Elucidation of Physiological, Transcriptomic and Metabolomic Salinity Response Mechanisms in Medicago sativa
Abstract
1. Introduction
2. Results
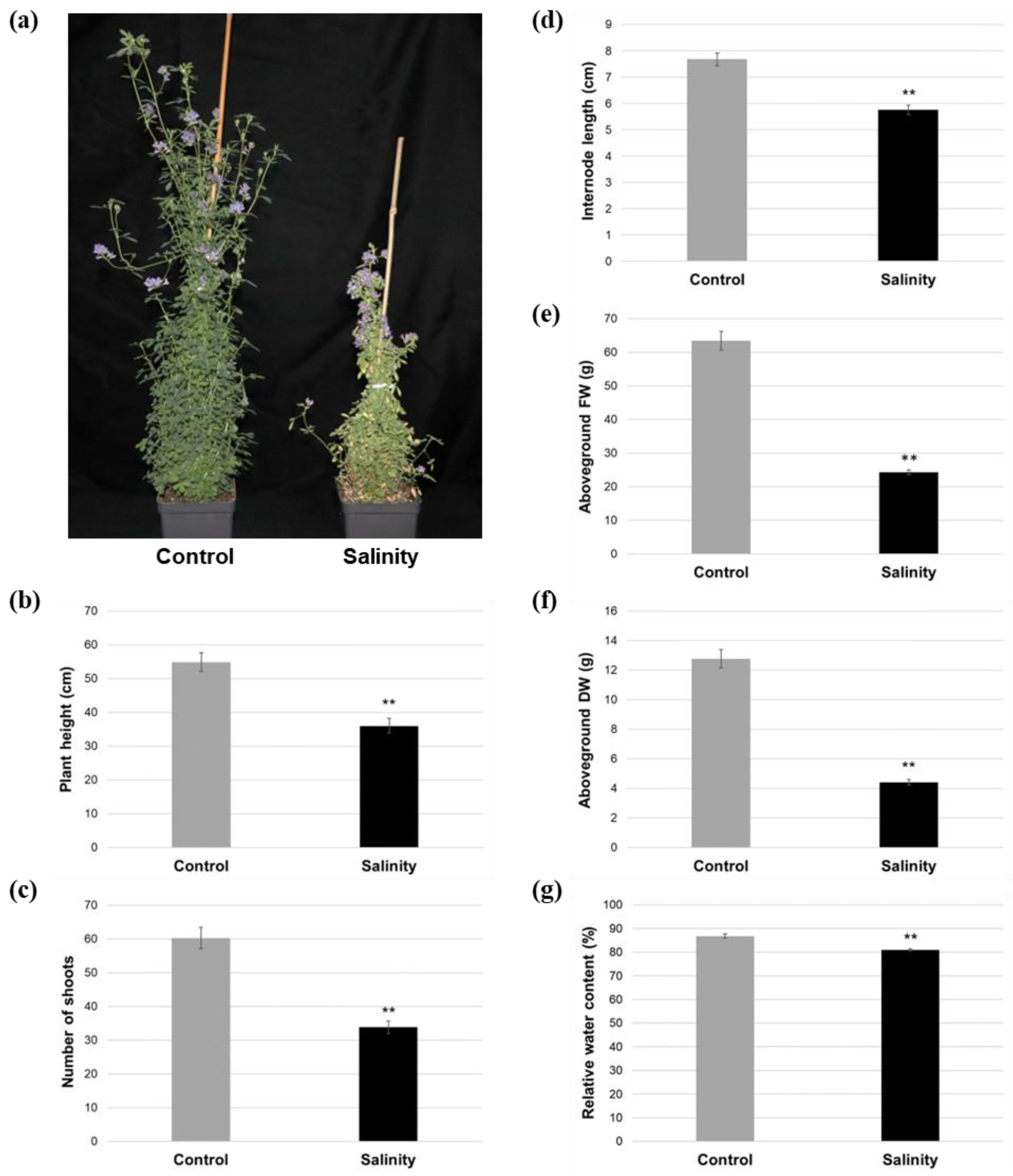
2.1. Salinity-Treated Alfalfa Plants Exhibited Reductions in Aboveground Growth and Water Content Compared to Control-Treated Plants
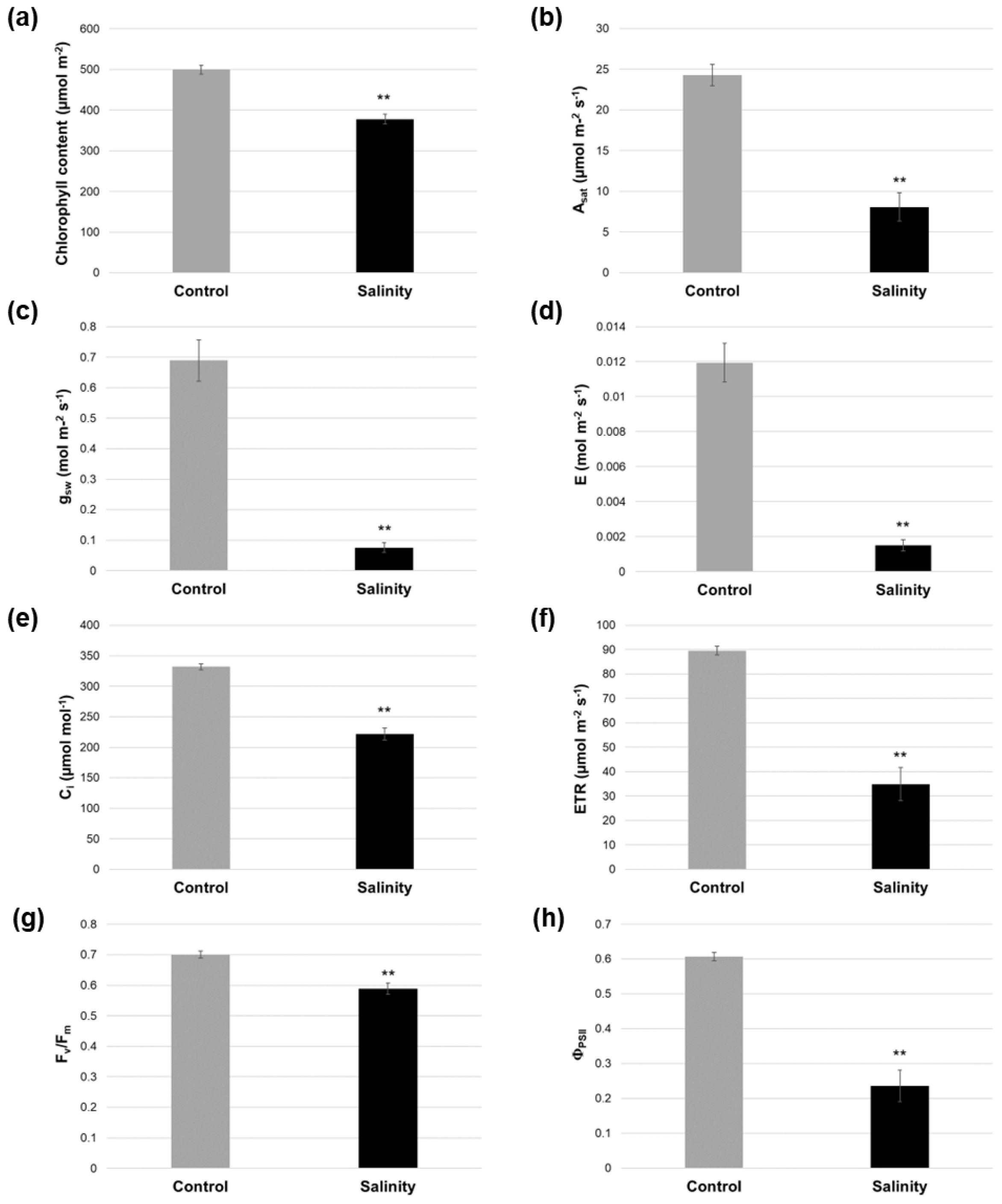
2.2. Salinity-Treated Alfalfa Plants Exhibited Reductions in Photosynthesis-Related Parameters Compared to Control-Treated Plants
2.3. Alfalfa Plants under Saline Stress Displayed Altered Osmoprotectant Levels and Antioxidant-Related Parameters Compared to Control Conditions
2.4. RNA-Seq Analysis of Leaf Tissue from Salinity- and Control-Treated Alfalfa Plants
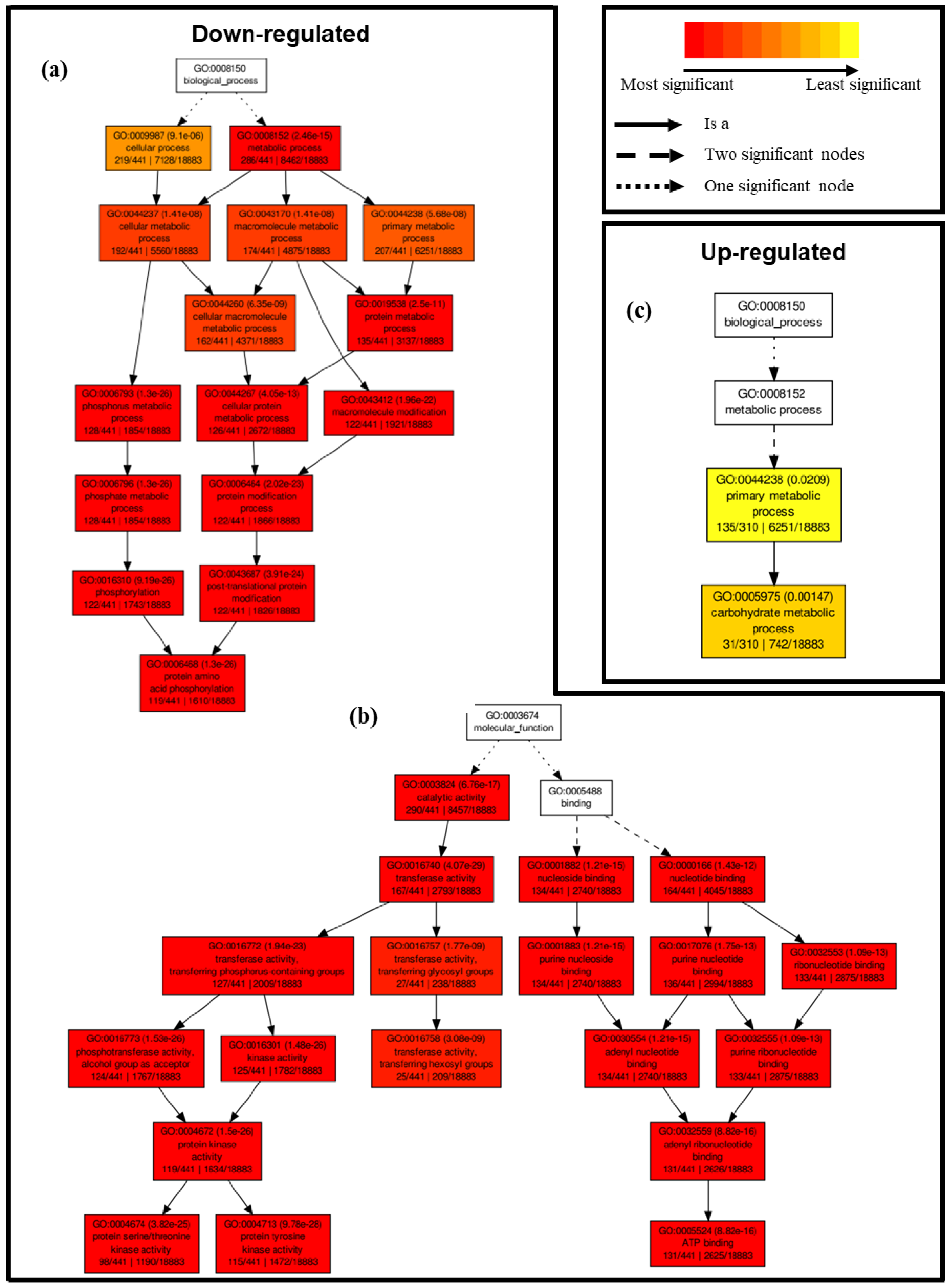
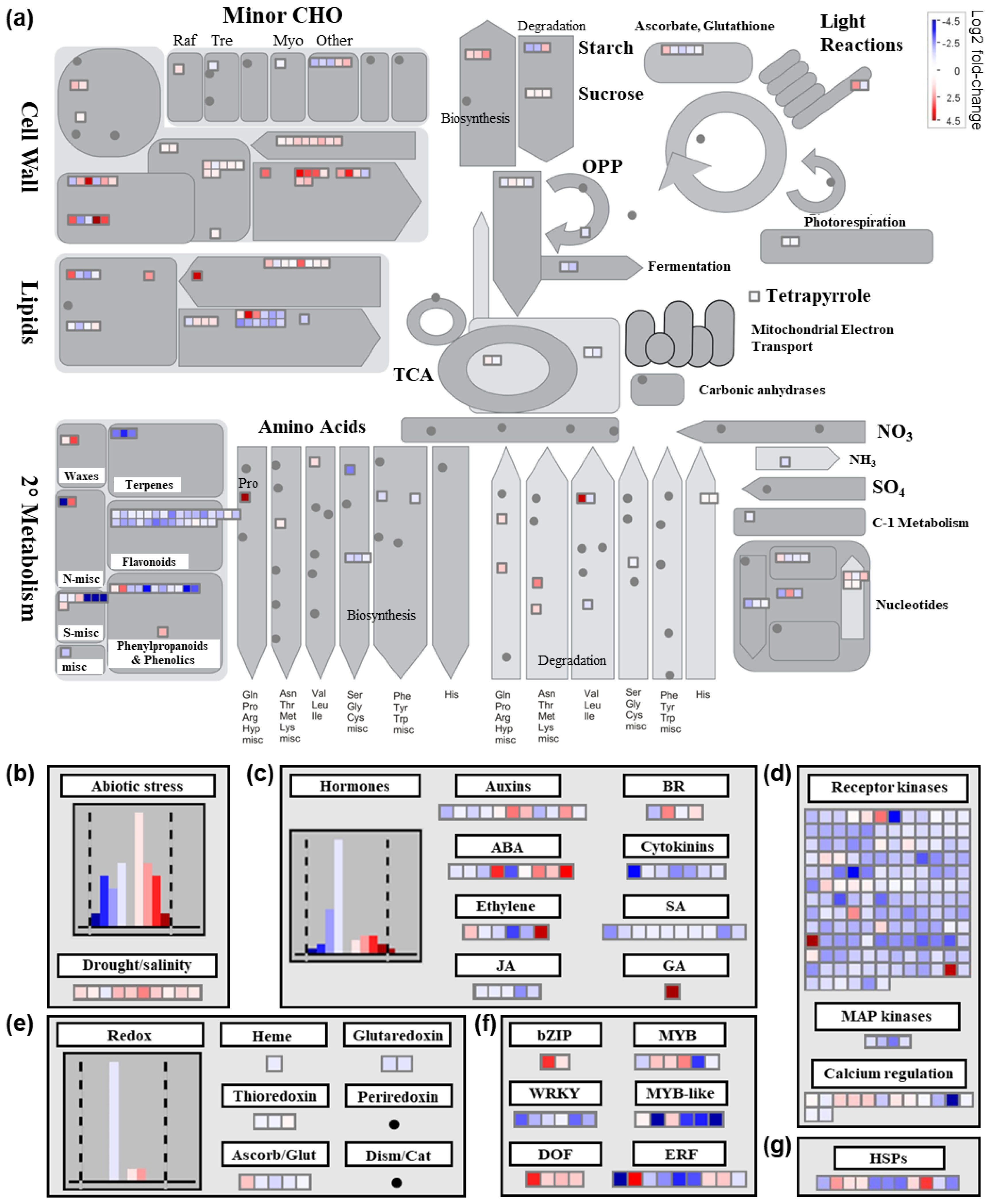
2.4.1. Differential Transcriptional Responses in General Metabolic Pathways between Saline and Control Conditions
2.4.2. Differential Transcriptional Responses in Abiotic Stress- and Redox-Related Pathways between Saline and Control Conditions
2.4.3. Differential Transcriptional Responses in Phytohormone Metabolism-Related Pathways and Genes Encoding Transcription Factors between Saline and Control Conditions
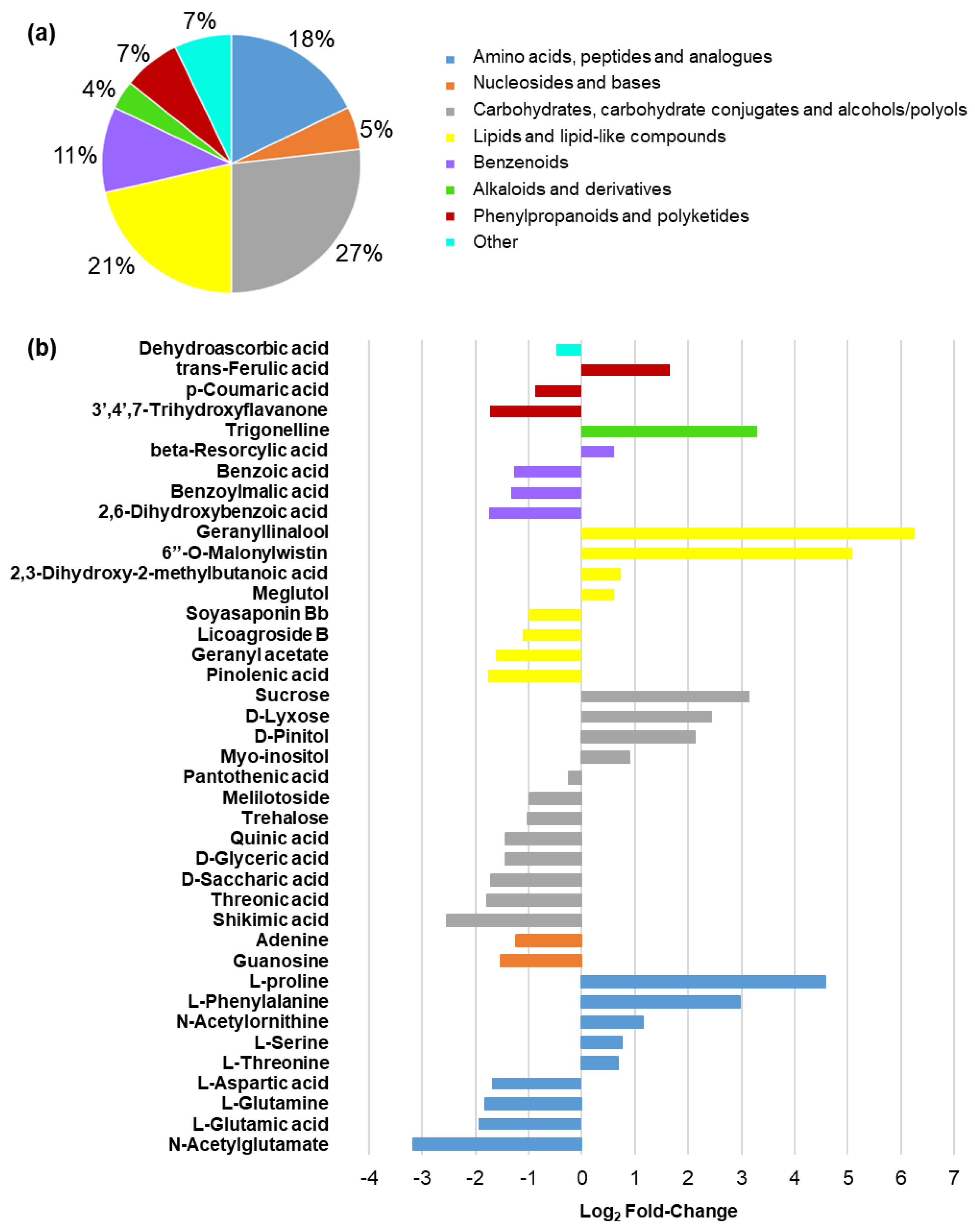
2.5. Metabolomic Response in Leaf Tissues from Salinity- and Control-Treated Alfalfa Plants
2.6. Joint Pathway Enrichment Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions
4.2. Assessment of Growth Characteristics
4.3. Measurement of Relative Water Content
4.4. Biochemical Assessments
4.5. Visualization of Reactive Oxygen Species
4.6. Determination of Total Antioxidant Capacity
4.7. Assessment of Chlorophyll Levels and Photosynthetic-Related Parameters
4.8. RNA-Seq Analysis
4.9. Validation of RNA-Seq Results Using Quantitative RT-PCR (qRT-PCR)
4.10. Metabolomic Analysis
4.11. Joint Pathway Analysis
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhattarai, S.; Biswas, D.; Fu, Y.-B.; Biligetu, B. Morphological, physiological, and genetic responses to salt stress in alfalfa: A review. Agronomy 2020, 10, 577. [Google Scholar] [CrossRef]
- Singer, S.D.; Hannoufa, A.; Acharya, S. Molecular improvement of alfalfa for enhanced productivity and adaptability in a changing environment. Plant Cell Environ. 2018, 41, 1955–1971. [Google Scholar] [CrossRef]
- Singer, S.D.; Weselake, R.J.; Acharya, S. Molecular enhancement of alfalfa: Improving quality traits for superior livestock performance and reduced environmental impact. Crop. Sci. 2018, 58, 55–71. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Global Map of Salt-Affected Soils; FAO: Rome, Italy, 2021; Available online: https://www.fao.org/3/cb7247en/cb7247en.pdf (accessed on 4 November 2022).
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Natural Res. For. 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2022: Impacts, Adaptation and Vulnerability. Working Group II Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. 2022. Available online: https://www.ipcc.ch/report/ar6/wg2/downloads/report/IPCC_AR6_WGII_SummaryVolume.pdf (accessed on 28 October 2022).
- Pulido-Bosch, A.; Rigol-Sanchez, J.P.; Vallejos, A.; Andreu, J.M.; Ceron, J.C.; Molina-Sanchez, L.; Sola, F. Impacts of agricultural irrigation on groundwater salinity. Environ. Earth Sci. 2018, 77, 197. [Google Scholar] [CrossRef]
- Putnam, D.H. Strategies for the improvement of water-use efficient irrigated alfalfa systems. In Proceedings of the California Alfalfa and Grain Symposium, Sacramento, CA, USA, 10–12 December 2012. [Google Scholar]
- Cornacchione, M.V.; Suarez, D.L. Evaluation of alfalfa (Medicago sativa L.) populations’ response to salinity stress. Crop. Sci. 2017, 57, 137–150. [Google Scholar] [CrossRef]
- Searchinger, T.; Waite, R.; Hanson, C.; Ranganathan, J. Creating a Sustainable Food Future: A Menu of Solutions to Feed Nearly 10 Billion People by 2050; World Resources Institute: Washington, DC, USA, 2019; Available online: https://files.wri.org/d8/s3fs-public/wrr-food-full-report.pdf (accessed on 30 August 2022).
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Bhattarai, S.; Lundell, S.; Biligetu, B. Effect of sodium chloride salt on germination, growth, and elemental composition of alfalfa cultivars with different tolerances to salinity. Agronomy 2022, 12, 2516. [Google Scholar] [CrossRef]
- Serraj, R.; Drevon, J.-J. Effects of salinity and nitrogen source on growth and nitrogen fixation in alfalfa. J. Plant Nutr. 1998, 21, 1805–1818. [Google Scholar] [CrossRef]
- Acharya, B.R.; Sandhu, D.; Ferreira, J.F.S. Physiological, morphological, and genetic responses of alfalfa to salinity. In The Alfalfa Genome. Compendium of Plant Genomes; Yu, L.S., Kole, C., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Chele, K.H.; Tinte, M.M.; Piater, L.A.; Dubery, I.A.; Tugizimana, F. Soil salinity, a serious environmental issue and plant responses: A metabolomics perspective. Metabolites 2021, 11, 724. [Google Scholar] [CrossRef]
- Kumar, J.; Singh, S.; Singh, M.; Srivastava, P.K.; Mishra, R.K.; Singh, V.P.; Prasad, S.M. Transcriptional regulation of salinity stress in plants: A short review. Plant Gene 2017, 11, 160–169. [Google Scholar] [CrossRef]
- Basu, S.; Kumar, A.; Benazir, I.; Kumar, G. Reassessing the role of ion homeostasis for improving salinity tolerance in crop plants. Physiol. Plant 2021, 171, 502–519. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- Pan, T.; Liu, M.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Nie, C.; Yu, M.; Kuznetsov, V.V.; Allakhverdiev, S.I.; Shabala, S. Non-stomatal limitation of photosynthesis by soil salinity. Crit. Rev. Environ. Sci. Technol. 2021, 51, 791–825. [Google Scholar] [CrossRef]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global plant-responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2014, 35, 425–437. [Google Scholar] [CrossRef]
- Bhattarai, S.; Fu, Y.-B.; Coulman, B.; Tanino, K.; Karunakaran, C.; Biligetu, B. Transcriptomic analysis of differentially expressed genes in leaves and roots of two alfalfa (Medicago sativa L.) cultivars with different salt tolerance. BMC Plant Biol. 2021, 21, 446. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Liu, X.; Li, D.; Gao, T.; Song, Y. Transcriptional profiling reveals that a MYB transcription factor MsMYB4 contributes to the salinity stress response of alfalfa. PLoS ONE 2018, 13, e0204033. [Google Scholar] [CrossRef]
- Kaundal, R.; Duhan, N.; Acharya, B.R.; Pudussery, M.V.; Ferreira, F.S.; Suarez, D.L.; Sandhu, D. Transcriptional profiling of two contrasting genotypes uncovers molecular mechanisms underlying salt tolerance in alfalfa. Sci. Rep. 2021, 11, 5210. [Google Scholar] [CrossRef]
- Lei, Y.; Xu, Y.; Hettenhausen, C.; Lu, C.; Shen, G.; Zhang, C.; Li, J.; Song, J.; Lin, H.; Wu, J. Comparative analysis of alfalfa (Medicago sativa L.) leaf transcriptomes reveals genotype-specific salt tolerance mechanisms. BMC Plant Biol. 2018, 18, 35. [Google Scholar] [CrossRef]
- Li, J.; Ma, M.; Sun, Y.; Lu, P.; Shi, H.; Guo, Z.; Zhu, H. Comparative physiological and transcriptome profiles uncover salt tolerance mechanisms in alfalfa. Front. Plant Sci. 2022, 13, 931619. [Google Scholar] [CrossRef]
- Luo, D.; Zhou, Q.; Wu, Y.; Chai, X.; Liu, W.; Wang, Y.; Yang, Q.; Wang, Z.; Liu, Z. Full-length transcript sequencing and comparative transcriptomic analysis to evaluate the contribution of osmotic and ionic stress components towards salinity tolerance in the roots of cultivated alfalfa (Medicago sativa L.). BMC Plant Biol. 2019, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.A.; Samac, D.A.; Yu, L.-X. Pan-transcriptome identifying master genes and regulation network in response to drought and salt stressed in alfalfa (Medicago sativa L.). Sci. Rep. 2021, 11, 17203. [Google Scholar] [CrossRef]
- Postnikova, O.A.; Shao, J.; Nemchinov, L.G. Analysis of the alfalfa root transcriptome in response to salinity stress. Plant Cell Physiol. 2013, 54, 1041–1055. [Google Scholar] [CrossRef]
- Xiong, X.; Wei, Y.-Q.; Chen, J.-H.; Liu, N.; Zhang, Y.-J. Transcriptome analysis of genes and pathways associated with salt tolerance in alfalfa under non-uniform stress. Plant Physiol. Biochem. 2020, 151, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Kataria, S.; Verma, S.K. Salinity stress responses and adaptive mechanisms in major glycophytic crops: The story so far. In Salinity Responses and Tolerance in Plants; Kumar, V., Wani, S., Suprasanna, P., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2018; Volume 1, pp. 1–39. [Google Scholar]
- Duan, H.; Tiika, R.J.; Tian, F.; Lu, Y.; Zhang, Q.; Hu, Y.; Cui, G.; Yang, H. Metabolomics analysis unveils important changes involved in the salt tolerance of Salicornia europaea. Front. Plant Sci. 2023, 13, 1097076. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.K.; Pandey, S.; Tanna, B.; Alkan, N.; Mishra, A. Comparative metabolomics unveils the role of metabolites and metabolic pathways in the adaptive mechanisms of shrubby halophytes. Environ. Exp. Bot. 2022, 202, 105030. [Google Scholar] [CrossRef]
- Singh, J.; Singh, V.; Dutt, V.; Walia, N.; Kumawat, G.; Jakhar, M.L.; Yadava, D.K.; Sharma, P.C. Insights into salt tolerance of mustard (Brassica juncea L. Czern&Coss): A metabolomics perspective. Environ. Exp. Bot. 2022, 194, 104760. [Google Scholar]
- Li, J.; Essemine, J.; Shang, C.; Zhang, H.; Zhu, X.; Yu, J.; Chen, G.; Qu, M.; Sun, D. Combined proteomics and metabolism analysis unravels prominent roles of antioxidant system in the prevention of alfalfa (Medicago sativa L.) against salt stress. Int. J. Mol. Sci. 2020, 21, 909. [Google Scholar] [CrossRef]
- Sarri, E.; Termentzi, A.; Abraham, E.M.; Papadopoulos, G.K.; Baira, E.; Machera, K.; Loukas, V.; Komaitis, F.; Tani, E. Salinity stress alters the secondary metabolic profile of M. sativa, M. arborea and their hybrid (alborea). Int. J. Mol. Sci. 2021, 22, 4882. [Google Scholar] [CrossRef]
- Li, M.; Yu, A.; Sun, Y.; Hu, Q.; Kang, J.; Chen, L.; Zhu, X.; Yang, Q.; Long, R. Lipid composition remodeling and storage lipid conversion play a critical role in salt tolerance in alfalfa (Medicago sativa L.) leaves. Environ. Exp. Bot. 2023, 205, 105144. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Cornacchione, M.V.; Liu, X.; Suarez, D.L. Nutrient composition, forage parameters, and antioxidant capacity of alfalfa (Medicago sativa, L.) in response to saline irrigation water. Agriculture 2015, 5, 577–597. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, E.; Razmjoo, J.; Zahedi, M.; Pessarakli, M. Selecting alfalfa cultivars for salt tolerance based on some physiochemical traits. Agronomy J. 2014, 106, 1758–1764. [Google Scholar] [CrossRef]
- Benabderrahim, M.A.; Guiza, M.; Haddad, M. Genetic diversity of salt tolerance in tetraploid alfalfa (Medicago sativa L.). Acta Physiol. Plant 2020, 42, 5. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Shomali, A.; Azad, N.; Hassani, B.; Lastochkina, O.; Li, T. Calcium signaling and salt tolerance are diversely entwined in plants. Plant Signal. Behav. 2019, 14, 1665455. [Google Scholar] [CrossRef]
- Acharya, S.N.; Steppuhn, H. Bridgeview alfalfa. Can. J. Plant Sci. 2012, 92, 203–206. [Google Scholar] [CrossRef]
- Redmann, R.E. Osmotic and specific ion effects on the germination of alfalfa. Can. J. Bot. 1974, 52, 803–808. [Google Scholar] [CrossRef]
- Al-Niemi, T.S.; Campbell, W.F.; Rumbaugh, M.D. Response of alfalfa cultivars to salinity during germination and post-germination growth. Crop. Sci. 1992, 32, 976–980. [Google Scholar] [CrossRef]
- Sandhu, D.; Cornacchione, M.V.; Ferreira, J.F.S.; Suarez, D.L. Variable salinity responses of 12 alfalfa genotypes and comparative expression analyses of salt-response genes. Sci. Rep. 2017, 7, 42958. [Google Scholar] [CrossRef]
- Esechie, H.A.; Al-Barhi, B.; Al-Gheity, S.; Al-Khanjari, S. Root and shoot growth in salinity-stressed alfalfa in response to nitrogen source. J. Plant Nutr. 2007, 25, 2559–2569. [Google Scholar] [CrossRef]
- Xiong, X.; Liu, N.; Wei, Y.-Q.; Bi, Y.-X.; Luo, J.-C.; Xu, R.-X.; Zhou, J.-Q.; Zhang, Y.-J. Effects of non-uniform root zone salinity on growth, ion regulation, and antioxidant defense system in two alfalfa cultivars. Plant Physiol. Biochem. 2018, 132, 434–444. [Google Scholar] [CrossRef]
- Kapulnik, Y.; Heuer, B. Forage production of four alfalfa (Medicago sativa) cultivars under salinity. Arid Soil Res. Rehabil. 1991, 5, 127–135. [Google Scholar] [CrossRef]
- Khavari-Nejad, R.A.; Chaparzadeh, N. The effects of NaCl and CaCl2 on photosynthesis and growth of alfalfa plants. Photosynthetica 1998, 35, 461–466. [Google Scholar] [CrossRef]
- Ma, D.; Cai, J.; Ma, Q.; Wang, W.; Zhao, L.; Li, J.; Su, L. Comparative time-course transcriptome analysis of two contrasting alfalfa (Medicago sativa L.) genotypes reveals tolerance mechanisms to salt stress. Front. Plant Sci. 2022, 13, 1070846. [Google Scholar] [CrossRef]
- Wungrampha, S.; Joshi, R.; Singla-Pareek, S.L.; Pareek, A. Photosynthesis and salinity: Are these mutually exclusive? Photosynthetica 2018, 56, 366–381. [Google Scholar] [CrossRef]
- Denlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Raina, S.K.; Sultan, S.M. Arabidopsis MAPK signaling pathways and their cross talks in abiotic stress response. J. Plant Biochem. Biotechnol. 2020, 29, 700–714. [Google Scholar] [CrossRef]
- Feng, W.; Kita, D.; Peaucell, A.; Caartwright, H.N.; Doan, V.; Duan, Q.; Liu, M.-C.; Maman, J.; Steinhorst, L.; Schmitz-Thom, I.; et al. The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr. Biol. 2018, 28, 666–675.e5. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, P.; Zhao, F.; Lu, L.; Long, X.; Hao, Z.; Shi, J.; Chen, J. The Liriodendron chinense MKK2 gene enhances Arabidopsis thaliana salt resistance. Forests 2020, 11, 1160. [Google Scholar] [CrossRef]
- Teige, M.; Scheikl, E.; Eulgem, T.; Dóczi, R.; Ichimura, K.; Shinozaki, K.; Dangl, J.L.; Hirt, H. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol. Cell 2004, 15, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-H.; Zhang, F.-J.; Sun, P.; Li, Z.-Y.; Zheng, P.-F.; Gu, K.-D.; Hao, Y.-J.; Zhang, Z.; You, C.-X. Apple receptor-like kinase FERONIA regulates salt tolerance and ABA sensitivity in Malus domestica. J. Plant Physiol. 2022, 270, 153616. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Anderson, C.T.; Xiao, C. Dynamics of pectic homogalacturonan in cellular morphogenesis and adhesion, wall integrity sensing and plant development. Nat. Plants 2022, 8, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ma, Y.; Chen, N.; Guo, S.; Liu, H.; Guo, X.; Chong, K.; Xu, Y. Overexpression of stress-inducible OSBURP16, the β subunit of polygalacturonase 1, decreases pectin content and cell adhesion and increases abiotic stress sensitivity in rice. Plant Cell Environ. 2013, 37, 1144–1158. [Google Scholar] [CrossRef]
- Shafi, A.; Gill, T.; Zahoor, I.; Ahuja, P.S.; Sreenivasulu, Y.; Kumar, S.; Singh, A.K. Ectopic expression of SOD and APX genes in Arabidopsis alters metabolic pools and genes related to secondary cell wall cellulose biosynthesis and improve salt tolerance. Mol. Biol. Rep. 2019, 46, 1985–2002. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2017, 217, 523–539. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Maathuis, F.J.M.; Ahmad, I.; Patishtan, J. Regulation of Na+ fluxes in plants. Front. Plant Sci. 2014, 5, 467. [Google Scholar] [CrossRef]
- Qui, Q.-S.; Guo, Y.; Dietrich, M.A.; Zhu, J.-K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. USA 2002, 99, 8436–8441. [Google Scholar]
- Oh, D.-H.; Leidi, E.; Zhang, Q.; Hwang, S.-M.; Li, Y.; Qintero, F.J.; Jiang, X.; D’Urzo, M.P.; Lee, S.Y.; Zhao, Y.; et al. Loss of halophytism by interference with SOS1 expression. Plant Physiol. 2009, 151, 210–222. [Google Scholar] [CrossRef]
- Sathee, L.; Sairam, R.K.; Chinnusamy, V.; Jha, S.K. Differential transcript abundance of salt overly sensitive (SOS) pathway genes is a determinant of salinity stress tolerance of wheat. Acta Physiol. Plant 2015, 37, 169. [Google Scholar] [CrossRef]
- Ali, A.; Maggio, A.; Bressan, R.A.; Yun, D.-J. Role and functional differences of HKT1-type transporters in plants under salt stress. Int. J. Mol. Sci. 2019, 20, 1059. [Google Scholar] [CrossRef] [PubMed]
- Hauser, F.; Horie, T. A conserved primary salt tolerance mechanism mediated by HKT transporters: A mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant Cell Environ. 2010, 33, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Nath, J.; Singh, A.K.; Pareek, A.; Joshi, R. Ion transporters and their regulatory signal transduction mechanisms for salinity tolerance in plants. Physiol. Plant 2022, 174, e13702. [Google Scholar] [CrossRef] [PubMed]
- Dahuja, A.; Kumar, R.R.; Sakhare, A.; Watts, A.; Singh, B.; Goswami, S.; Sachdev, A.; Praveen, S. Role of ATP-binding cassette transporters in maintaining plant homeostasis under abiotic and biotic stresses. Physiol. Plant 2021, 171, 785–801. [Google Scholar] [CrossRef]
- Anower, M.R.; Peel, M.D.; Mott, I.W.; Wu, Y. Physiological processes associated with salinity tolerance in an alfalfa half-sib family. J. Agron. Crop. Sci. 2017, 203, 506–518. [Google Scholar] [CrossRef]
- Sairam, R.K.; Rao, K.V.; Srivastava, G.C. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.K.; Tripathi, D.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M. Reactive oxygen species (ROS): Beneficial companions of plants’ developmental processes. Front. Plant Sci. 2016, 7, 1299. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Ann. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Ashrafi, E.; Razmjoo, J.; Zahedi, M.; Pessarakli, M. Screening alfalfa for salt tolerance based on lipid peroxidation and antioxidant enzymes. Agron. J. 2015, 107, 167–173. [Google Scholar] [CrossRef]
- Hou, C.; Li, X.; Tian, D.; Xu, B.; Zhang, C.; Ren, J.; Chen, N. Evaluation of the effects of water and salinity stress on the growth and biochemistry of alfalfa (Medicago sativa L.) at the branching stage. Sustainability 2022, 14, 10262. [Google Scholar] [CrossRef]
- Dewhirst, R.A.; Fry, S.C. The oxidation of dehydroascorbic acid and 2,3-diketogulonate by distinct reactive oxygen species. Biochem. J. 2018, 475, 3451–3470. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Karalija, E.; Šola, I.; Bok, V.V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Yates, P.S.; Roberson, J.; Ramsue, L.K.; Song, B.-H. Bridging the gaps between plant and human health: A systematic review of soyasaponins. J. Agric. Food Chem. 2021, 69, 14387–14401. [Google Scholar] [CrossRef]
- Bian, S.; Jiang, Y. Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Sci. Hort. 2009, 120, 264–270. [Google Scholar] [CrossRef]
- Luna, C.M.; Pastori, G.M.; Driscoll, S.; Groten, K.; Bernard, S.; Foyer, C.H. Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J. Exp. Bot. 2004, 56, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.D.; Subedi, U.; Lehmann, M.; Burton Hughes, K.; Feyissa, B.A.; Hannoufa, A.; Shan, B.; Chen, G.; Kader, K.; Ortega Polo, R.; et al. Identification of differential drought response mechanisms in Medicago sativa subsp. sativa and falcata through comparative assessments at the physiological, biochemical, and transcriptional levels. Plants 2021, 10, 2107. [Google Scholar] [CrossRef] [PubMed]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Valderrama, R.; Mata-Pérez, C.; Padilla, M.N.; Corpas, F.J.; Barroso, J.B. Antioxidant systems are regulated by nitric oxide-mediated post-translational modifications (NO-PTMs). Front. Plant Sci. 2016, 7, 152. [Google Scholar] [CrossRef]
- Palma, J.M.; Mateos, R.M.; Lopez-Jaramillo, F.J.; Ruiz, M.R.; González-Gordo, S.; Lechuga-Sancho, A.; Corpas, F.J. Plant catalases as NO and H2S targets. Redox Biol. 2020, 34, 101525. [Google Scholar] [CrossRef]
- Li, D.-M.; Nie, Y.-X.; Zhang, J.; Yin, J.-S.; Wang, X.-J.; Bai, J.-G. Ferulic acid pretreatment enhances dehydration-stress tolerance of cucumber seedlings. Biol. Plant 2013, 57, 711–717. [Google Scholar] [CrossRef]
- Yildiztugay, E.; Ozfidan-Konacki, C.; Karahan, H.; Kucukoduk, M.; Turkan, I. Ferulic acid confers tolerance against excess boron by regulating ROS levels and inducing antioxidant system in wheat leaves (Triticum aestivum). Environ. Exp. Bot. 2019, 161, 193–202. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020, 25, 2227–2230. [Google Scholar] [CrossRef] [PubMed]
- Holsteens, K.; Jaegere, I.D.; Wynants, A.; Prinsen, E.L.J.; de Poel, B.V. Mild and severe salt stress responses are age-dependently regulated by abscisic acid in tomato. Front. Plant Sci. 2022, 13, 982622. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.M.; Freund, M.; Wager, B.M.; Knoblauch, M.; Fromm, J.; Mueller, H.M.; Ache, P.; Krischke, M.; Mueller, M.J.; Müller, T.; et al. Under salt stress guard cells rewire ion transport and abscisic acid signaling. New Phytol. 2021, 231, 1040–1055. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic acid biosynthesis in plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef]
- Bao, A.-K.; Du, B.-Q.; Touil, L.; Kang, P.; Wang, Q.-L.; Wang, S.-M. Co-expression of tonoplast cation/H+ antiporter and H+-pyrophosphatase from xerophyte Zygophyllum xanthoxylum improves alfalfa plant growth under salinity, drought and field conditions. Plant Biotechnol. J. 2015, 14, 964–975. [Google Scholar] [CrossRef]
- Liu, X.-P.; Hawkins, C.; Peel, M.D.; Yu, L.-Y. Genetic loci associated with salt tolerance in advanced breeding populations of tetraploid alfalfa using genome-wide association studies. Plant Genome 2019, 12, 180026. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2020, 251, 3. [Google Scholar] [CrossRef]
- Gao, Y.; Long, R.; Kang, J.; Wang, Z.; Zhang, T.; Sun, H.; Li, X.; Yang, Q. Comparative proteomic analysis reveals that antioxidant system and soluble sugar metabolism contribute to salt tolerance in alfalfa (Medicago sativa L.) leaves. J. Proteome Res. 2019, 18, 191–203. [Google Scholar] [CrossRef]
- Azizpour, K.; Shakiba, M.R.; Khosh Kholg Sima, N.A.; Alvari, H.; Mogaddam, M.; Esfandiari, E.; Pessarakli, M. Physiological response of spring durum wheat genotypes to salinity. J. Plant Nutr. 2009, 33, 859–873. [Google Scholar] [CrossRef]
- Praxedes, S.C.; de Lacerda, C.F.; Ferreira, T.M.; Prisco, J.T.; DaMatta, F.M.; Gomes-Filho. Salt tolerance is unrelated to carbohydrate metabolism in cowpea cultivars. Acta Physiol. Plant 2011, 33, 887–896. [Google Scholar] [CrossRef]
- Ponnu, J.; Wahl, V.; Schmid, M. Trehalose-6-phosphate: Connecting plant metabolism and development. Front. Plant Sci. 2011, 2, 70. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-W.; Zang, B.-S.; Deng, X.-W.; Wang, X.-P. Overexpression of the trehaolose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice. Planta 2011, 234, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Suárez, R.; Calderón, C.; Iturriaga, G. Enhanced tolerance to multiple abiotic stresses in transgenic alfalfa accumulating trehalose. Crop. Sci. 2009, 49, 1791–1799. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Ahn, C.-H.; Hossain, A.; Lee, E.; Kanth, B.K.; Park, P.B. Increased salt and drought tolerance by D-pinitol production in transgenic Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2018, 504, 315–320. [Google Scholar] [CrossRef]
- Hu, L.; Zhou, K.; Liu, Y.; Yang, S.; Zhang, J.; Gong, X.; Ma, F. Overexpression of MdMIPS1 enhances salt tolerance by improving osmosis, ion balance, and antioxidant activity in transgenic apple. Plant Sci. 2020, 301, 110654. [Google Scholar] [CrossRef]
- Kusuda, H.; Koga, W.; Kusano, M.; Oikawa, A.; Saito, K.; Hirai, M.Y.; Yoshida, K.T. Ectopic expression of myo-inositol 3-phosphate synthase induces a wide range of metabolic changes and confers salt tolerance in rice. Plant Sci. 2015, 232, 49–56. [Google Scholar] [CrossRef]
- Li, Y.; Ding, M.; Cui, C.; An, Q.; Wu, J.; Zhou, G.; Wan, Y.; Bao, W. Overexpression of a gene encoding trigonelline synthase from Areca catechu L. promotes drought resilience in transgenic Arabidopsis. Plants 2022, 11, 487. [Google Scholar] [CrossRef]
- Morales, A.J.; Bajgain, P.; Garver, Z.; Maughan, P.J.; Udall, J.A. Physiological responses of Chenopodium quinoa to salt stress. Int. J. Plant Physiol. Biochem. 2011, 3, 219–232. [Google Scholar]
- Bertrand, A.; Gatzke, C.; Bipfubusa, M.; Lévesque, V.; Chalifour, F.P.; Claessens, A.; Rocher, S.; Tremblay, G.F.; Beauchamp, C.J. Physiological and Biochemical Responses to Salt Stress of Alfalfa Populations Selected for Salinity Tolerance and Grown in Symbiosis with Salt-Tolerant Rhizobium. Agronomy 2020, 10, 569. [Google Scholar] [CrossRef]
- Ratiu, I.A.; Al-Suod, H.; Ligor, M.; Monedeiro, F.; Buszewski, B. Effects of growth conditions and cultivability on the content of cyclitols in Medicago sativa. Int. J. Environ. Sci. Technol. 2021, 18, 33–48. [Google Scholar] [CrossRef]
- Tramontano, W.A.; Jouve, D. Trigonelline accumulation in salt-stressed legumes and the role of other osmoregulators as cell cycle control agents. Phytochemistry 1997, 44, 1037–1040. [Google Scholar] [CrossRef]
- Rai, A.N.; Penna, S. Molecular evolution of plant P5C5 gene involved in proline biosynthesis. Mol. Biol. Rep. 2013, 40, 6429–6435. [Google Scholar] [CrossRef]
- Farissi, M.; Ghoulam, C.; Bouizgaren, A. Changes in water deficit saturation and photosynthetic pigments of alfalfa populations under salinity and assessment of proline role in salt tolerance. Agric. Sci. Res. 2013, 3, 29–35. [Google Scholar]
- Torabi, M.; Halim, M.R.A. Variation of root and shoot growth and free proline accumulation in Iranian alfalfa ecotypes under salt stress. J. Food Agric. Environ. 2010, 8, 323–327. [Google Scholar]
- Guan, C.; Huang, Y.-H.; Cui, X.; Liu, S.-J.; Zhou, Y.-Z.; Zhang, Y.-W. Overexpression of gene encoding the key enzyme involved in proline-biosynthesis (PuP5CS) to improve salt tolerance in switchgrass (Panicum virgatuum L.). Plant Cell Rep. 2018, 37, 1187–1199. [Google Scholar] [CrossRef]
- Hmida-Sayari, A.; Gargouri-Bouzid, R.; Bidani, A.; Jaoua, L.; Savouré, A.; Jaoua, S. Overexpression of Δ1-pyrroline-5-carboxylate synthetase increases proline production and confers salt tolerance in transgenic potato plants. Plant Sci. 2005, 169, 746–752. [Google Scholar] [CrossRef]
- Surekha, C.; Kumari, K.N.; Aruna, L.V.; Suneetha, G.; Arundhati, A.; Kishor, P.B.K. Expression of the Vigna aconitifolia P5CSF129A gene in transgenic pigeonpea enhances proline accumulation and salt tolerance. Plant Cell Tissue Organ Cult. 2014, 116, 27–36. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.-P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef]
- Rai, V.K. Role of amino acids in plant responses to stresses. Biol. Plant 2002, 45, 481–487. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Zhou, M.; Shabala, S. How does stomatal density and residual transpiration contribute to osmotic stress tolerance? Plants 2023, 12, 494. [Google Scholar] [CrossRef] [PubMed]
- Bourdenx, B.; Bernard, A.; Domergue, F.; Pascal, S.; Léger, A.; Roby, D.; Pervent, M.; Vile, D.; Haslam, R.P.; Napier, J.A.; et al. Overexpression of Arabidopsis ECERIFERUM1 promotes wax very-long-chain alkane biosynthesis and influences plant response to biotic and abiotic stresses. Plant Physiol. 2011, 156, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.B.; Suh, M.C. Arabidopsis 3-ketoacyl-CoA synthase 4 is essential for root and pollen tube growth. J. Plant Biol. 2021, 64, 155–165. [Google Scholar] [CrossRef]
- Lü, S.; Song, T.; Kosma, D.K.; Parsons, E.P.; Rowland, O.; Jenks, M.A. Arabidopsis CER8 encodes LONG-CHAIN ACYL-COA SYNTHETASE 1 (LACS1) that has overlapping functions with LACS2 in plant wax and cutin synthesis. Plant J. 2009, 59, 553–564. [Google Scholar] [CrossRef]
- Ge, S.; Liu, D.; Chu, M.; Liu, X.; Wei, Y.; Che, X.; Zhu, L.; He, L.; Xu, J. Dynamic and adaptive membrane lipid remodeling in leaves of sorghum under salt stress. Crop. J. 2022, 10, 1557–1569. [Google Scholar] [CrossRef]
- Tasseva, G.; Richard, L.; Zachowski, A. Regulation of phosphatidylcholine biosynthesis under salt stress involves choline kinases in Arabidopsis thaliana. FEBS Lett. 2004, 566, 115–120. [Google Scholar] [CrossRef]
- Xiao, R.; Zou, Y.; Guo, X.; Li, H.; Lu, H. Fatty acid desaturases (FADs) modulate multiple lipid metabolism pathways to improve plant resistance. Mol. Biol. Rep. 2022, 49, 9997–10011. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.-N.; Meng, Q.; Fan, H.; Sui, N. The roles of chloroplast membrane lipids in abiotic stress responses. Plant Signal. Behav. 2020, 15, 1807152. [Google Scholar] [CrossRef]
- Hong, Y.; Zhang, W.; Wang, X. Phospholipase D and phosphatidic acid signalling in plant responses to drought and salinity. Plant Cell Environ. 2010, 33, 627–635. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Hassan, F.A.S. How salt stress-responsive proteins regulate plant adaptation to saline conditions. Plant Mol. Biol. 2021, 108, 175–224. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.-H.; Zhang, H.-X.; Ali, M.; Gai, W.-X.; Cheng, G.-X.; Yu, Q.-H.; Yang, S.-B.; Li, X.-X.; Gong, Z.-H. A small heat shock protein CaHsp25.9 positively regulates heat, salt, and drought stress tolerance in pepper (Capsicum annuum L.). Plant Physiol. Biochem. 2019, 142, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Lim, S.; Baek, W.; Lee, S.C. The pepper late embryogenesis abundant protein CaLEA1 acts in regulating abscisic acid signaling, drought and salt stress response. Physiol. Plant 2015, 154, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Mishra, S.; Awasthi, J.P.; Sahoo, L.; Panda, S.K. Enhanced drought and salinity tolerance in transgenic mustard [Brassica juncea (L.) Czern & Coss.] overexpressing Arabidopsis group 4 late embryogenesis abundant gene (AtLEA4-1). Environ. Exp. Bot. 2016, 128, 99–111. [Google Scholar]
- Wang, A.; Yu, X.; Mao, Y.; Liu, Y.; Liu, G.; Liu, Y.; Niu, X. Overexpression of a small heat-shock-protein gene enhances tolerance to abiotic stresses in rice. Plant Breed. 2015, 134, 384–393. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, Z.; Zhang, D.; Zhang, J.; Di, H.; Wu, F.; Wang, Y. Co-transforming bar and CsLEA enhanced tolerance to drought and salt stress in transgenic alfalfa (Medicago sativa L.). Biochem. Biophys. Res. Commun. 2016, 472, 75–82. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, Q.; Kang, J.; Sun, Y.; Gruber, M.; Chao, Y. Isolation and functional characterization of a Medicago sativa L. gene, MsLEA3-1. Mol. Biol. Rep. 2012, 39, 2883–2892. [Google Scholar] [CrossRef]
- Lee, K.-W.; Cha, J.-Y.; Kim, K.-H.; Kim, Y.-G.; Lee, B.-H.; Lee, S.-H. Overexpression of alfalfa mitochondrial HSP23 in prokaryotic and eukaryotic model systems confers enhanced tolerance to salinity and arsenic stress. Biotechnol. Lett. 2012, 34, 167–174. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Yang, Q.; Fang, F.; Kang, J.; Zhang, T. Isolation and characterization of a gene from Medicago sativa L., encoding a bZIP transcription factor. Mol. Biol. Rep. 2013, 40, 1227–1239. [Google Scholar] [CrossRef]
- Song, Y.; Lv, J.; Qiu, N.; Bai, Y.; Yang, N.; Dong, W. The constitutive expression of alfalfa MsMYB2L enhances salinity and drought tolerance of Arabidopsis thaliana. Plant Physiol. Biochem. 2019, 141, 300–305. [Google Scholar] [CrossRef]
- Chen, T.; Yang, Q.; Zhang, X.; Ding, W.; Gruber, M. An alfalfa (Medicago sativa L.) ethylene response factor gene, MsERF11, enhances salt tolerance in transgenic Arabidopsis. Plant Cell Rep. 2012, 31, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yang, Q.; Gruber, M.; Kang, J.; Sun, Y.; Ding, W.; Zhang, T.; Zhang, X. Expression of an alfalfa (Medicago sativa L.) ethylene response factor gene MsERF8 in tobacco plants enhances resistance to salinity. Mol. Biol. Rep. 2012, 39, 6067–6075. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, L.; Chen, J.; Tao, L.; An, Y.; Cai, H.; Guo, C. Overexpression of the alfalfa WRKY11 gene enhances salt tolerance in soybean. PLoS ONE 2018, 13, e0192382. [Google Scholar] [CrossRef] [PubMed]
- Boodley, J.W.; Sheldrake, R., Jr. Cornell Peat-Lite Mixes for Commercial Plant Growing; New York State College of Agriculture and Life Sciences, Cornell University: Ithaca, NY, USA, 1972. [Google Scholar]
- Singer, S.D.; Burton Hughes, K.; Subedi, U.; Dhariwal, G.K.; Kader, K.; Acharya, S.; Chen, G.; Hannoufa, A. The CRISPR/Cas9-mediated modulation of SQUAMOSA PROMOTER-BINDING PROTEIN-LIKE 8 in alfalfa leads to distinct phenotypic outcomes. Front. Plant Sci. 2022, 12, 774146. [Google Scholar] [CrossRef]
- Ewels, P.A.; Peltzer, A.; Filinger, S.; Patel, H.; Alneberg, J.; Wilm, A.; Garcia, M.U.; Di Tommaso, P.; Nahnsen, S. The nf-core framework for community-curated bioinformatics pipelines. Nat. Biotechnol. 2020, 38, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Ewels, P.; Peltzer, A.; Botvinnik, O.; Sturm, G.; Moreno, D.; Vemuri, P.; silviamorins; Pantano, L.; Binzer-Panchal, M.; et al. nf-core/rnaseq: Nf-core/rnaseq v3.10.1—Plastered Rhodium Rudolph (3.10.1). Zenodo. 2023. Available online: https://zenodo.org/record/7505987#.ZGtEprdByUk (accessed on 28 July 2021).
- Di Tommaso, P.; Chatzou, M.; Floden, E.W.; Barja, P.P.; Palumbo, E.; Notredame, C. Nextflow enables reproducible computational workflows. Nat. Biotechnol. 2017, 35, 316–319. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Li, W. RSeQC: Quality control of RNA-seq experiments. Bioinformatics 2012, 28, 2184–2185. [Google Scholar] [CrossRef]
- García-Alcalde, F.; Okonechnikov, K.; Carbonell, J.; Cruz, L.M.; Götz, S.; Tarazona, S.; Dopazo, J.; Meyer, T.F.; Conesa, A. Qualimap: Evaluating next-generation sequencing alignment data. Bioinformatics 2012, 28, 2678–2679. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2009, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wilke, C.O.; Wickham, H.; Wilke, M.C.O. Package ‘Cowplot’. Streamlined Plot Theme and Plot Annotations for ‘ggplot2’. 2019. Available online: https://cran.r-project.org/web/packages/cowplot/index.html (accessed on 26 September 2022).
- Tian, T.; Liu, Y.; Yan, H.; You, Q.; Yi, X.; Du, Z.; Xu, W.; Su, Z. agriGO v2.0: A GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 2017, 45, W122–W129. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fu, Y.; Ban, L.; Wang, Z.; Feng, G.; Li, J.; Gao, H. Selection of reliable reference genes for quantitative real-time RT-PCR in alfalfa. Genes Genet. Syst. 2015, 90, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ideda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Yu, H.; Huan, T. MAFFIN: Metabolomics sample normalization using maximal density fold change with high-quality metabolic features and corrected signal intensities. Bioinformatics 2022, 38, 3429–3437. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists. Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 His Majesty the King in Right of Canada. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singer, S.D.; Lehmann, M.; Zhang, Z.; Subedi, U.; Burton Hughes, K.; Lim, N.Z.-L.; Ortega Polo, R.; Chen, G.; Acharya, S.; Hannoufa, A.; et al. Elucidation of Physiological, Transcriptomic and Metabolomic Salinity Response Mechanisms in Medicago sativa. Plants 2023, 12, 2059. https://doi.org/10.3390/plants12102059
Singer SD, Lehmann M, Zhang Z, Subedi U, Burton Hughes K, Lim NZ-L, Ortega Polo R, Chen G, Acharya S, Hannoufa A, et al. Elucidation of Physiological, Transcriptomic and Metabolomic Salinity Response Mechanisms in Medicago sativa. Plants. 2023; 12(10):2059. https://doi.org/10.3390/plants12102059
Chicago/Turabian StyleSinger, Stacy D., Madeline Lehmann, Zixuan Zhang, Udaya Subedi, Kimberley Burton Hughes, Nathaniel Z.-L. Lim, Rodrigo Ortega Polo, Guanqun Chen, Surya Acharya, Abdelali Hannoufa, and et al. 2023. "Elucidation of Physiological, Transcriptomic and Metabolomic Salinity Response Mechanisms in Medicago sativa" Plants 12, no. 10: 2059. https://doi.org/10.3390/plants12102059
APA StyleSinger, S. D., Lehmann, M., Zhang, Z., Subedi, U., Burton Hughes, K., Lim, N. Z.-L., Ortega Polo, R., Chen, G., Acharya, S., Hannoufa, A., & Huan, T. (2023). Elucidation of Physiological, Transcriptomic and Metabolomic Salinity Response Mechanisms in Medicago sativa. Plants, 12(10), 2059. https://doi.org/10.3390/plants12102059








