Soybean Yield and Nutrition Grown on the Straw of Grain Sorghum Inoculated with Azospirillum brasilense and Intercropped with BRS Paiaguás Grass
Abstract
1. Introduction
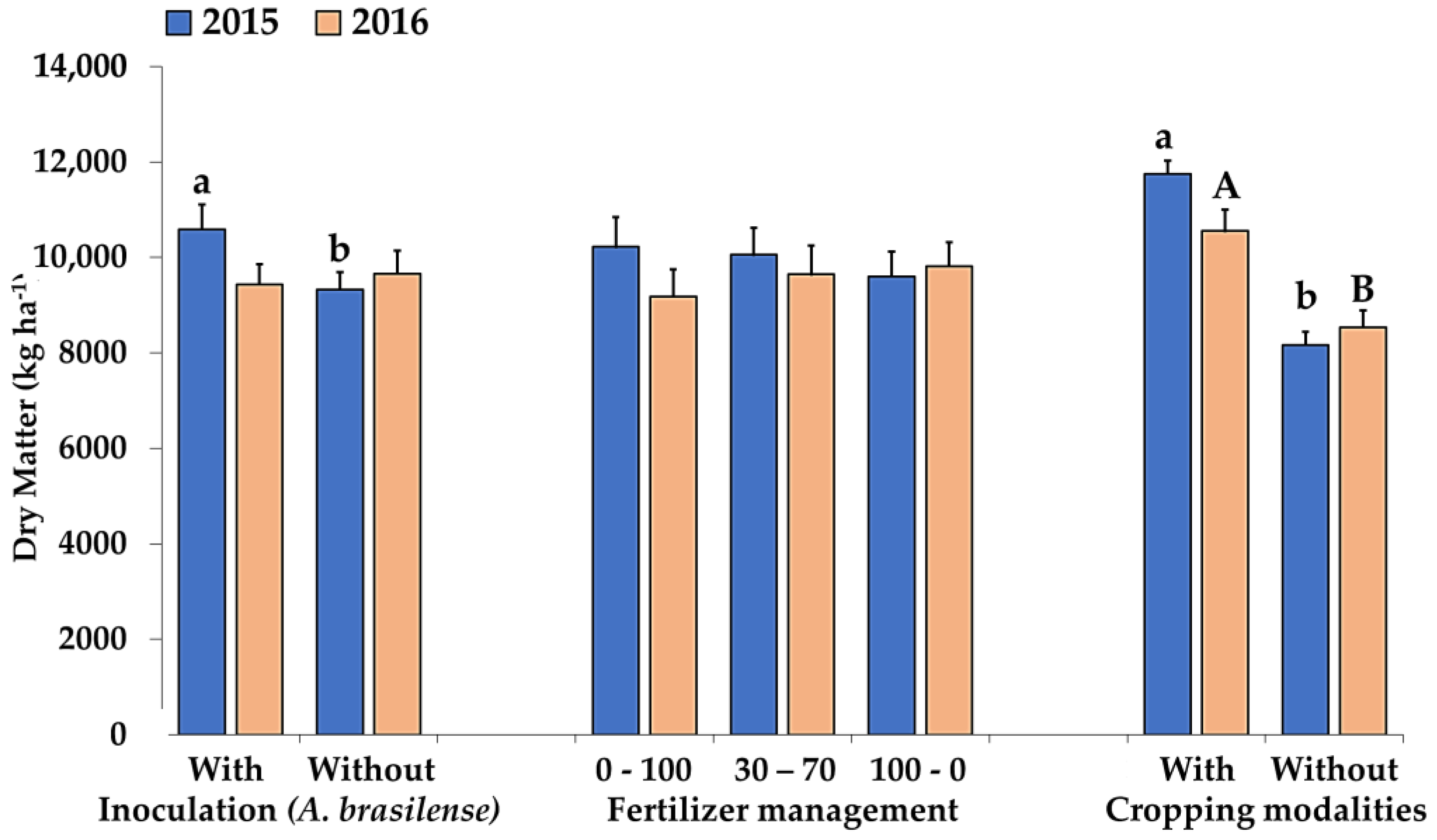
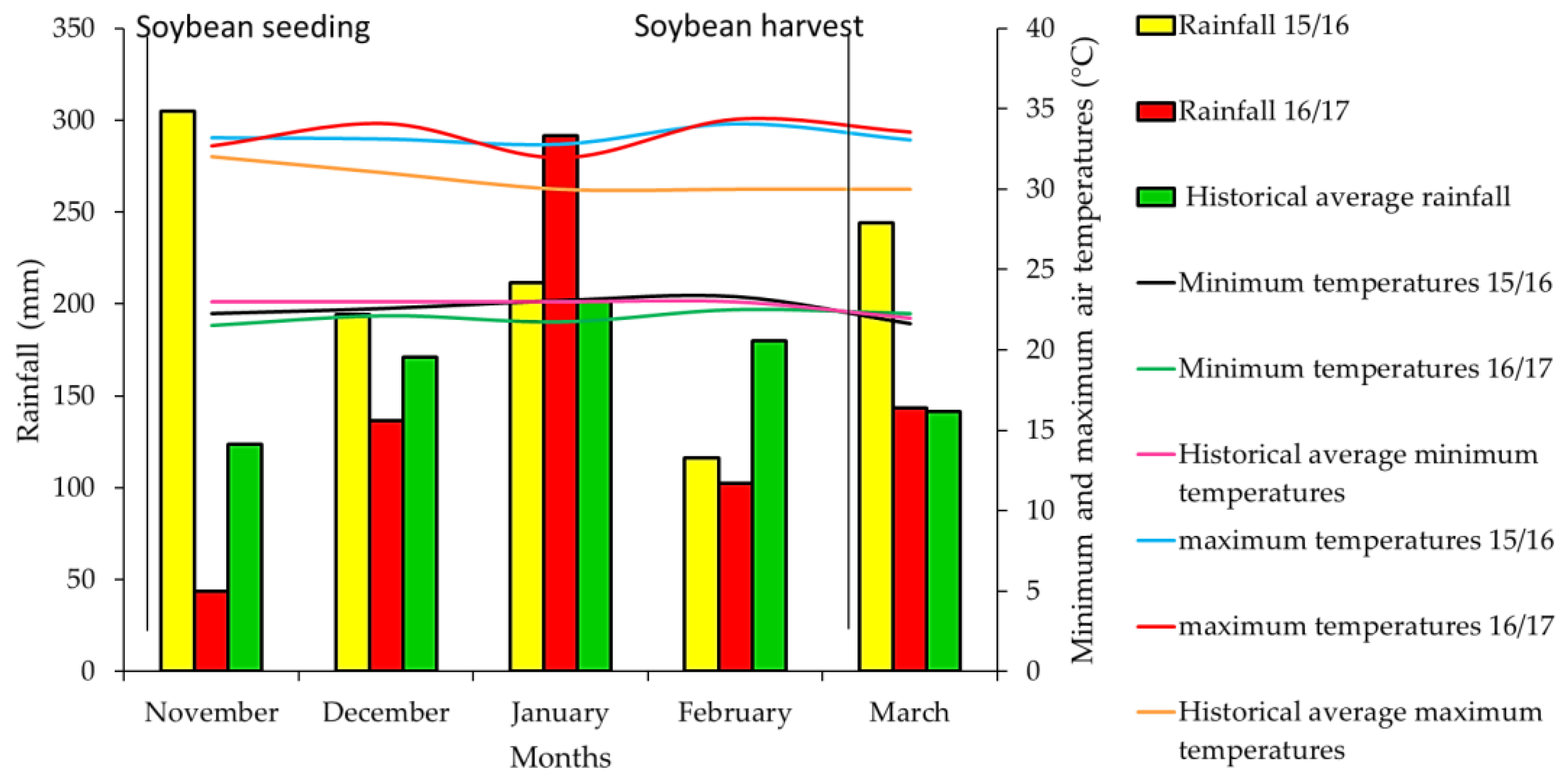
2. Results and Discussion
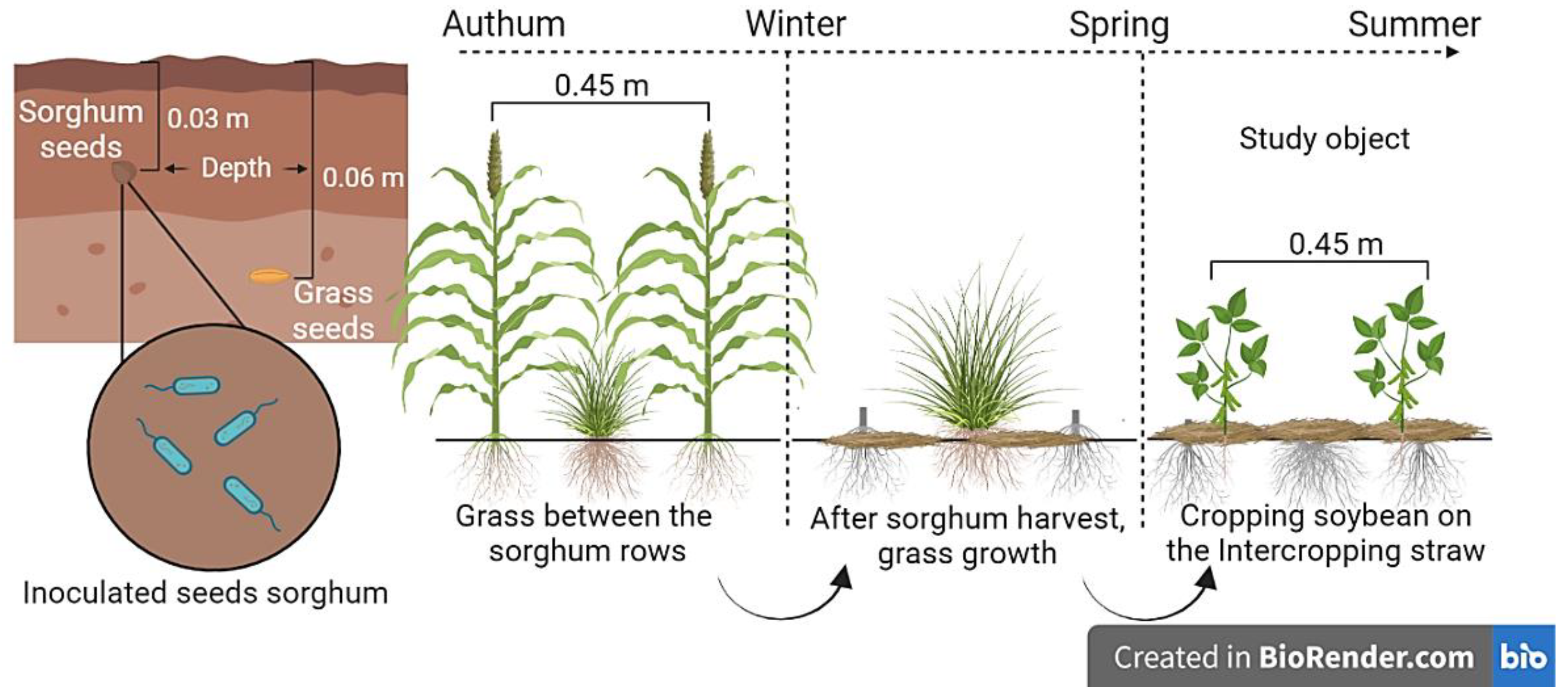
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. Day of 8 Billion. 2022. Available online: https://www.un.org/en/dayof8billion (accessed on 17 November 2022).
- Food and Agriculture Organization of the United Nations. Save and Grow—A Policymaker’s Guide to the Sustainable Intensification of Smallholder Crop Production; FAO: Roma, Italy, 2011; p. 102. [Google Scholar]
- Food and Agriculture Organization of the United Nations OECD-FAO. Agricultural Outlook 2020–2029. 2020. Available online: https://www.oecd-ilibrary.org/agriculture-and-food/oecd-fao-agricultural-outlook-2020-2029_1112c23b-en (accessed on 17 November 2022).
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveirra, V.Á.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araújo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa Solos: Brasília, Brasil, 2018; 355p. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; de Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Borghi, E.; Crusciol, C.A.C.; Nascente, A.S.; Sousa, V.V.; Martins, P.O.; Mateus, G.P.; Costa, C. Sorghum Grain Yield, Forage Biomass Production and Revenue as Affected by Intercropping Time. Eur. J. Agron. 2013, 51, 130–139. [Google Scholar] [CrossRef]
- Mateus, G.P.; Crusciol, C.A.C.; Pariz, C.M.; Borghi, E.; Costa, C.; Martello, J.M.; Franzluebbers, A.J.; Castilhos, A.M. Sidedress Nitrogen Application Rates to Sorghum Intercropped with Tropical Perennial Grasses. Agron. J. 2016, 108, 433–447. [Google Scholar] [CrossRef]
- Pingali, P.L. Green Revolution: Impacts, Limits, And the Path Ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef]
- Rada, N. Assessing Brazil’s Cerrado Agricultural Miracle. Food Policy 2013, 38, 146–155. [Google Scholar] [CrossRef]
- Andreotti, M.; Araldi, M.; Guimarães, V.F.; Furlani, E.; Buzetti, S. Produtividade do Milho Safrinha e Modificações Químicas de um Latossolo em Sistema Plantio Direto em Função de Espécies de Cobertura Após Calagem Superficial. Acta Sci. Agron. 2008, 30, 109–115. [Google Scholar] [CrossRef]
- Costa, N.R.; Andreotti, M.; Crusciol, C.A.C.; Pariz, C.M.; Bossolani, J.W.; Pascoaloto, I.M.; da Rocha Lima, C.G.; dos Santos Batista Bonini, C.; de Castilhos, A.M.; Calonego, J.C. Soybean Yield and Nutrition after Tropical Forage Grasses. Nutr. Cycl. Agroecosystems 2021, 121, 31–49. [Google Scholar] [CrossRef]
- Ceccon, G.; Borghi, E.; Crusciol, C.A.C. Modalidades e Métodos de Implantação do Consórcio Milho-Braquiária. In Consórcio Milho-Braquiária; Ceccon, G., Ed.; Embrapa: Brasília, Brazil, 2013; pp. 25–46. [Google Scholar]
- Maia, G.A.; de Pinho Costa, K.A.; da Costa Severiano, E.; Epifanio, P.S.; Neto, J.F.; Ribeiro, M.G.; Fernandes, P.B.; Guimarães Silva, J.F.; Gonçalves, W.G. Yield and Chemical Composition of Brachiaria Forage Grasses in the Offseason after Corn Harvest. Am. J. Plant Sci. 2014, 5, 933–941. [Google Scholar] [CrossRef]
- Galdos, M.V.; Brown, E.; Rosolem, C.A.; Pires, L.F.; Hallett, P.D.; Mooney, S.J. Brachiaria Species Influence Nitrate Transport in Soil by Modifying Soil Structure with Their Root System. Sci. Rep. 2020, 10, 5072. [Google Scholar] [CrossRef]
- Magalhães, P.C.; Souza, T.C.; May, A.; Lima Filho, D.F.; Moreira, J.A.A.; Leite, C.E.P.; Albuquerque, C.J.B.; Freitas, R.S. Exigências Edafoclimáticas e Fisiologia da Produçã. In Sorgo: Do Plantio à Colheita, 1st ed.; Borém, A., Pimentel, L., Pereira, R., Eds.; UFV: Viçosa, Brazil, 2014; pp. 58–88. [Google Scholar]
- Magalhães, P.C.; de Souza, T.C.; Schaffert, R.E. Ecofisiologia. Cultivo do Sorgo; Embrapa Milho e Sorgo: Brasília, Brazil, 2015. [Google Scholar]
- Bogiani, J.C.; Ferreira, A.C.B. Plantas de Cobertura no Sistema Soja-Milho-Algodão no Cerrado; Informações Agronômicas; International Plant Nutrition Institute: Piracicaba, Brazil, 2017. [Google Scholar]
- Hadebe, S.T.; Modi, A.T.; Mabhaudhi, T. Drought Tolerance and Water Use of Cereal Crops: A Focus on Sorghum as a Food Security Crop in Sub-Saharan Africa. J. Agron. Crop Sci. 2017, 203, 177–191. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Foloni, J.S.S.; Tiritan, C.S. Root Growth and Nutrient Accumulation in Cover Crops as Affected by Soil Compaction. Soil Tillage Res. 2002, 65, 109–115. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Neto, L.O.; Costa, V.E.; da Silva Grassmann, C. Ruzigrass Root Persistence and Soybean Root Growth. Plant Soil 2019, 442, 333–341. [Google Scholar] [CrossRef]
- Crusciol, C.A.C.; Nascente, A.S.; Mateus, G.P.; Borghi, E.; Leles, E.P.; Santos, N.C.B. Effect of Intercropping on Yields of Corn with Different Relative Maturities and Palisadegrass. Agron. J. 2013, 105, 599–606. [Google Scholar] [CrossRef]
- Crusciol, C.A.C.; Marques, R.R.; Filho, A.C.A.C.; Soratto, R.P.; Costa, C.H.M.; Neto, J.F.; Castro, G.S.A.; Pariz, C.M.; de Castilhos, A.M. Annual Crop Rotation of Tropical Pastures with No-till Soil as Affected by Lime Surface Application. Eur. J. Agron. 2016, 80, 88–104. [Google Scholar] [CrossRef]
- Xu, Z.; Li, C.; Zhang, C.; Yu, Y.; van der Werf, W.; Zhang, F. Intercropping maize and soybean increases efficiency of land and fertilizer nitrogen use: A meta-analysis. Field Crops Res. 2020, 246, 107661. [Google Scholar] [CrossRef]
- SoyStats. A Reference Guide to Important Soybean Facts and Figures. American Soybean Association. Available online: http://soystats.com/international-world-soybean-production/ (accessed on 21 April 2023).
- Companhia Nacional de Abastecimento. Acompanhamento da Safra Brasileira: Boletim da Safra de Grãos. 2023. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos (accessed on 21 April 2023).
- Ministério da Agricultura e Pecuária. VBP. 2022. Available online: https://www.gov.br/agricultura/pt-br/assuntos/noticias-2022/valor-da-producao-agropecuaria-de-2022-esta-estimado-em-r-1-241-trilhao-1 (accessed on 21 April 2023).
- Companhia Nacional de Abastecimento. Acompanhamento da Safra Brasileira: Grãos. 2023. Available online: https://www.conab.gov.br/info-agro/safras/graos (accessed on 21 April 2023).
- Nascente, A.S.; Stone, L.F.; Crusciol, C.A.C. Soil Chemical Properties Affected by Cover Crops under No-Tillage System. Rev. Ceres 2015, 62, 401–409. [Google Scholar] [CrossRef]
- Calonego, J.C.; Raphael, J.P.A.; Rigon, J.P.G.; de Oliveira Neto, L.; Rosolem, C.A. Soil Compaction Management and Soybean Yields with Cover Crops under No-till and Occasional Chiseling. Eur. J. Agron. 2017, 85, 31–37. [Google Scholar] [CrossRef]
- Jensen, E.S.; Carlsson, G.; Hauggaard-Nielsen, H. Intercropping of Grain Legumes and Cereals Improves the Use of Soil N Resources and Reduces the Requirement for Synthetic Fertilizer N: A Global-Scale Analysis. Agron. Sustain. Dev. 2020, 40, 5. [Google Scholar] [CrossRef]
- Costa, N.R.; Andreotti, M.; Crusciol, C.A.C.; Pariz, C.M.; Bossolani, J.W.; de Castilhos, A.M.; do Nascimento, C.A.C.; da Lima, C.G.R.; Bonini, C. dos S.B.; Kuramae, E.E. Can Palisade and Guinea Grass Sowing Time in Intercropping Systems Affect Soybean Yield and Soil Chemical Properties? Front. Sustain. Food Syst. 2020, 4, 81. [Google Scholar] [CrossRef]
- Fibach-Paldi, S.; Burdman, S.; Okon, Y. Key Physiological Properties Contributing to Rhizosphere Adaptation and Plant Growth Promotion Abilities of Azospirillum brasilense. FEMS Microbiol. Lett. 2012, 326, 99–108. [Google Scholar] [CrossRef]
- Kochar, M.; Srivastava, S. Surface Colonization by Azospirillum brasilense SM in the Indole-3-Acetic Acid Dependent Growth Improvement of Sorghum. J. Basic Microbiol. 2012, 52, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Cecagno, R.; Fritsch, T.E.; Schrank, I.S. The Plant Growth-Promoting Bacteria Azospirillum amazonense: Genomic Versatility and Phytohormone Pathway. Biomed Res. Int. 2015, 2015, 898592. [Google Scholar] [CrossRef] [PubMed]
- Hungria, M. Inoculação com Azospirillum brasilense: Inovação em Rendimento a Baixo Custo. Documentos 2011, 325, 36. [Google Scholar]
- Nakao, A.H.; Andreotti, M.; de Soares, D.A.; Modesto, V.C.; Dickmann, L. Intercropping Urochloa brizantha and Sorghum Inoculated with Azospirillum brasilense for Silage. Rev. Cienc. Agron. 2018, 49, 501–511. [Google Scholar] [CrossRef]
- Tahir, S.; Marschner, P. Clay Addition to Sandy Soil Reduces Nutrient Leaching—Effect of Clay Concentration and Ped Size. Commun. Soil Sci. Plant Anal. 2017, 48, 1813–1821. [Google Scholar] [CrossRef]
- Lorensini, F.; Ceretta, C.A.; Brunetto, G.; Cerini, J.B.; Lourenzi, C.R.; de Conti, L.; Tiecher, T.L.; Schapanski, D.E. Disponibilidade de Nitrogênio de Fontes Minerais e Orgânicas Aplicadas em uum Argissolo Cultivado com Videira. Rev. Ceres 2014, 61, 241–247. [Google Scholar] [CrossRef]
- de Asevedo Soares, D.; Andreotti, M.; Vicentini, M.E.; Freitas, L.A.; Modesto, V.C.; Nakao, A.H.; Dickmann, L.; Filho, M.C.M.T. Grain Sorghum Grown as Second Crop and Inoculated with Azospirillum brasilense Associated with Nitrogen Fertilization. Rev. Agric. Neotrop. 2021, 8, e5117. [Google Scholar] [CrossRef]
- Farinelli, R.; Lemos, L.B. Produtividade e Eficiência Agronômica do Milho em Função da Adubação Nitrogenada e Manejos do Solo. Rev. Bras. Milho e Sorgo 2010, 9, 135–146. [Google Scholar] [CrossRef]
- Lorensini, F.; Ceretta, C.A.; Girotto, E.; Cerini, J.B.; Lourenzi, C.R.; de Conti, L.; Trindade, M.M.; de Melo, G.W.; Brunetto, G. Lixiviação e Volatilização de Nitrogênio em um Argissolo Cultivado com Videira Submetida à Adubação Nitrogenada. Cienc. Rural 2012, 42, 1173–1179. [Google Scholar] [CrossRef]
- Silva, D.F.; Pegoraro, R.F.; Maia, V.M.; Kondo, M.K.; Souza, G.L.O.D.; Mota, M.F.C. Volatilização de Amônia do Solo Após Doses de Ureia com Inibidores de Urease e de Nitrificação na Cultura do Abacaxi. Rev. Ceres 2017, 64, 327–335. [Google Scholar] [CrossRef]
- Ajeigbe, H.A.; Akinseye, F.M.; Ayuba, K.; Jonah, J. Productivity and Water Use Efficiency of Sorghum [Sorghum Bicolor (L.) Moench] Grown under Different Nitrogen Applications in Sudan Savanna Zone, Nigeria. Int. J. Agron. 2018, 2018, 7676058. [Google Scholar] [CrossRef]
- Arshad, M. Fortnightly Dynamics and Relationship of Growth, Dry Matter Partition and Productivity of Maize Based Sole and Intercropping Systems at Different Elevations. Eur. J. Agron. 2021, 130, 126377. [Google Scholar] [CrossRef]
- Modesto, V.C.; Andreotti, M.; Nakao, A.H.; de Soares, D.A.; de Froio, L.L.; Dickmann, L.; Pascoaloto, I.M.; Fernandes, I.M.D.M. Yield and Production Components of Corn Under Straw of Marandu Palisade Grass Inoculated with Azospirillum brasilense in the Low-Land Cerrado. Front. Sustain. Food Syst. 2021, 4, 617065. [Google Scholar] [CrossRef]
- Quaggio, J.A.; Cantarella, H.; Rosolem, C.A.; Crusciol, C.A.C. Soja. In Recomendações de adubação e calagem para o Estado de São Paulo; Cantarella, H., Quaggio, J.A., Mattos Jr, D., Boaretto, R.M., van Raij, B., Eds.; Instituto Agronômico/Fundação IAC: Campinas, Brazil, 2022; pp. 209–212. [Google Scholar]
- Malavolta, E.A. Elementos de Nutrição Mineral de Plantas; Ceres: São Paulo, Brazil, 1980. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK, 1995. [Google Scholar]
- Rosolem, C.A.; Calonego, J.C.; Foloni, J.S.S. Lixiviação de Potássio da Palha de Espécies de Cobertura de Solo de Acordo com a Quantidade de Chuva Aplicada. Rev. Bras. Ciência do Solo 2003, 27, 355–362. [Google Scholar] [CrossRef]
- Pariz, C.M.; Andreotti, M.; Buzetti, S.; Bergamaschine, A.F.; de Ulian, N.A.; Furlan, L.C.; de Meirelles, P.R.L.; Cavasano, F.A. Straw Decomposition of Nitrogen-Fertilized Grasses Intercropped with Irrigated Maize in an Integrated Crop-Livestock System. Rev. Bras. Ciência do Solo 2011, 35, 2029–2037. [Google Scholar] [CrossRef]
- Vogt, A.A.B.J.G.A.; Trezzi, M.M. Cultura do Milho Irrigado. EMBRAPA Technol. Inf. 2003, 1125, 81–87. [Google Scholar]
- da Silva Ricce, W.; Alves, S.J.; Prete, C.E.C. Época de Dessecação de Pastagem de Inverno e Produtividade de Grãos de Soja. Pesqui. Agropecu. Bras. 2011, 46, 1220–1225. [Google Scholar] [CrossRef]
- Aratani, R.G.; de Maria, I.C.; de Castro, O.M.; Peche Filho, A.; Duarte, A.P.; Kanthack, R.A.D. Desempenho de Semeadoras-Adubadoras de Soja Em Latossolo Vermelho Muito Argiloso Com Palha Intacta de Milho. Rev. Bras. Eng. Agrícola e Ambient. 2006, 10, 517–522. [Google Scholar] [CrossRef]
- Orlowski, J.M.; Gregg, G.L.; Lee, C.D. Early-Season Lactofen Application Has Limited Effect on Soybean Branch and Mainstem Yield Components. Crop Sci. 2016, 56, 432–438. [Google Scholar] [CrossRef]
- Ribeiro, A.B.M.; Bruzi, A.T.; Zuffo, A.M.; Zambiazzi, E.V.; Soares, I.O.; Vilela, N.J.D.; de Pereira, J.L.A.R.; Moreira, S.G. Productive Performance of Soybean Cultivars Grown in Different Plant Densities. Ciência Rural 2017, 47, 1–8. [Google Scholar] [CrossRef]
- Xu, C.; Li, R.; Song, W.; Wu, T.; Sun, S.; Hu, S.; Han, T.; Wu, C. Responses of Branch Number and Yield Component of Soybean Cultivars Tested in Different Planting Densities. Agriculture 2021, 11, 69. [Google Scholar] [CrossRef]
- Moitinho, M.R.; Ferraudo, A.S.; Panosso, A.R.; da Bicalho, E.S.; Teixeira, D.D.B.; de Barbosa, M.A.; Tsai, S.M.; Borges, B.M.F.; de Souza Cannavan, F.; de Souza, J.A.M.; et al. Effects of Burned and Unburned Sugarcane Harvesting Systems on Soil CO2 Emission and Soil Physical, Chemical, and Microbiological Attributes. Catena 2021, 196, 104903. [Google Scholar] [CrossRef]
- Pariz, C.M.; Costa, N.R.; Costa, C.; Crusciol, C.A.C.; de Castilhos, A.M.; de Meirelles, P.R.L.; Calonego, J.C.; Andreotti, M.; de Souza, D.M.; Cruz, I.V.; et al. An Innovative Corn to Silage-Grass-Legume Intercropping System With Oversown Black Oat and Soybean to Silage in Succession for the Improvement of Nutrient Cycling. Front. Sustain. Food Syst. 2020, 4, 544996. [Google Scholar] [CrossRef]
- Bláha, L. Importance Of Root-Shoot Ratio For Crops Production. Agron. Agric. Sci. 2019, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, C.A.; Arango, J.; Arevalo, A.; Nuñez, J.; Cardoso, J.A.; Dungait, J.A.J. Smart Forage Selection Could Significantly Improve Soil Health in the Tropics. Sci. Total Environ. 2019, 688, 609–621. [Google Scholar] [CrossRef]
- Galindo-Castañeda, T.; Lynch, J.P.; Six, J.; Hartmann, M. Improving Soil Resource Uptake by Plants Through Capitalizing on Synergies Between Root Architecture and Anatomy and Root-Associated Microorganisms. Front. Plant Sci. 2022, 13, 827369. [Google Scholar] [CrossRef]
- Kunrath, T.R.; de Carvalho, P.C.F.; Cadenazzi, M.; Bredemeier, C.; Anghinoni, I. Grazing Management in an Integrated Crop-Livestock System: Soybean Development and Grain Yield. Rev. Cienc. Agron. 2015, 46, 645–653. [Google Scholar] [CrossRef]
- Wu, H.; Cai, A.; Xing, T.; Huai, S.; Zhu, P.; Xu, M.; Lu, C. Fertilization Enhances Mineralization of Soil Carbon and Nitrogen Pools by Regulating the Bacterial Community and Biomass. J. Soils Sediments 2021, 21, 1633–1643. [Google Scholar] [CrossRef]
- Yang, X.; You, L.; Hu, H.; Chen, Y. Conversion of Grassland to Cropland Altered Soil Nitrogen-Related Microbial Communities at Large Scales. Sci. Total Environ. 2022, 816, 151645. [Google Scholar] [CrossRef]
- Nakao, A.H.; Andreotti, M.; de Asevedo Soares, D.; Modesto, V.C.; Pechoto, E.A.P.; Freitas, L.A. Soybean in Succession to the Residue of the Sorghum/Paiaguas Grass Straw with Azospirillum brasilense. Rev. Ceres 2019, 66, 395–401. [Google Scholar] [CrossRef]
- Montenegro, A.A.A.; Abrantes, J.R.C.B.; de Lima, J.L.M.P.; Singh, V.P.; Santos, T.E.M. Impact of Mulching on Soil and Water Dynamics under Intermittent Simulated Rainfall. Catena 2013, 109, 139–149. [Google Scholar] [CrossRef]
- De Borges, T.K.S.; de Montenegro, A.A.A.; dos Santos, T.E.M.; da Silva, D.D.; Silva Junior, V.d.P.e. Influência de Práticas Conservacionistas na Umidade do Solo e no Cultivo do Milho (Zea Mays L.) em Semiárido Nordestino. Rev. Bras. Ciência do Solo 2014, 38, 1862–1873. [Google Scholar] [CrossRef]
- De Resende, Á.V.; Fontoura, S.M.V.; Borghi, E.; dos Santos, F.C.; Kappes, C.; Moreira, S.G.; de Oliveira Junior, A.; Borin, A.L.D.C. Solos de Fertilidade Construída: Características, Funcionamento e Manejo. Inf. Agronômicas 2016, 1–19. [Google Scholar]
- Francis Clar, J.T.; Anex, R.P. Flux Intensity and Diurnal Variability of Soil N2O Emissions in a Highly Fertilized Cropping System. Soil Sci. Soc. Am. J. 2020, 84, 1983–1994. [Google Scholar] [CrossRef]
- Silva, I.R.; Mendonça, E.S. Matéria Orgânica do Solo. In Fertilidade do Solo; Novais, R.F., Alvares, V.H.V., Barros, N.F., Fontes, R.L.F., Cantarutti, R.B., Neves, J.C.L., Eds.; SBCS: Viçosa, Brazil, 2007; pp. 275–374. [Google Scholar]
- Raij, B.V.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Chemical Analysis to Evaluate the Fertility of Tropical Soils; Instituto Agronomico de Campinas: Campinas, Brazil, 2001; ISBN 8585564059. [Google Scholar]
- Kluthcouski, J.; Cobucci, T.; Aidar, H.; Yokoyama, L.P.; de Oliveira, I.P.; da Costa, J.L.S.; da Silva, J.G.; Vilela, L.; de Barcellos, A.O.; de Magnabosco, C.U. Sistema Santa Fé-Tecnologia Embrapa: Integração Lavoura-Pecuária Pelo Consórcio de Culturas Anuais com Forrageiras, em Áreas de Lavoura, nos Sistemas Direto e Convencional. Circ. Técnica 2000, 1–28. [Google Scholar]
- Cantarella, H.; Raij, B.V.; Sawazaki, E. Sorgo-Granífero, Forrageiro e Vassou. In Boletim Técnico 100: Recomendação de Adubação e Calagem para o Estado de São Paulo; Raij, B.V., Cantarella, H., Quaggio, J.A., Furlani, A.M.C., Eds.; Instituto Agronômico: Campinas, Brazil, 1997; pp. 66–67. [Google Scholar]
- Fehr, W.R.; Caviness, C.E. Stages of Soybean Development. Spec. Rep. 1977, 80, 11. [Google Scholar]
- Ambrosano, E.J.; Tanaka, R.T.; Mascarenhas, H.A.A.; Raij, B.V.; Quaggio, J.A.; Cantarella, H. Legumes and Oilseeds. In Recommendations for Fertilization and Liming in the State of São Paulo; Raij, B.V., Cantarella, H., Quaggio, J.A., Furlani, A.M.C., Eds.; Instituto Agronômico: Campinas, Brazil, 1997; pp. 189–203. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações; POTAFOS: Piracicaba, Brazil, 1997. [Google Scholar]
- Shapiro, A.S.S.; Wilk, M.B. Biometrika Trust an Analysis of Variance Test for Normality (Complete Samples) Published by: Oxford University Press on Behalf of Biometrika Trust Stable. Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Levene, H. Robust Tests for Equality of Variances. In Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; Olkin, I., Ghurye, S.G., Hoeffding, W., Madow, W.G., Mann, H.B., Eds.; Stanford University Press: Stanford, CA, USA, 1960; pp. 278–292. [Google Scholar]
- R Development Core Team A Language and Environment for Statistical Compunting. Available online: https://www.r-project.org/ (accessed on 19 March 2021).
- Pimentel-Gomes, F. Curso de Estatística Experimental, 15th ed.; Fealq: Piracicaba, Brazil, 2000. [Google Scholar]
- Ferreira, D.F. SISVAR: Um Programa Para Análises e Ensino de Estatística. Rev. Científica Symp. 2008, 6, 36–41. [Google Scholar]
| 2015/16 | |||||||
| Treatments | SPAD | N | P | K | Ca | Mg | S |
| Units | -------------------------------------g kg−1------------------------------------- | ||||||
| Inoculation | |||||||
| With | 49.1 ± 0.27 | 37 ± 0.82 b | 3.5 ± 0.05 | 19 ± 0.54 b | 6.0 ± 0.16 b | 4.3 ± 0.11 | 2.1 ± 0.05 |
| Without | 50.0 ± 0.35 | 41 ± 0.93 a | 3.6 ± 0.07 | 21 ± 0.48 a | 7.0 ± 0.21 a | 4.2 ± 0.04 | 2.1 ± 0.06 |
| Cropping modalities | |||||||
| Intercropped | 49.8 ± 0.31 a | 39 ± 1.22 | 3.5 ± 0.07 | 20 ± 0.52 | 6.4 ± 0.22 a | 4.5 ± 0.09 a | 2.1 ± 0.06 |
| Monoculture | 48.8 ± 0.29 b | 39 ± 0.65 | 3.6 ± 0.05 | 20 ± 0.54 | 6.0 ± 0.16 b | 4.1 ± 0.05 b | 2.1 ± 0.06 |
| Fertilizer management * | |||||||
| 0–100% | 49.4 ± 0.38 | 39 ± 1.47 | 3.6 ± 0.10 | 20 ± 0.70 ab | 6.1 ± 0.20 b | 4.4 ± 0.13 a | 2.2 ± 0.08 |
| 30–70% | 49.2 ± 0.28 | 39 ± 1.35 | 3.5 ± 0.07 | 19 ± 0.71 b | 6.8 ± 0.30 a | 4.4 ± 0.09 a | 2.2 ± 0.08 |
| 100–0% | 49.4 ± 0.48 | 39 ± 0.61 | 3.6 ± 0.07 | 21 ± 0.38 a | 6.0 ± 0.30 b | 4.2 ± 0.06 b | 2.0 ± 0.02 |
| CV(%) | 3 | 8 | 6 | 10 | 9 | 4 | 11 |
| 2016/17 | |||||||
| Treatments | SPAD | N | P | K | Ca | Mg | S |
| Units | ---------------------------------------g kg−1--------------------------------------- | ||||||
| Inoculation | |||||||
| With | 40.3 ± 0.46 | 46 ± 0.61 b | 3.6 ± 0.04 b | 13 ± 0.22 b | 5.5 ± 0.12 b | 3.5 ± 0.06 | 2.5 ± 0.03 a |
| Without | 40.6 ± 0.57 | 49 ± 0.41 a | 4.1 ± 0.09 a | 15 ± 0.37 a | 5.8 ± 0.09 a | 3.4 ± 0.05 | 2.3 ± 0.05 b |
| Cropping modalities | |||||||
| Intercropped | 40.8 ± 0.43 | 46 ± 0.60 b | 3.7 ± 0.06 b | 13 ± 0.27 b | 5.7 ± 0.10 | 3.5 ± 0.05 a | 2.40 ± 0.03 |
| Monoculture | 40.3 ± 0.60 | 49 ± 0.44 a | 4.0 ± 0.10 a | 14 ± 0.39 a | 5.6 ± 0.11 | 3.3 ± 0.06 b | 2.3 ± 0.05 |
| Fertilizer management * | |||||||
| 0–100% | 40.4 ± 0.73 | 48 ± 0.61 | 4.0 ± 0.14 | 13 ± 0.42 | 5.6 ± 0.12 | 3.4 ± 0.08 | 2.4 ± 0.04 |
| 30–70% | 40.9 ± 0.66 | 47 ± 0.77 | 3.7 ± 0.09 | 13 ± 0.51 | 5.9 ± 0.13 | 3.4 ± 0.07 | 2.3 ± 0.06 |
| 100–0% | 40.1 ± 0.52 | 47 ± 0.74 | 3.9 ± 0.09 | 13 ± 0.30 | 5.6 ± 0.12 | 3.4 ± 0.07 | 2.3 ± 0.06 |
| CV(%) | 7 | 5 | 7 | 4 | 7 | 6 | 7 |
| Cropping Modalities | |||
| A. brasilense | Intercropped | Monoculture | |
| (A) | Plants ha−1 | ||
| With | 323,045 ± 6389 Bb | 347,531 ± 5847 Aa | |
| Without | 344,444 ± 4260 Aa | 337,654 ± 6925 Aa | |
| (B) | Grain yield kg ha−1 | ||
| With | 3887 ± 60 Aa | 3946 ± 58 Aa | |
| Without | 3464 ± 93 Bb | 3973 ± 74 Aa | |
| Nitrogen fertilizer management | |||
| Cropping modalities | 0–100% * | 30–70% | 100–0% |
| (C) | Grain yield, kg ha−1 | ||
| Intercropped | 3686 ± 221 | 3494 ± 217 | 3808 ± 64 |
| Monoculture | 3587 ± 148 ab | 4053 ± 134 a | 3322 ± 128 b |
| 2015/16 | |||||||
| Treatments | FPS | PH | FPI | NPP | NGP | HGW | GY (2) |
| Inoculation | (plants ha−1) | (m) | (cm) | - | - | (g) | (kg ha−1) |
| With | 335,288 ± 4945 | 1.05 ± 0.02 | 18.4 ± 0.29 a | 39 ± 0.83 | 2.3 ± 0.04 | 14.6 ± 0.22 a | 3916 ± 041 |
| Without | 341,049 ± 4038 | 1.07 ± 0.01 | 16.9 ± 0.28 b | 41 ± 0.93 | 2.3 ± 0.08 | 13.8 ± 0.27 b | 3719 ± 78 |
| Cropping modalities | |||||||
| Intercropped | 333,745 ± 4368 | 1.06 ± 0.01 | 17.3 ± 0.31 | 40 ± 0.85 | 2.3 ± 0.06 | 14.0 ± 0.19 | 3775 ± 69 |
| Monoculture | 342,593 ± 4550 | 1.05 ± 0.02 | 17.9 ± 0.32 | 40 ± 0.96 | 2.2 ± 0.06 | 14.3 ± 0.32 | 3959 ± 46 |
| Fertilizer management * | |||||||
| 0–100% | 343,519 ± 6077 | 1.07 ± 0.01 | 17.5 ± 0.35 | 40 ± 1.38 | 2.2 ± 0.09 | 14.0 ± 0.32 | 3769 ± 82 ab |
| 30–70% | 334,259 ± 5625 | 1.05 ± 0.02 | 17.9 ± 0.46 | 40 ± 0.76 | 2.3 ± 0.06 | 14.0 ± 0.35 | 3728 ± 83 b |
| 100–0% | 336,728 ± 4876 | 1.06 ± 0.02 | 17.5 ± 0.37 | 41 ± 1.13 | 2.3 ± 0.08 | 15.0 ± 0.28 | 3955 ± 66 a |
| CV(%) | 6 | 6 | 8 | 11 | 14 | 9 | 6 |
| 2016/17 | |||||||
| Treatments | FPS | PH | FPI | NPP | NGP | HGW | GY (3) |
| Inoculation | (plants ha−1) | (m) | (cm) | - | - | (g) | (kg ha−1) |
| With | 286,806 ± 5857 a | 1.05 ± 0.71 | 16.8 ± 0.28 | 39 ± 1.04 | 2.6 ± 0.06 | 15.0 ± 0.53 | 3749 ± 114 |
| Without | 267,130 ± 6662 b | 1.05 ± 1.13 | 16.2 ± 0.18 | 41 ± 0.84 | 2.5 ± 0.06 | 14.2 ± 0.32 | 3568 ± 95 |
| Cropping modalities | |||||||
| Intercropped | 287,963 ± 6315 a | 1.07 ± 0.73 a | 16.1 ± 0.24 b | 39 ± 1.09 | 2.5 ± 0.05 | 14.5 ± 0.47 | 3663 ± 110 |
| Monoculture | 265,972 ± 6059 b | 1.03 ± 1.01 b | 16.8 ± 0.22 a | 41 ± 0.78 | 2.5 ± 0.07 | 14.7 ± 0.41 | 3654 ± 103 |
| Fertilizer management * | |||||||
| 0–100% | 274,306 ± 7098 | 1.05 ± 1.33 | 16.5 ± 0.30 | 40 ± 0.87 | 2.6 ± 0.10 | 14.3 ± 0.44 | 3636 ± 129 |
| 30–70% | 279,861 ± 8664 | 1.07 ± 1.03 | 16.6 ± 0.32 | 40 ± 0.75 | 2.5 ± 0.05 | 14.9 ± 0.61 | 3724 ± 156 |
| 100–0% | 279,861 ± 8595 | 1.04 ±1.02 | 16.4 ± 0.29 | 40 ± 1.70 | 2.5 ± 0.07 | 14.6 ± 0.56 | 3615 ± 103 |
| CV(%) | 11 | 4 | 7 | 13 | 12 | 16 | 12 |
| Depth | pH | SOM a | P | H + Al | K+ | Ca2+ | Mg2+ | CEC b | BS c |
|---|---|---|---|---|---|---|---|---|---|
| m | CaCl2 | g dm−3 | mg dm−3 | ---------------mmolc dm−3 --------------- | % | ||||
| 0–0.20 | 5.5 | 22 | 17 | 28 | 1.4 | 26 | 18 | 73.1 | 62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Asevedo Soares, D.; Modesto, V.C.; Nakao, A.H.; Soares, W.R.; Freitas, L.A.; Dickmann, L.; Pascoaloto, I.M.; Andreotti, M. Soybean Yield and Nutrition Grown on the Straw of Grain Sorghum Inoculated with Azospirillum brasilense and Intercropped with BRS Paiaguás Grass. Plants 2023, 12, 2007. https://doi.org/10.3390/plants12102007
de Asevedo Soares D, Modesto VC, Nakao AH, Soares WR, Freitas LA, Dickmann L, Pascoaloto IM, Andreotti M. Soybean Yield and Nutrition Grown on the Straw of Grain Sorghum Inoculated with Azospirillum brasilense and Intercropped with BRS Paiaguás Grass. Plants. 2023; 12(10):2007. https://doi.org/10.3390/plants12102007
Chicago/Turabian Stylede Asevedo Soares, Deyvison, Viviane Cristina Modesto, Allan Hisashi Nakao, Wellington Rosa Soares, Leandro Alves Freitas, Lourdes Dickmann, Isabô Melina Pascoaloto, and Marcelo Andreotti. 2023. "Soybean Yield and Nutrition Grown on the Straw of Grain Sorghum Inoculated with Azospirillum brasilense and Intercropped with BRS Paiaguás Grass" Plants 12, no. 10: 2007. https://doi.org/10.3390/plants12102007
APA Stylede Asevedo Soares, D., Modesto, V. C., Nakao, A. H., Soares, W. R., Freitas, L. A., Dickmann, L., Pascoaloto, I. M., & Andreotti, M. (2023). Soybean Yield and Nutrition Grown on the Straw of Grain Sorghum Inoculated with Azospirillum brasilense and Intercropped with BRS Paiaguás Grass. Plants, 12(10), 2007. https://doi.org/10.3390/plants12102007








