Development of a Pressurized Green Liquid Extraction Procedure to Recover Antioxidant Bioactive Compounds from Strawberry Tree Fruit (Arbutus unedo L.)
Abstract
:1. Introduction
2. Results and Discussion
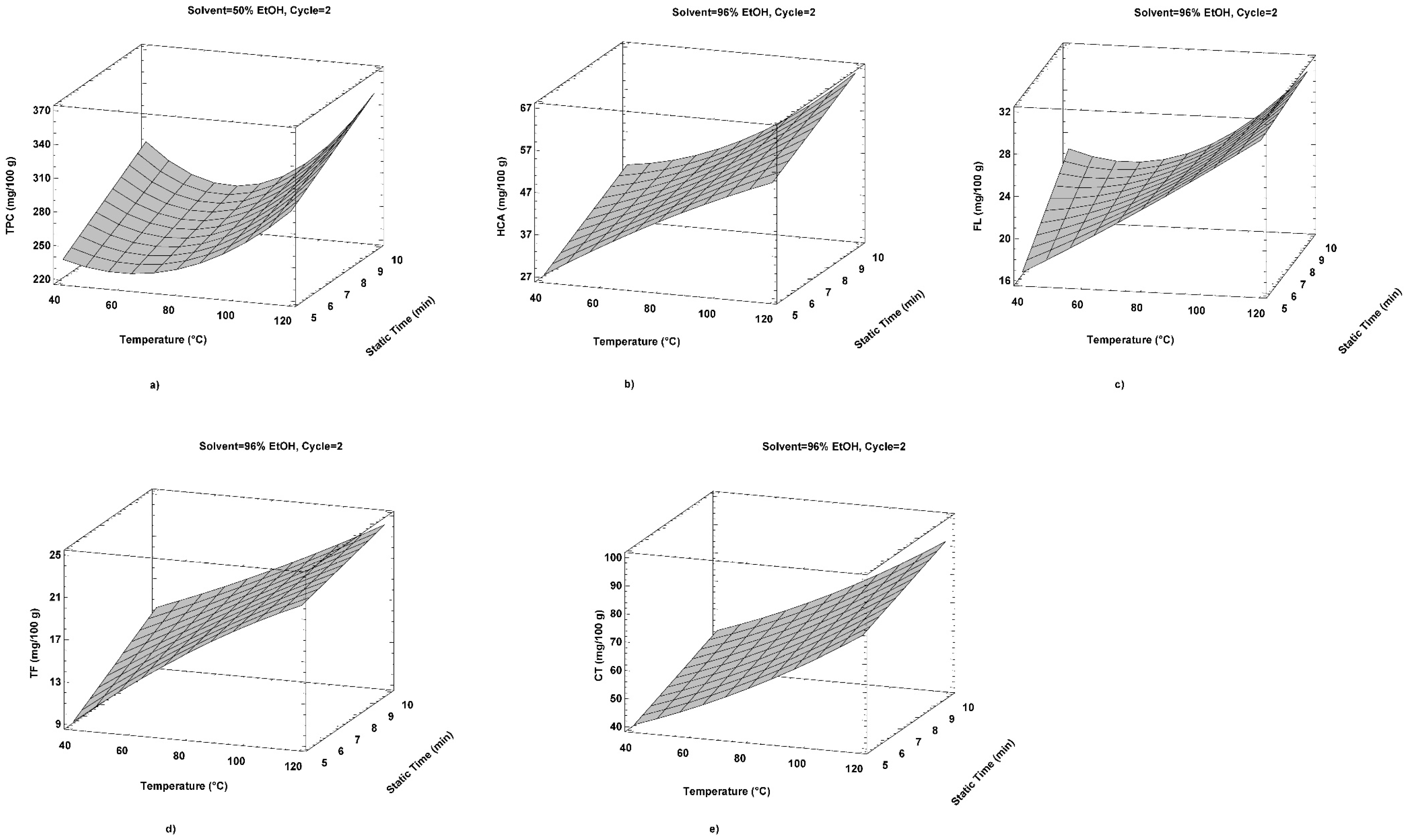
2.1. The Influence of PLE Extraction Parameters on Polyphenolic Compound Content and Antioxidant Potential
2.2. Correlation of Temperature with Polyphenolic Compounds and Antioxidant Capacity
2.3. Optimization of PLE Processing Parameters for Antioxidant Bioactive Compounds Extraction
3. Materials and Methods
3.1. Chemical and Standards
3.2. Plant Material
Pressurized Liquid Extraction (PLE)
3.3. Determination of Total Phenolic Content (TPC)
3.4. Determination of Total Flavonoids Content (TF)
3.5. Determination of Total Hydroxycinnamic Acids (HCA) and Total Flavonols Content (FL)
3.6. Determination of Condensed Tannins (CT)
3.7. Determination of In Vitro Antioxidant Capacity
3.7.1. 2,2-Diphenyl-1-picrylhydrazyl Method (DPPH)
3.7.2. Ferric Reducing Antioxidant Power Method (FRAP)
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bebek Markovinović, A.; Brčić Karačonji, I.; Jurica, K.; Lasić, D.; Skendrović Babojelić, M.; Duralija, B.; Šic Žlabur, J.; Putnik, P.; Bursać Kovačević, D. Strawberry tree fruits and leaves (Arbutus unedo L.) as raw material for sustainable functional food processing: A Review. Horticulturae 2022, 8, 881. [Google Scholar] [CrossRef]
- Dakic, D.; Sladojevic, S.; Lolic, T.; Stefanovic, D. Process Mining Possibilities and Challenges: A Case Study. In Proceedings of the SISY 2019—IEEE 17th International Symposium on Intelligent Systems and Informatics, Subotica, Serbia, 14–19 September 2019; pp. 161–166. [Google Scholar]
- Jurica, K. Phenolic Compounds in Strawberry Tree (Arbutus unedo L.) and Their Biological Effects; Department of Biology, Faculty of Science, University of Zagreb: Zagreb, Croatia, 2016. [Google Scholar]
- Morales, D. Use of strawberry tree (Arbutus unedo) as a source of functional fractions with biological activities. Foods 2022, 11, 3838. [Google Scholar] [CrossRef]
- El Haouari, M.; Assem, N.; Changan, S.; Kumar, M.; Daştan, S.D.; Rajkovic, J.; Taheri, Y.; Sharifi-Rad, J.; Kabra, A. An insight into phytochemical, pharmacological, and nutritional properties of Arbutus unedo L. from Morocco. Evid. Based Complement. Altern. Med. 2021, 2021, 1794621. [Google Scholar] [CrossRef] [PubMed]
- Jurica, K.; Brčić Karačonji, I.; Tariba, B.; Živković, T.; Brajenović, N.; Pizent, A. A multielement profile of Croatian strawberry tree (Arbutus unedo L.) fruit and leaves. In Proceedings of the ISTERH 2015 Conference “Recent Advances in Trace Element Research in Health and Disease”, Dubrovnik, Croatia, 18–22 October 2015. [Google Scholar]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional foods: Product development, technological trends, efficacy testing, and safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [PubMed]
- Pavlić, B.; Šojić, B.; Teslić, N.; Putnik, P.; Bursać Kovačević, D. Extraction of bioactive compounds and essential oils from herbs using green technologies. In Aromatic Herbs in Food; Galanakis, C.M., Ed.; Academic Press: Cambridge, UK, 2021; pp. 233–262. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- López, C.J.; Caleja, C.; Prieto, M.A.; Sokovic, M.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Stability of a cyanidin-3-O-glucoside extract obtained from Arbutus unedo L. and incorporation into wafers for colouring purposes. Food Chem. 2019, 275, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.; Rodrigues, S.; Mendes, S.; Maranhão, P.; Ganhão, R. Impact of aqueous extract of Arbutus unedo fruits on limpets (Patella spp.) pâté during storage: Proximate composition, physicochemical quality, oxidative stability, and microbial development. Foods 2020, 9, 807. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B. An overview of the recent trends on the waste valorization techniques for food wastes. J. Environ. Manag. 2019, 233, 352–370. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A knowledge base for the recovery of natural phenols with different solvents. Int. J. Food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef]
- Bursać Kovačević, D.; Barba, F.J.; Granato, D.; Galanakis, C.M.; Herceg, Z.; Dragović-Uzelac, V.; Putnik, P. Pressurized hot water extraction (PHWE) for the green recovery of bioactive compounds and steviol glycosides from Stevia rebaudiana Bertoni leaves. Food Chem. 2018, 254, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Barba, F.J.; Španić, I.; Zorić, Z.; Dragović-Uzelac, V.; Bursać Kovačević, D. Green extraction approach for the recovery of polyphenols from Croatian olive leaves (Olea europea). Food Bioprod. Process. 2017, 106, 19–28. [Google Scholar] [CrossRef]
- García, M.C.; Lombardo-Cristina, V.; Marina, M.L. Multifunctional and Collaborative Protection of Proteins, Peptides, Phenolic Compounds, and Other Molecules against Oxidation in Apricot Seeds Extracts. Antioxidants 2022, 11, 2354. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A.; Ibañez, E. Pressurized Liquid Extraction. In Liquid-Phase Extraction; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–398. [Google Scholar] [CrossRef]
- Izcara, S.; Morante-Zarcero, S.; Casado, N.; Sierra, I. Study of the phenolic compound profile of Arbutus unedo L. fruits at different ripening stages by HPLC-TQ-MS/MS. Appl. Sci. 2021, 11, 11616. [Google Scholar] [CrossRef]
- Pallauf, K.; Rivas-Gonzalo, J.C.; del Castillo, M.D.; Cano, M.P.; de Pascual-Teresa, S. Characterization of the antioxidant composition of strawberry tree (Arbutus unedo L.) fruits. J. Food Compos. Anal. 2008, 21, 273–281. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Gliszczyńska-Świgło, A. Binary ethanol–water solvents affect phenolic profile and antioxidant capacity of flaxseed extracts. Eur. Food Res. Technol. 2015, 242, 777–786. [Google Scholar] [CrossRef]
- Scarano, P.; Guida, R.; Zuzolo, D.; Tartaglia, M.; Prigioniero, A.; Postiglione, A.; Pinto, G.; Illiano, A.; Amoresano, A.; Schicchi, R.; et al. An endemic plant of the mediterranean area: Phytochemical characterization of strawberry tree (Arbutus unedo L.) fruits extracts at different ripening stages. Front. Nutr. 2022, 9, 915994. [Google Scholar] [CrossRef]
- Jacotet-Navarro, M.; Laguerre, M.; Fabiano-Tixier, A.-S.; Tenon, M.; Feuillère, N.; Bily, A.; Chemat, F. What is the best ethanol-water ratio for the extraction of antioxidants from rosemary? Impact of the solvent on yield, composition, and activity of the extracts. Electrophoresis 2018, 39, 1946–1956. [Google Scholar] [CrossRef]
- Carabias-Martínez, R.; Rodríguez-Gonzalo, E.; Revilla-Ruiz, P.; Hernández-Méndez, J. Pressurized liquid extraction in the analysis of food and biological samples. J. Chromatogr. A 2005, 1089, 1–17. [Google Scholar] [CrossRef]
- Putnik, P. Influence of acidity and extraction time on the recovery of flavonoids from grape skin pomace optimized by response surface methodology. Chem. Biochem. Eng. Q. J. 2017, 30, 455–464. [Google Scholar] [CrossRef]
- Putnik, P.; Bursać Kovačević, D.; Dragović-Uzelac, V. Optimizing acidity and extraction time for polyphenolic recovery and antioxidant capacity in grape pomace skin extracts with response surface methodology approach. J. Food Process. Preserv. 2016, 40, 1256–1263. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef] [PubMed]
- Šic Žlabur, J.; Voća, S.; Dobričević, N.; Pliestić, S.; Galić, A.; Boričević, A.; Borić, N. Ultrasound-assisted extraction of bioactive compounds from lemon balm and peppermint leaves. Int. Agrophysics 2016, 30, 95–104. [Google Scholar] [CrossRef]
- Dimitrova, L.; Petrova, M.; Geneva, M.; Stancheva, I.R.A.; Zayova, E.L.Y. Antioxidant activity of in vitro propagated Stevia rebaudiana Bertoni plants of different origins. Turk. J. Biol. 2013, 37, 106–113. [Google Scholar] [CrossRef]
- Yang, J.; Ou, X.; Zhang, X.; Zhou, Z.; Ma, L. Effect of different solvents on the measurement of phenolics and the antioxidant activity of mulberry (Morus atropurpurea Roxb.) with accelerated solvent extraction. J. Food Sci. 2017, 82, 605–612. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Pollini, L.; Cossignani, L.; Juan, C.; Mañes, J. Extraction of phenolic compounds from fresh apple pomace by different non-conventional techniques. Molecules 2021, 26, 4272. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Martín-Álvarez, P.J.; Señoráns, F.J.; Cifuentes, A.; Ibáñez, E. Optimization of accelerated solvent extraction of antioxidants from Spirulina platensis microalga. Food Chem. 2005, 93, 417–423. [Google Scholar] [CrossRef]
- Rajha, H.N.; Louka, N.; Darra, N.E.; Hobaika, Z.; Boussetta, N.; Vorobiev, E.; Maroun, R.G. Multiple response optimization of high temperature, low time aqueous extraction process of phenolic compounds from grape byproducts. Food Nutr. Sci. 2014, 5, 351–360. [Google Scholar] [CrossRef]
- Yuan, B.; Danao, M.-G.C.; Stratton, J.E.; Weier, S.A.; Weller, C.L.; Lu, M. High pressure processing (HPP) of aronia berry purée: Effects on physicochemical properties, microbial counts, bioactive compounds, and antioxidant capacities. Innov. Food Sci. Emerg. Technol. 2018, 47, 249–255. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2020, 10, 3. [Google Scholar] [CrossRef]
- Howard, L.R.; Clark, J.R.; Brownmiller, C. Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. J. Sci. Food Agric. 2003, 83, 1238–1247. [Google Scholar] [CrossRef]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Shortle, E.; O’Grady, M.N.; Gilroy, D.; Furey, A.; Quinn, N.; Kerry, J.P. Influence of extraction technique on the anti-oxidative potential of hawthorn (Crataegus monogyna) extracts in bovine muscle homogenates. Meat Sci. 2014, 98, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F. An automated, specific, spectrophotometric method for measuring ascorbic acid in plasma (EFTSA). Clin. Biochem. 1996, 29, 111–116. [Google Scholar] [CrossRef]
| PLE Variables | n | TPC 1 | HCA 2 | FL 3 | TF 4 | CT 5 | DPPH 6 | FRAP 7 |
|---|---|---|---|---|---|---|---|---|
| Solvent Type | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | |
| Distilled water | 24 | 58.95 ± 2.49 c | 4.05 ± 0.41 c | 2.47 ± 0.32 c | 5.86 ± 0.08 c | 14.83 ± 0.40 c | 224.07 ± 0.07 b | 798.01 ± 11.35 b |
| 50% EtOH | 24 | 253.83 ± 2.49 a | 26.2 ± 0.41 b | 10.02 ± 0.32 b | 9.77 ± 0.08 b | 55.77 ± 0.40 b | 220.93 ± 0.07 c | 1221.1 ± 11.35 c |
| 96% EtOH | 24 | 237.01 ± 2.49 b | 44.18 ± 0.41 a | 23.70 ± 0.32 a | 16.78 ± 0.08 a | 62.68 ± 0.40 a | 225.82 ± 0.07 a | 962.93 ± 11.35 a |
| Temperature | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | |
| 40 °C | 24 | 161.24 ± 2.49 c | 16.25 ± 0.41 c | 7.43 ± 0.32 c | 7.75 ± 0.08 c | 31.27 ± 0.40 c | 226.23 ± 0.07 a | 774.28 ± 11.35 c |
| 80 °C | 24 | 179.44 ± 2.49 b | 22.15 ± 0.41 b | 10.26 ± 0.32 b | 10.24 ± 0.08 b | 44.13 ± 0.40 b | 224.29 ± 0.07 b | 913.95 ± 11.35 b |
| 120 °C | 24 | 209.11 ± 2.49 a | 36.03 ± 0.41 a | 18.50 ± 0.32 a | 14.41 ± 0.08 a | 57.88 ± 0.40 a | 220.31 ± 0.07 c | 1293.9 ± 11.35 a |
| Static Extraction Time | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | |
| 5 min | 36 | 173.79 ± 2.04 b | 22.61 ± 0.34 b | 10.67 ± 0.26 b | 9.94 ± 0.06 b | 41.18 ± 0.33 b | 224.64 ± 0.05 a | 944.63 ± 9.27 b |
| 10 min | 36 | 192.73 ± 2.04 a | 27.01 ± 0.34 a | 13.45 ± 0.26 a | 11.67 ± 0.06 a | 47.68 ± 0.33 a | 222.58 ± 0.05 b | 1043.4 ± 9.27 a |
| Number of Cycles | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p = 0.23 ‡ | p ≤ 0.01 † | |
| 1 | 36 | 169.88 ± 2.04 b | 22.78 ± 0.34 b | 11.36 ± 0.26 b | 9.99 ± 0.06 b | 41.39 ± 0.33 b | 223.56 ± 0.05 a | 862.89 ± 9.27 b |
| 2 | 36 | 196.65 ± 2.04 a | 26.85 ± 0.34 a | 12.76 ± 0.26 a | 11.62 ± 0.06 a | 47.46 ± 0.33 a | 223.66 ± 0.05 a | 1125.2 ± 9.27 a |
| Dataset average | 72 | 183.26 ± 1.44 | 24.81 ± 0.24 | 12.06 ± 0.18 | 10.80 ± 0.05 | 44.23 ± 0.40 | 223.61 ± 0.04 | 994.03 ± 6.56 |
| T | TPC | HCA | FL | TF | CT | DPPH | FRAP | |
|---|---|---|---|---|---|---|---|---|
| T | 1.00 | 0.21 | 0.40 * | 0.40 * | 0.47 * | 0.43 * | −0.58 * | 0.62 * |
| TPC | 1.00 | 0.80 * | 0.67 * | 0.71 * | 0.92 * | −0.27 * | 0.64 * | |
| HCA | 1.00 | 0.95 * | 0.95 * | 0.91 * | −0.19 | 0.48 * | ||
| FL | 1.00 | 0.93 * | 0.80 * | −0.18 | 0.35 * | |||
| TF | 1.00 | 0.85 * | −0.12 | 0.46 * | ||||
| CT | 1.00 | −0.28 * | 0.66 * | |||||
| DPPH | 1.00 | −0.57 * | ||||||
| FRAP | 1.00 |
| PLE Variables | TPC 1 | HCA 2 | FL 3 | TF 4 | CT 5 |
|---|---|---|---|---|---|
| Extraction Solvent | 50% EtOH | 96% EtOH | 96% EtOH | 96% EtOH | 96% EtOH |
| Temperature (°C) | 120 | 120 | 120 | 120 | 120 |
| Static Extraction Time (min) | 10 | 10 | 10 | 10 | 10 |
| Number of Cycles | 2 | 2 | 2 | 2 | 2 |
| Predicted values (mg 100 g−1) | 351.65 | 66.36 | 31.06 | 24.29 | 92.24 |
| Run | Solvent Type | Temperature (°C) | Static Extraction Time (min) | Number of Cycles |
|---|---|---|---|---|
| 1 | Distilled water | 40 | 5 | 1 |
| 2 | Distilled water | 40 | 5 | 2 |
| 3 | Distilled water | 40 | 10 | 1 |
| 4 | Distilled water | 40 | 10 | 2 |
| 5 | Distilled water | 80 | 5 | 1 |
| 6 | Distilled water | 80 | 5 | 2 |
| 7 | Distilled water | 80 | 10 | 1 |
| 8 | Distilled water | 80 | 10 | 2 |
| 9 | Distilled water | 120 | 5 | 1 |
| 10 | Distilled water | 120 | 5 | 2 |
| 11 | Distilled water | 120 | 10 | 1 |
| 12 | Distilled water | 120 | 10 | 2 |
| 13 | 50% EtOH 1 | 40 | 5 | 1 |
| 14 | 50% EtOH | 40 | 5 | 2 |
| 15 | 50% EtOH | 40 | 10 | 1 |
| 16 | 50% EtOH | 40 | 10 | 2 |
| 17 | 50% EtOH | 80 | 5 | 1 |
| 18 | 50% EtOH | 80 | 5 | 2 |
| 19 | 50% EtOH | 80 | 10 | 1 |
| 20 | 50% EtOH | 80 | 10 | 2 |
| 21 | 50% EtOH | 120 | 5 | 1 |
| 22 | 50% EtOH | 120 | 5 | 2 |
| 23 | 50% EtOH | 120 | 10 | 1 |
| 24 | 50% EtOH | 120 | 10 | 2 |
| 25 | 96% EtOH 2 | 40 | 5 | 1 |
| 26 | 96% EtOH | 40 | 5 | 2 |
| 27 | 96% EtOH | 40 | 10 | 1 |
| 28 | 96% EtOH | 40 | 10 | 2 |
| 29 | 96% EtOH | 80 | 5 | 1 |
| 30 | 96% EtOH | 80 | 5 | 2 |
| 31 | 96% EtOH | 80 | 10 | 1 |
| 32 | 96% EtOH | 80 | 10 | 2 |
| 33 | 96% EtOH | 120 | 5 | 1 |
| 34 | 96% EtOH | 120 | 5 | 2 |
| 35 | 96% EtOH | 120 | 10 | 1 |
| 36 | 96% EtOH | 120 | 10 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bebek Markovinović, A.; Milošević, S.; Teslić, N.; Pavlić, B.; Putnik, P.; Brčić Karačonji, I.; Jurica, K.; Lasić, D.; Bursać Kovačević, D. Development of a Pressurized Green Liquid Extraction Procedure to Recover Antioxidant Bioactive Compounds from Strawberry Tree Fruit (Arbutus unedo L.). Plants 2023, 12, 2006. https://doi.org/10.3390/plants12102006
Bebek Markovinović A, Milošević S, Teslić N, Pavlić B, Putnik P, Brčić Karačonji I, Jurica K, Lasić D, Bursać Kovačević D. Development of a Pressurized Green Liquid Extraction Procedure to Recover Antioxidant Bioactive Compounds from Strawberry Tree Fruit (Arbutus unedo L.). Plants. 2023; 12(10):2006. https://doi.org/10.3390/plants12102006
Chicago/Turabian StyleBebek Markovinović, Anica, Sanja Milošević, Nemanja Teslić, Branimir Pavlić, Predrag Putnik, Irena Brčić Karačonji, Karlo Jurica, Dario Lasić, and Danijela Bursać Kovačević. 2023. "Development of a Pressurized Green Liquid Extraction Procedure to Recover Antioxidant Bioactive Compounds from Strawberry Tree Fruit (Arbutus unedo L.)" Plants 12, no. 10: 2006. https://doi.org/10.3390/plants12102006
APA StyleBebek Markovinović, A., Milošević, S., Teslić, N., Pavlić, B., Putnik, P., Brčić Karačonji, I., Jurica, K., Lasić, D., & Bursać Kovačević, D. (2023). Development of a Pressurized Green Liquid Extraction Procedure to Recover Antioxidant Bioactive Compounds from Strawberry Tree Fruit (Arbutus unedo L.). Plants, 12(10), 2006. https://doi.org/10.3390/plants12102006