Plants. 2023; 12(10):1997. https://doi.org/10.3390/plants12101997
Chicago/Turabian StyleNoumi, Emira, Iqrar Ahmad, Mohd Adnan, Abderrahmen Merghni, Harun Patel, Najla Haddaji, Nouha Bouali, Khulood Fahad Alabbosh, Siwar Ghannay, Kaïss Aouadi, and et al. 2023. "GC/MS Profiling, Antibacterial, Anti-Quorum Sensing, and Antibiofilm Properties of Anethum graveolens L. Essential Oil: Molecular Docking Study and In-Silico ADME Profiling" Plants 12, no. 10: 1997. https://doi.org/10.3390/plants12101997
APA StyleNoumi, E., Ahmad, I., Adnan, M., Merghni, A., Patel, H., Haddaji, N., Bouali, N., Alabbosh, K. F., Ghannay, S., Aouadi, K., Kadri, A., Polito, F., Snoussi, M., & De Feo, V. (2023). GC/MS Profiling, Antibacterial, Anti-Quorum Sensing, and Antibiofilm Properties of Anethum graveolens L. Essential Oil: Molecular Docking Study and In-Silico ADME Profiling. Plants, 12(10), 1997. https://doi.org/10.3390/plants12101997












