New Insight into the Function of Dopamine (DA) during Cd Stress in Duckweed (Lemna turionifera 5511)
Abstract
1. Introduction
2. Results
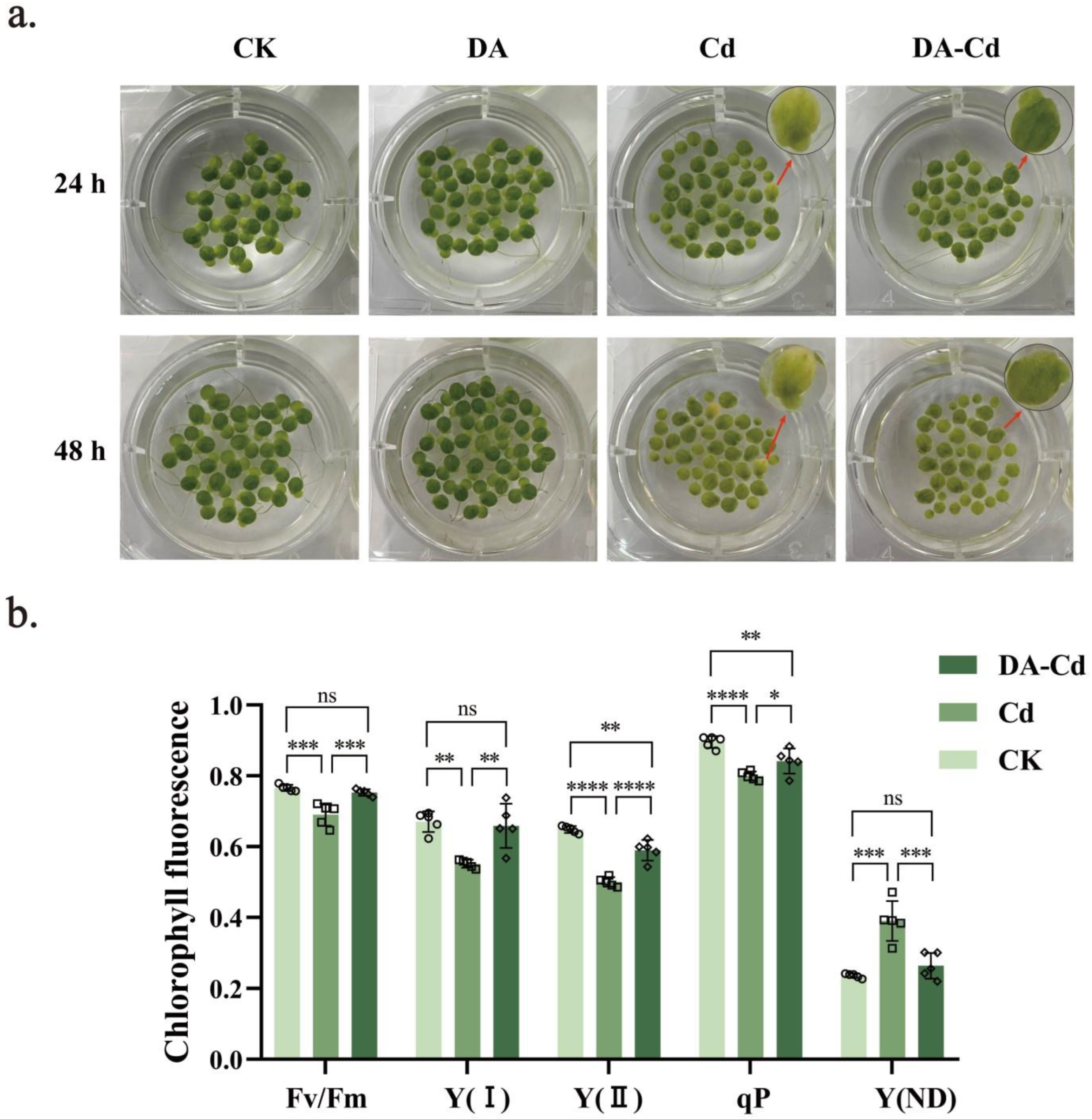
2.1. Effects of DA on the Frond and Root of Duckweed under Cd Stress
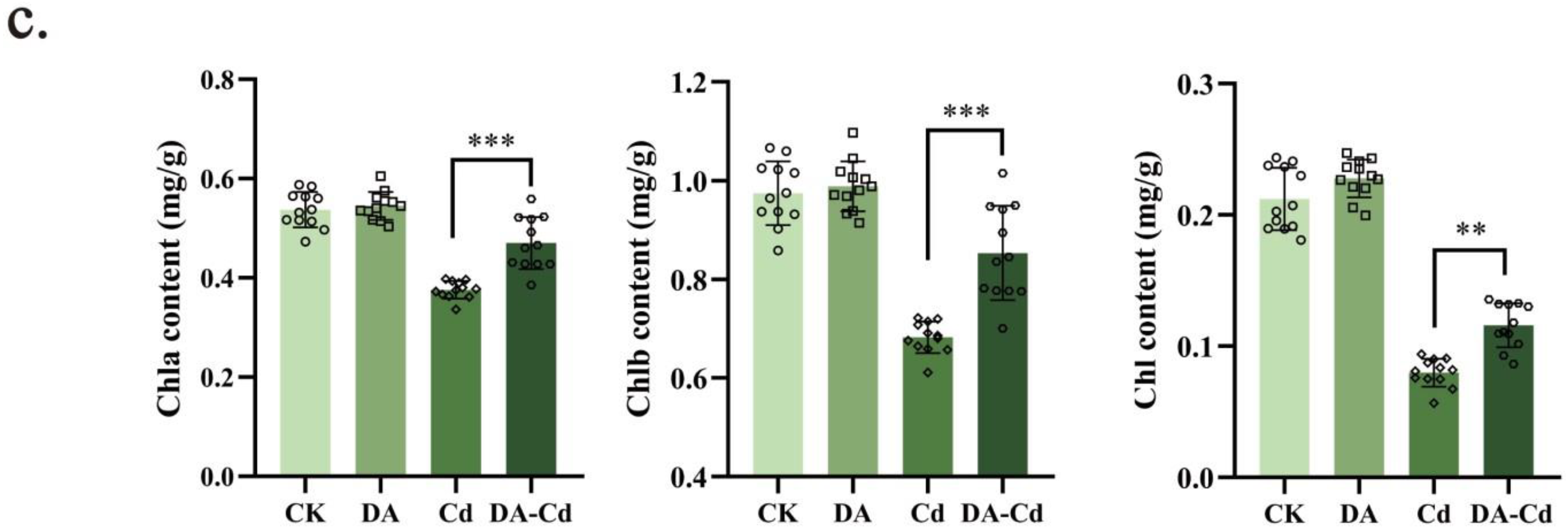
2.2. Chlorophyll Content and Photosynthesis of Duckweed Were Improved by DA under Cd Stress
2.3. Cd Accumulation Increased under DA Treatment
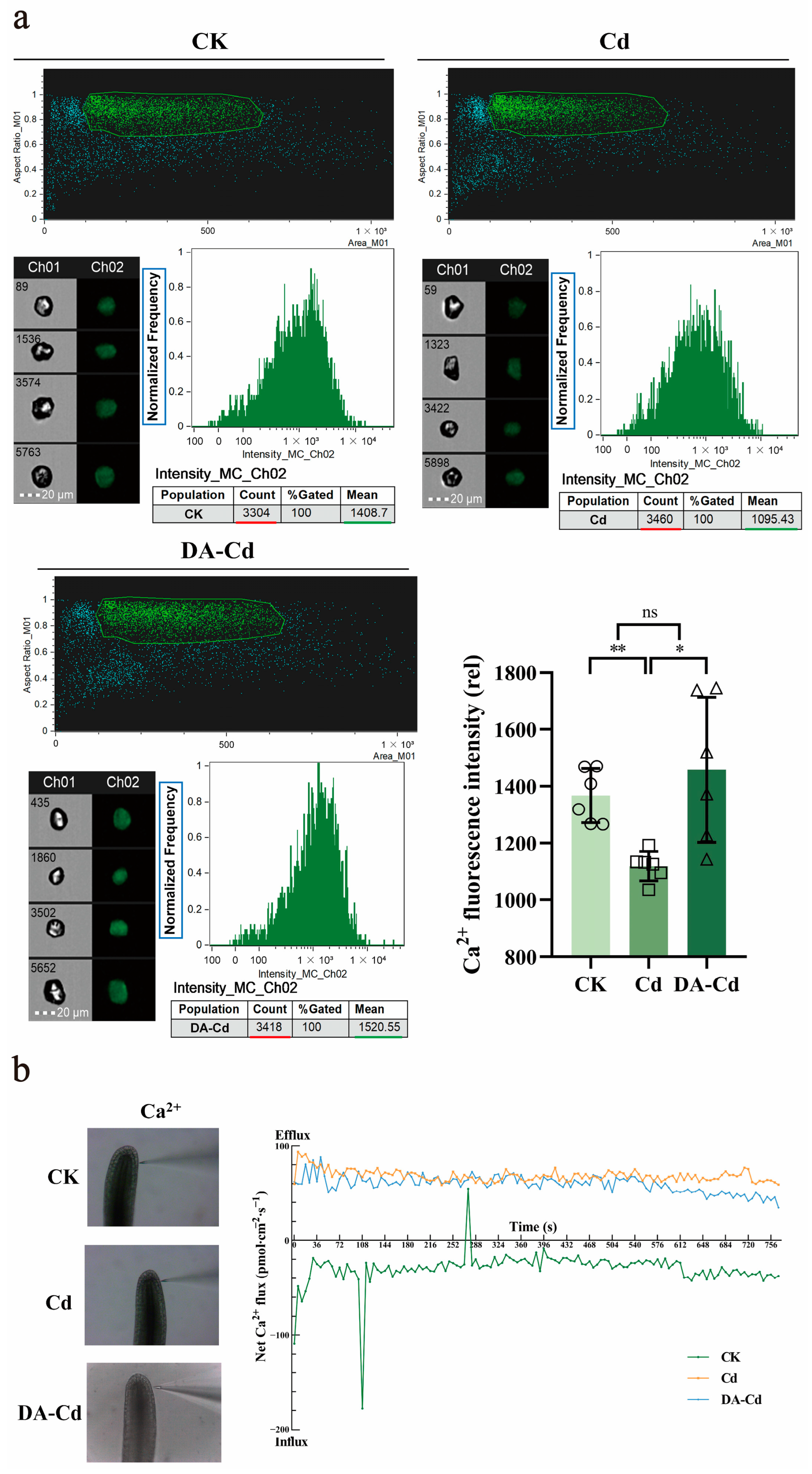
2.4. DA Recovered Ca2+ Content by Mediating Ca2+ Flux under Cd Stress
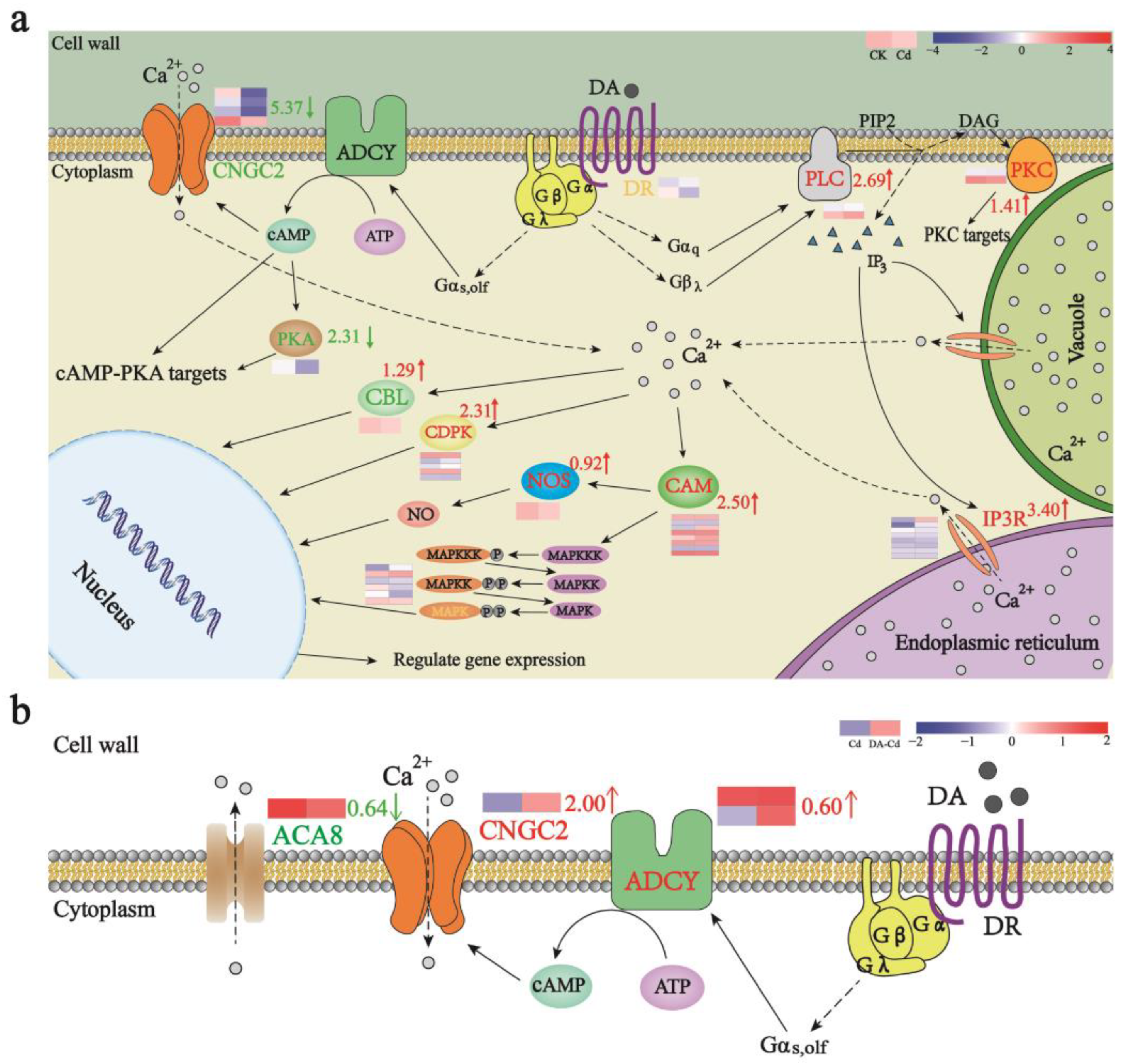
2.5. DA Addition Promoted Proteins Related to Cd Resistance and Accumulation
2.6. A Possible Site of DA Targets in Plants Is CNGC2
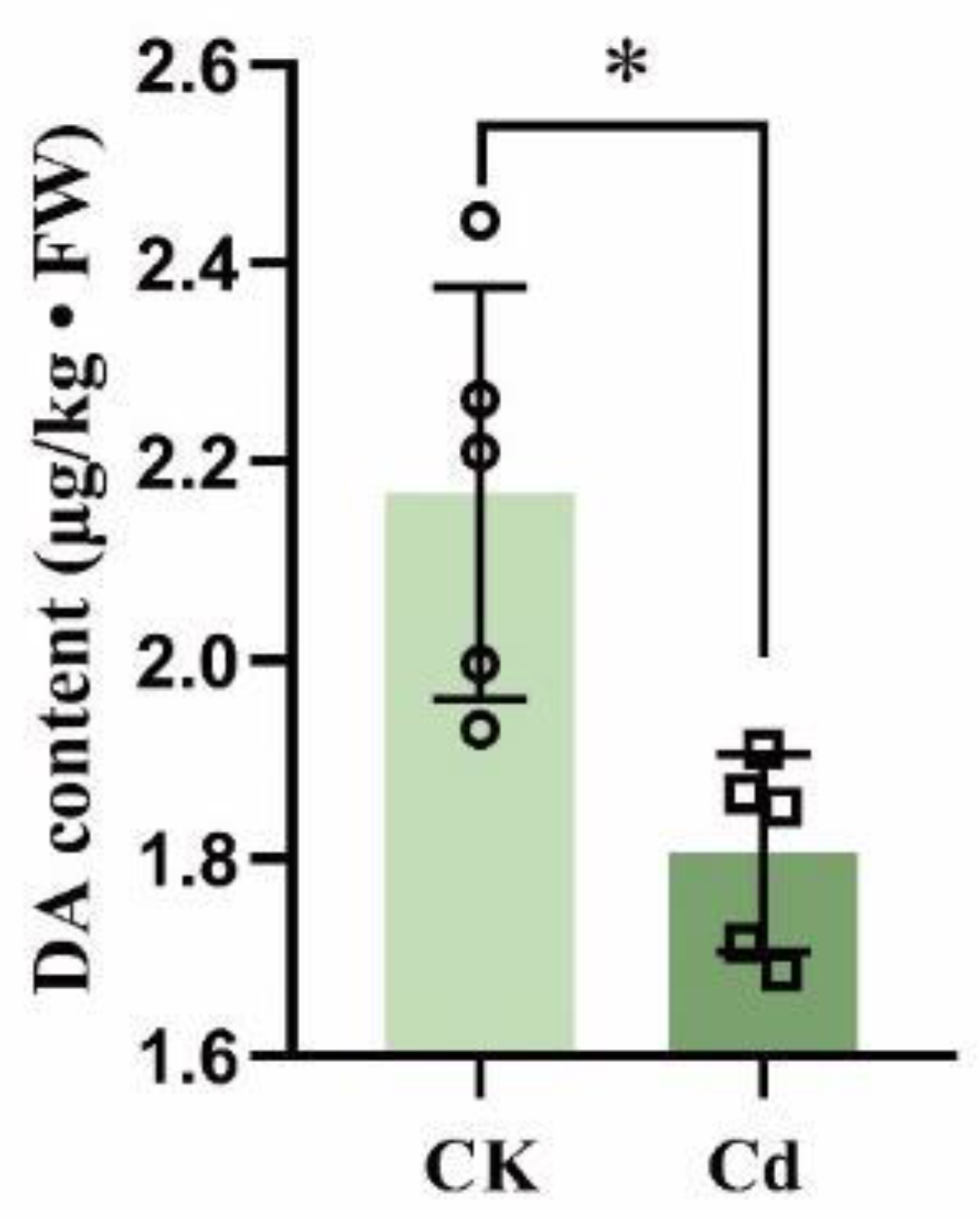
2.7. Endogenous DA Content Decreased under Cd Stress
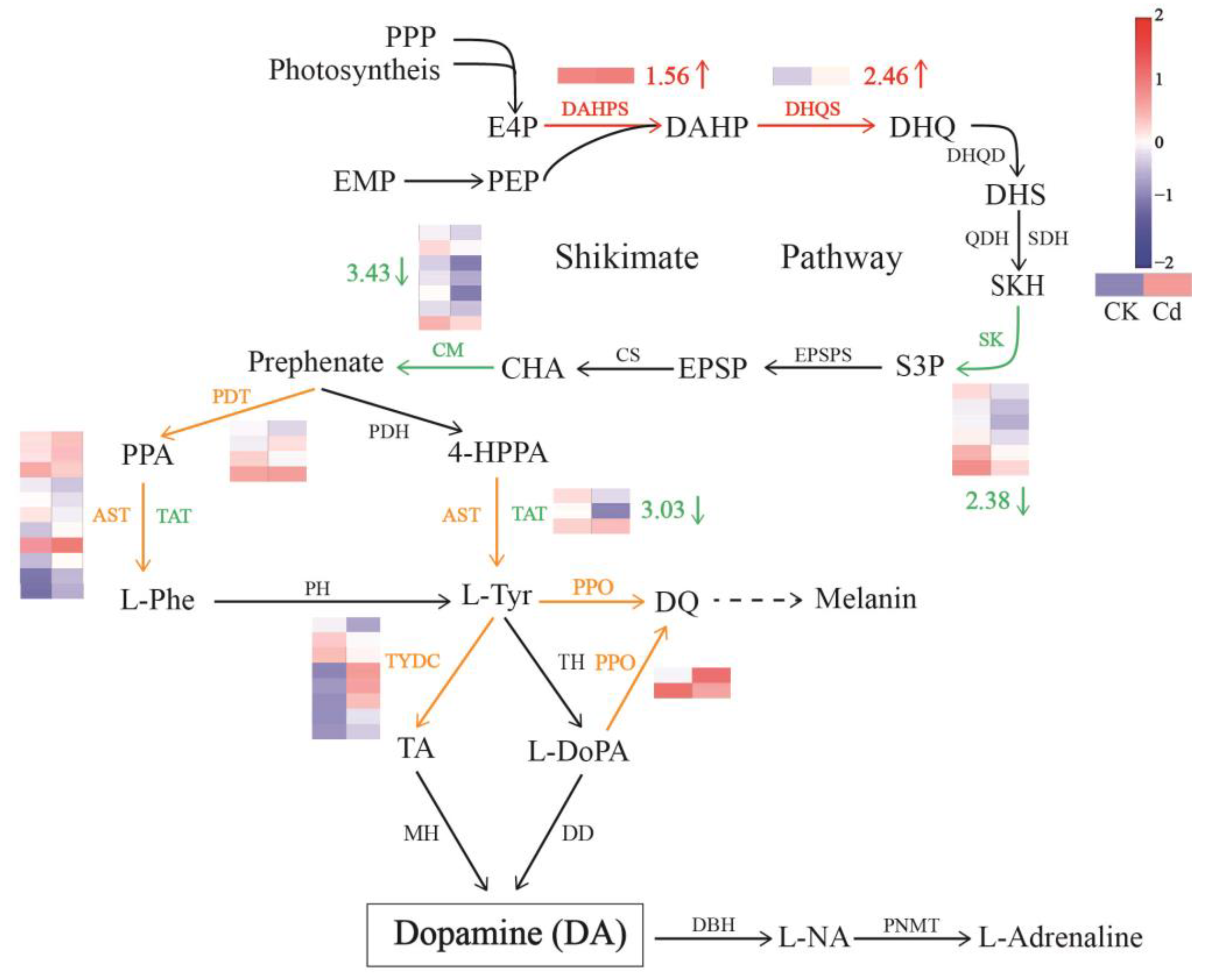
2.8. DA Biosynthesis Pathway Was Inhibited by Cd Stress
3. Discussion
4. Materials and Methods
4.1. Plant and Tissue Culture Conditions
4.2. DA Treatment Concentration under Cd Stress
4.3. Measurement of Chlorophyll Fluorescence
4.4. Measurement of Chlorophyll Content
4.5. DA Content Measurements
4.6. Measurement of Cd Content in Duckweed
4.7. Flow Cytometric Analysis of Intracellular Ca2+ and Cd2+ Content
4.8. Measurement of Cd2+ and Ca2+ Flux
4.9. RNA Sequencing
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Cheng, M.; Kopittke, P.M.; Wang, A.; Tang, C. Salinity decreases Cd translocation by altering Cd speciation in the halophytic Cd-accumulator Carpobrotus rossii. Ann. Bot. 2019, 123, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, A.A.; Filippini, T.; Ajsuvakova, O.P.; Skalnaya, M.G.; Aaseth, J.; Bjørklund, G.; Gatiatulina, E.R.; Popova, E.V.; Nemereshina, O.N.; Huang, P.-T.; et al. Cadmium and atherosclerosis: A review of toxicological mechanisms and a meta-analysis of epidemiologic studies. Environ. Res. 2018, 162, 240–260. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Habib, M.; Kakavand, S.N.; Zahid, Z.; Zahra, N.; Sharif, R.; Hasanuzzaman, M. Phytoremediation of Cadmium: Physiological, Biochemical, and Molecular Mechanisms. Biology 2020, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.; Chauvin, A.; Pascaud, F.; Kellenberger, S.; Farmer, E.E. GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signaling. Nature 2013, 500, 422–426. [Google Scholar] [CrossRef]
- Toyota, M.; Spencer, D.; Sawai-Toyota, S.; Jiaqi, W.; Zhang, T.; Koo, A.J.; Howe, G.A.; Gilroy, S. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 2018, 361, 1112–1115. [Google Scholar] [CrossRef]
- Yang, L.; Ren, Q.; Ma, X.; Wang, M.; Sun, J.; Wang, S.; Wu, X.; Chen, X.; Wang, C.; Li, Q.; et al. New insight into the effect of riluzole on cadmium tolerance and accumulation in duckweed (Lemna turionifera). Ecotoxicol. Environ. Saf. 2022, 241, 113783. [Google Scholar] [CrossRef]
- Yang, L.; Yao, J.; Sun, J.; Shi, L.; Chen, Y.; Sun, J. The Ca2+ signaling, Glu, and GABA responds to Cd stress in duckweed. Aquat. Toxicol. 2020, 218, 105352. [Google Scholar] [CrossRef]
- Su, Y.; Qin, C.; Begum, N.; Ashraf, M.; Zhang, L. Acetylcholine ameliorates the adverse effects of cadmium stress through mediating growth, photosynthetic activity and subcellular distribution of cadmium in tobacco (Nicotiana benthamiana). Ecotoxicol. Environ. Saf. 2020, 198, 110671. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, T.; Liu, W.; Liu, Y.; Zhao, Y.; Liu, Y.; Li, W.; Ding, K.; Ma, F.; Li, C. Functions of dopamine in plants: A review. Plant Signal. Behav. 2020, 15, 1827782. [Google Scholar] [CrossRef]
- Liang, B.; Gao, T.; Zhao, Q.; Ma, C.; Chen, Q.; Wei, Z.; Li, C.; Li, C.; Ma, F. Effects of Exogenous Dopamine on the Uptake, Transport, and Resorption of Apple Ionome Under Moderate Drought. Front. Plant Sci. 2018, 9, 755. [Google Scholar] [CrossRef]
- Jiao, X.; Li, Y.; Zhang, X.; Liu, C.; Liang, W.; Li, C.; Ma, F.; Li, C. Exogenous Dopamine Application Promotes Alkali Tolerance of Apple Seedlings. Plants 2019, 8, 580. [Google Scholar] [CrossRef]
- Liang, B.; Li, C.; Ma, C.; Wei, Z.; Wang, Q.; Huang, D.; Chen, Q.; Li, C.; Ma, F. Dopamine alleviates nutrient deficiency-induced stress in Malus hupehensis. Plant Physiol. Biochem. 2017, 119, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yuan, X.; Zhang, Z.; Wang, Y.; Ma, F.; Li, C. Dopamine Enhances the Resistance of Apple to Valsa mali Infection. Phytopathology 2022, 112, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Wang, Y.; Mao, Q.; Wu, M.; Yan, Y.; Ren, J.; Wang, X.; Liu, A.; Chen, S. Dopamine alleviates bisphenol A-induced phytotoxicity by enhancing antioxidant and detoxification potential in cucumber. Environ. Pollut. 2020, 259, 113957. [Google Scholar] [CrossRef]
- Belkacemi, L.; Darmani, N.A. Dopamine receptors in emesis: Molecular mechanisms and potential therapeutic function. Pharmacol. Res. 2020, 161, 105124. [Google Scholar] [CrossRef] [PubMed]
- Steiner, U.; Schliemann, W.; Strack, D. Assay for tyrosine hydroxylation activity of tyrosinase from betalain-forming plants and cell cultures. Anal. Biochem. 1996, 238, 72–75. [Google Scholar] [CrossRef]
- Kong, K.H.; Lee, J.L.; Park, H.J.; Cho, S.H. Purification and characterization of the tyrosinase isozymes of pine needles. IUBMB Life 1998, 45, 717–724. [Google Scholar] [CrossRef]
- Zhou, X.; Pardue, M.T.; Iuvone, P.M.; Qu, J. Dopamine signaling and myopia development: What are the key challenges. Prog. Retin. Eye Res. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef]
- Gao, T.; Zhang, Z.; Liu, X.; Wu, Q.; Chen, Q.; Liu, Q.; van Nocker, S.; Ma, F.; Li, C. Physiological and transcriptome analyses of the effects of exogenous dopamine on drought tolerance in apple. Plant Physiol. Biochem. 2020, 148, 260–272. [Google Scholar] [CrossRef]
- Skirycz, A.; Świędrych, A.; Szopa, J. Expression of human dopamine receptor in potato (Solanum tuberosum) results in altered tuber carbon metabolism. BMC Plant Biol. 2005, 5, 1. [Google Scholar] [CrossRef]
- Stomp, A.-M. The duckweeds: A valuable plant for biomanufacturing. Biotechnol. Annu. Rev. 2005, 11, 69–99. [Google Scholar] [CrossRef] [PubMed]
- Ekperusi, A.O.; Sikoki, F.D.; Nwachukwu, E.O. Application of common duckweed (Lemna minor) in phytoremediation of chemicals in the environment: State and future perspective. Chemosphere 2019, 223, 285–309. [Google Scholar] [CrossRef]
- Yu, C.; Sun, C.; Yu, L.; Zhu, M.; Xu, H.; Zhao, J.; Ma, Y.; Zhou, G. Comparative analysis of duckweed cultivation with sewage water and SH media for production of fuel ethanol. PLoS ONE 2014, 9, e115023. [Google Scholar] [CrossRef]
- Hou, W.; Chen, X.; Song, G.; Wang, Q.; Chang, C.C. Effects of copper and cadmium on heavy metal polluted waterbody restoration by duckweed (Lemna minor). Plant Physiol. Biochem. 2007, 45, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, X.; Chang, C.; Jia, D.; Wei, Z.; Li, C.; Ma, F. Dopamine alleviates salt-induced stress in Malus hupehensis. Physiol. Plant 2015, 153, 584–602. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, W.U.; Shah, A.A.; Yasin, N.A.; Ali, A.; Rizwan, M.; Ali, S. Dopamine Alleviates Hydrocarbon Stress in Brassica oleracea through Modulation of Physio-Biochemical Attributes and Antioxidant Defense Systems. Chemosphere 2021, 270, 128633. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, Q.; Gao, T.; Wang, H.; Zhang, Z.; Liang, B.; Wei, Z.; Liu, C.; Ma, F. The mitigation effects of exogenous melatonin on replant disease in apple. J. Pineal Res. 2018, 65, e12523. [Google Scholar] [CrossRef]
- Zhou, S.; Li, M.; Guan, Q.; Liu, F.; Zhang, S.; Chen, W.; Yin, L.; Qin, Y.; Ma, F. Physiological and proteome analysis suggest critical roles for the photosynthetic system for high water-use efficiency under drought stress in Malus. Plant Sci. 2015, 236, 44–60. [Google Scholar] [CrossRef]
- Allahverdiyeva, Y.; Mamedov, F.; Suorsa, M.; Styring, S.; Vass, I.; Aro, E.-M. Insights into the function of PsbR protein in Arabidopsis thaliana. Biochim. Biophys. Acta 2007, 1767, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ye, T.; Gao, X.; Chen, R.; Xu, J.; Xie, C.; Zhu, J.; Deng, X.; Wang, P.; Xu, Z. Molecular characterization and functional analysis of the OsPsbR gene family in rice. Mol. Genet. Genom. 2017, 292, 271–281. [Google Scholar] [CrossRef]
- Jensen, P.E.; Haldrup, A.; Zhang, S.; Scheller, H.V. The PSI-O subunit of plant photosystem I is involved in balancing the excitation pressure between the Two photosystems. J. Biol. Chem. 2004, 279, 24212–24217. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Shimakawa, G.; Sétif, P. Role of the two PsaE isoforms on O2 reduction at photosystem I in Arabidopsis thaliana. Biochim. Biophys. Acta (BBA) Bioenerg. 2020, 1861, 148089. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Jin, L.; Wang, X. Cadmium absorption and transportation pathways in plants. Int. J. Phytoremediat. 2017, 19, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.R.; Dos Reis, A.R.; Prado, E.R.; Lavres, J.; Pompeu, G.B.; Azevedo, R.A.; Gratão, P.L. New insights into cadmium stressful-conditions: Role of ethylene on selenium-mediated antioxidant enzymes. Ecotoxicol. Environ. Saf. 2019, 186, 109747. [Google Scholar] [CrossRef]
- Jiang, M.; Jiang, J.; Li, S.; Li, M.; Tan, Y.; Song, S.; Shu, Q.; Huang, J. Glutamate alleviates cadmium toxicity in rice via suppressing cadmium uptake and translocation. J. Hazard. Mater. 2020, 384, 121319. [Google Scholar] [CrossRef]
- Agarwal, P.; Mitra, M.; Banerjee, S.; Roy, S. MYB4 transcription factor, a member of R2R3-subfamily of MYB domain protein, regulates cadmium tolerance via enhanced protection against oxidative damage and increases expression of PCS1 and MT1C in Arabidopsis. Plant Sci. 2020, 297, 110501. [Google Scholar] [CrossRef]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef]
- Cosio, C.; Martinoia, E.; Keller, C. Hyperaccumulation of Cadmium and Zinc in Thlaspi caerulescens and Arabidopsis halleri at the Leaf Cellular Level. Plant Physiol. 2004, 134, 716–725. [Google Scholar] [CrossRef]
- Kieffer, P.; Schröder, P.; Dommes, J.; Hoffmann, L.; Renaut, J.; Hausman, J.-F. Proteomic and enzymatic response of poplar to cadmium stress. J. Proteom. 2009, 72, 379–396. [Google Scholar] [CrossRef]
- Perfus-Barbeoch, L.; Leonhardt, N.; Vavasseur, A.; Forestier, C. Heavy metal toxicity: Cadmium permeates through calcium channels and disturbs the plant water status. Plant J. 2002, 32, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Martinoia, E.; Lee, Y. Vacuolar Transporters for Cadmium and Arsenic in Plants and their Applications in Phytoremediation and Crop Development. Plant Cell Physiol. 2018, 59, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Kumazaki, K.; Wada, M.; Taniguchi, R.; Nakane, T.; Yamashita, K.; Hirata, K.; Ishitani, R.; Ito, K.; Nishizawa, T.; et al. Crystal structure of plant vacuolar iron transporter VIT1. Nat. Plants 2019, 5, 308–315. [Google Scholar] [CrossRef]
- Brembu, T.; Jørstad, M.; Winge, P.; Valle, K.C.; Bones, A.M. Genome-wide profiling of responses to cadmium in the diatom Phaeodactylum tricornutum. Environ. Sci. Technol. 2011, 45, 7640–7647. [Google Scholar] [CrossRef]
- Bellini, E.; Maresca, V.; Betti, C.; Castiglione, M.R.; Fontanini, D.; Capocchi, A.; Sorce, C.; Borsò, M.; Bruno, L.; Sorbo, S.; et al. The Moss Leptodictyum riparium Counteracts Severe Cadmium Stress by Activation of Glutathione Transferase and Phytochelatin Synthase, but Slightly by Phytochelatins. Int. J. Mol. Sci. 2020, 21, 1583. [Google Scholar] [CrossRef]
- Xu, H.; Yu, C.; Xia, X.; Li, M.; Li, H.; Wang, Y.; Wang, S.; Wang, C.; Ma, Y.; Zhou, G. Comparative transcriptome analysis of duckweed (Landoltia punctata) in response to cadmium provides insights into molecular mechanisms underlying hyperaccumulation. Chemosphere 2018, 190, 154–165. [Google Scholar] [CrossRef]
- Jian, S.; Luo, J.; Liao, Q.; Liu, Q.; Guan, C.; Zhang, Z. NRT1.1 Regulates Nitrate Allocation and Cadmium Tolerance in Arabidopsis. Front. Plant Sci. 2019, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Fu, Y.L.; Pike, S.M.; Bao, J.; Tian, W.; Zhang, Y.; Chen, C.Z.; Zhang, Y.; Li, H.M.; Huang, J.; et al. The Arabidopsis nitrate transporter NRT1.8 functions in nitrate removal from the xylem sap and mediates cadmium tolerance. Plant Cell 2010, 22, 1633–1646. [Google Scholar] [CrossRef]
- Chen, C.Z.; Lv, X.F.; Li, J.Y.; Yi, H.Y.; Gong, J.M. Arabidopsis NRT1.5 is another essential component in the regulation of nitrate reallocation and stress tolerance. Plant Physiol. 2012, 159, 1582–1590. [Google Scholar] [CrossRef]
- Shi, H.; Liu, W.; Yao, Y.; Wei, Y.; Chan, Z. Alcohol dehydrogenase 1 (ADH1) confers both abiotic and biotic stress resistance in Arabidopsis. Plant Sci. 2017, 262, 24–31. [Google Scholar] [CrossRef]
- Arena, C.; Figlioli, F.; Sorrentino, M.C.; Izzo, L.G.; Capozzi, F.; Giordano, S.; Spagnuolo, V. Ultrastructural, protein and photosynthetic alterations induced by Pb and Cd in Cynara cardunculus L., and its potential for phytoremediation. Ecotoxicol. Environ. Saf. 2017, 145, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wei, Y.; Li, N.; Zeng, J.; Han, Y.; Zuo, Z.; Wang, S.; Zhu, Y.; Zhang, Y.; Sun, J.; et al. Declined cadmium accumulation in Na+/H+ antiporter (NHX1) transgenic duckweed under cadmium stress. Ecotoxicol. Environ. 2019, 182, 109397. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Han, H.; Liu, M.; Zuo, Z.; Zhou, K.; Lü, J.; Zhu, Y.; Bai, Y.; Wang, Y. Overexpression of the Arabidopsis photorespiratory pathway gene, serine: Glyoxylate aminotransferase (AtAGT1), leads to salt stress tolerance in transgenic duckweed (Lemna minor). Plant Cell Tissue Organ Cult. 2013, 113, 407–416. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Shi, L.; Yu, J.; Yao, J.; Sun, J.; Zhao, L.; Sun, J. Enhanced Cd accumulation by Graphene oxide (GO) under Cd stress in duckweed. Aquat. Toxicol. 2020, 229, 105579. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Han, Y.; Wu, D.; Yong, W.; Liu, M.; Wang, S.; Liu, W.; Lu, M.; Wei, Y.; Sun, J. Salt and cadmium stress tolerance caused by overexpression of the Glycine Max Na+/H+ Antiporter (GmNHX1) gene in duckweed (Lemna turionifera 5511). Aquat. Toxicol. 2017, 192, 127–135. [Google Scholar] [CrossRef]
- Du, P.; Yin, B.; Cao, Y.; Han, R.; Ji, J.; He, X.; Liang, B.; Xu, J. Beneficial Effects of Exogenous Melatonin and Dopamine on Low Nitrate Stress in Malus hupehensis. Front. Plant Sci. 2022, 12, 807472. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, W.; Xu, S.; Shi, H.; Wen, D.; Huang, Y.; Peng, L.; Deng, T.; Du, R.; Li, F.; et al. Increasing ammonium nutrition as a strategy for inhibition of cadmium uptake and xylem transport in rice (Oryza sativa L.) exposed to cadmium stress. Environ. Exp. Bot. 2018, 155, 734–741. [Google Scholar] [CrossRef]
- Huang, S.; Xin, S.; Xie, G.; Han, J.; Liu, Z.; Wang, B.; Zhang, S.; Wu, Q.; Cheng, X. Mutagenesis reveals that the rice OsMPT3 gene is an important osmotic regulatory factor. Crop J. 2020, 8, 465–479. [Google Scholar] [CrossRef]





| Description | GeneID | DA_Cd_ Read Count | Cd_ Read Count | log2FoldChange | pval | padj |
|---|---|---|---|---|---|---|
| Photosystem II 10kDa protein (psbR) | Cluster-842.10293 | 2091.08 | 797.41 | 1.39 | 9.20 × 10−7 | 1.48 × 10−4 |
| Photosystem II 22kDa protein (psbS) | Cluster-842.9755 | 6179.71 | 2787.42 | 1.15 | 7.87 × 10−12 | 7.21 × 10−9 |
| Photosystem I subunit IV (psaE) | Cluster-842.8276 | 3329.22 | 1550.97 | 1.10 | 3.25 × 10−6 | 4.13 × 10−4 |
| Photosystem I subunit (PsaO) | Cluster-842.10000 | 5863.39 | 2741.13 | 1.10 | 3.44 × 10−8 | 9.32 × 10−6 |
| Light-harvesting complex I chlorophyll a/b binding protein 3 (LHCA3) | Cluster-842.7185 | 2683.81 | 1203.95 | 1.16 | 2.38 × 10−8 | 6.65 × 10−6 |
| Light-harvesting complex II chlorophyll a/b binding protein 1 (LHCB1) | Cluster-842.7154 | 8094.49 | 2867.23 | 1.50 | 3.09 × 10−11 | 2.23 × 10−8 |
| Light-harvesting complex II chlorophyll a/b binding protein 6 (LHCB6) | Cluster-842.3135 | 455.67 | 191.30 | 1.25 | 1.35 × 10−4 | 7.88 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Yang, Y.; Ma, X.; He, Y.; Ren, Q.; Huang, Y.; Wang, J.; Xue, Y.; Yang, R.; Guo, Y.; et al. New Insight into the Function of Dopamine (DA) during Cd Stress in Duckweed (Lemna turionifera 5511). Plants 2023, 12, 1996. https://doi.org/10.3390/plants12101996
Wang W, Yang Y, Ma X, He Y, Ren Q, Huang Y, Wang J, Xue Y, Yang R, Guo Y, et al. New Insight into the Function of Dopamine (DA) during Cd Stress in Duckweed (Lemna turionifera 5511). Plants. 2023; 12(10):1996. https://doi.org/10.3390/plants12101996
Chicago/Turabian StyleWang, Wenqiao, Yunwen Yang, Xu Ma, Yuman He, Qiuting Ren, Yandi Huang, Jing Wang, Ying Xue, Rui Yang, Yuhan Guo, and et al. 2023. "New Insight into the Function of Dopamine (DA) during Cd Stress in Duckweed (Lemna turionifera 5511)" Plants 12, no. 10: 1996. https://doi.org/10.3390/plants12101996
APA StyleWang, W., Yang, Y., Ma, X., He, Y., Ren, Q., Huang, Y., Wang, J., Xue, Y., Yang, R., Guo, Y., Sun, J., Yang, L., & Sun, Z. (2023). New Insight into the Function of Dopamine (DA) during Cd Stress in Duckweed (Lemna turionifera 5511). Plants, 12(10), 1996. https://doi.org/10.3390/plants12101996





