Silver Nanoparticles of Artemisia sieberi Extracts: Chemical Composition and Antimicrobial Activities
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of A. sieberi
2.2. Biosynthesis and Characterization of A. sieberi AgNPs
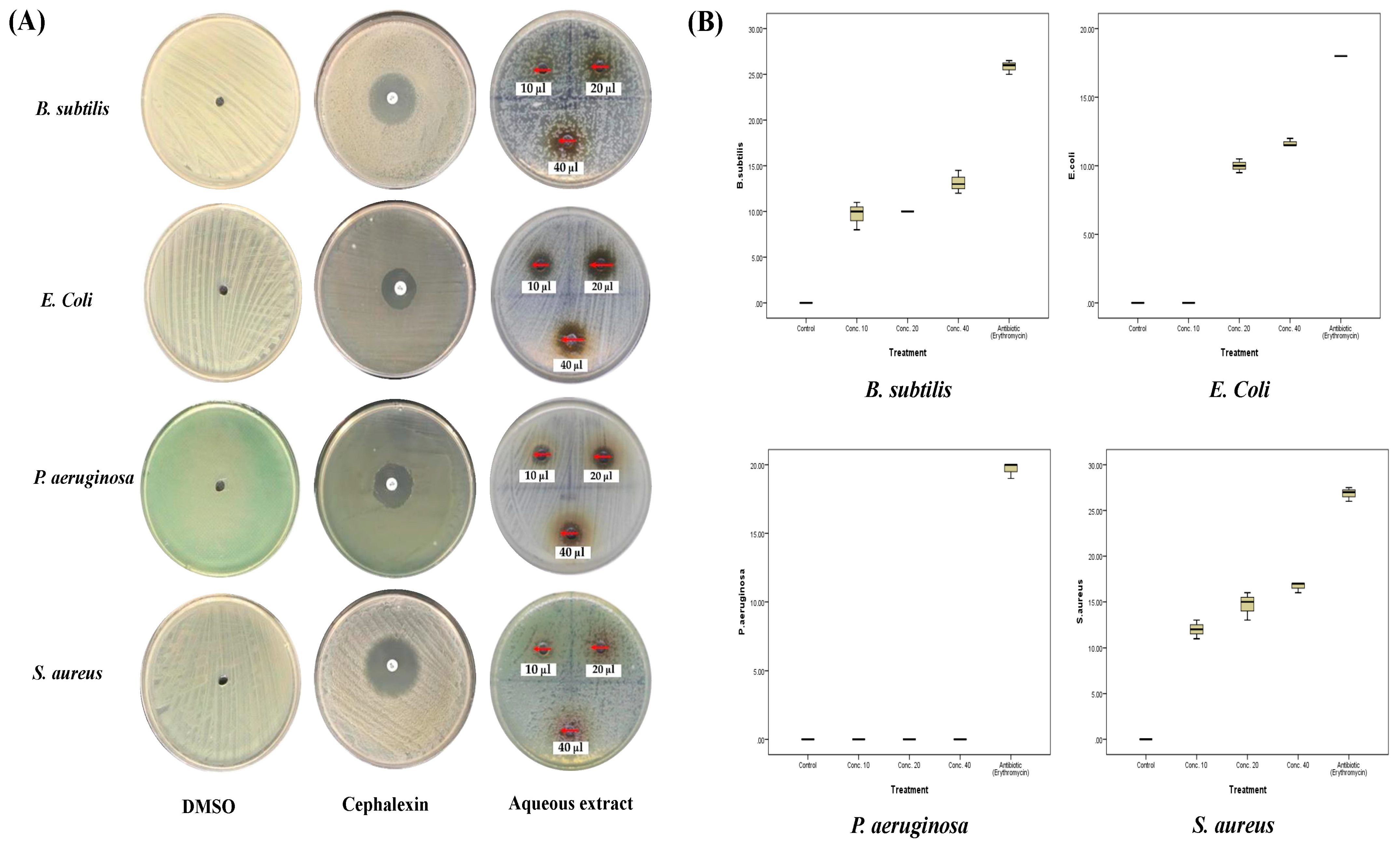
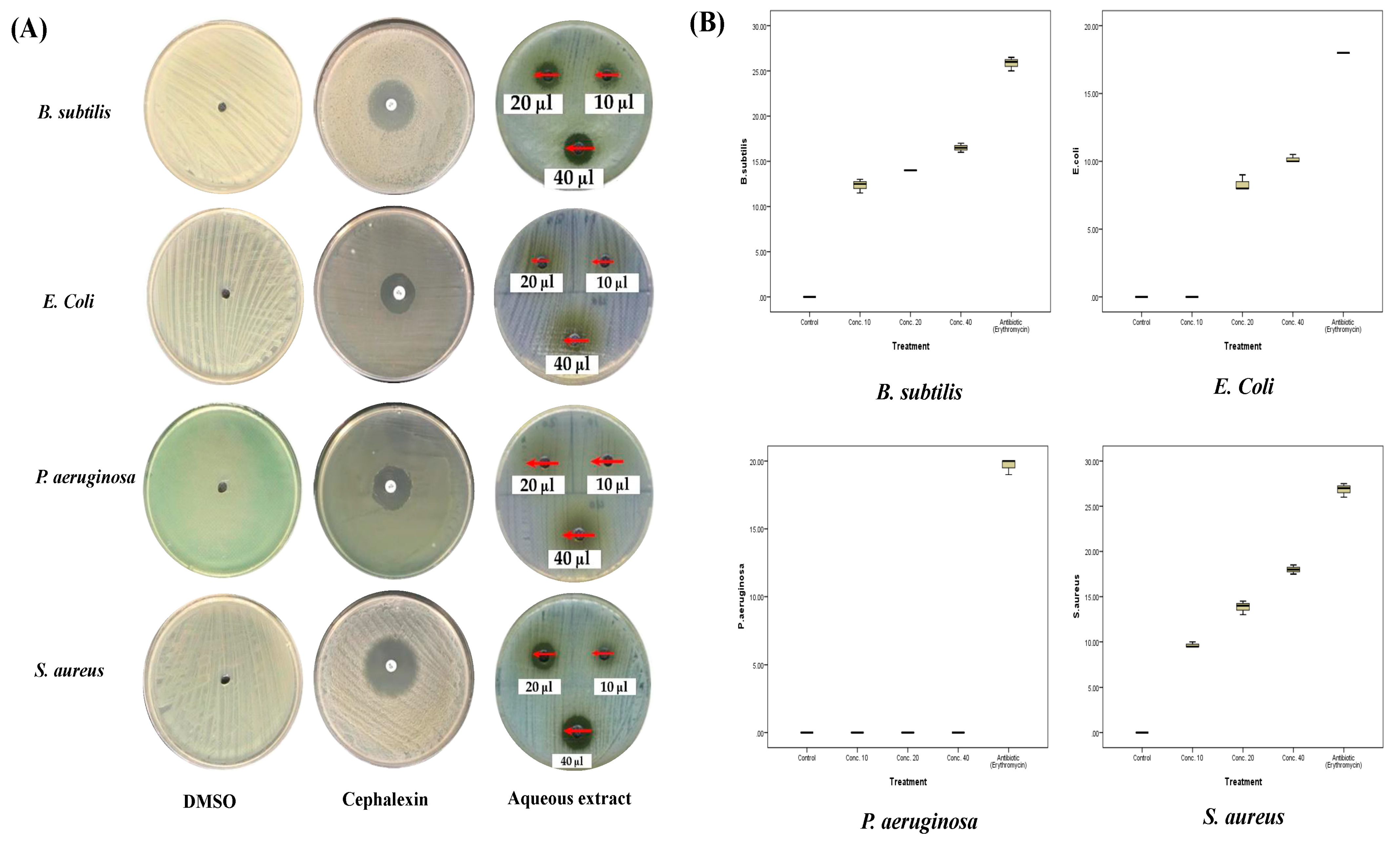
2.3. Antibacterial Activities of A. sieberi
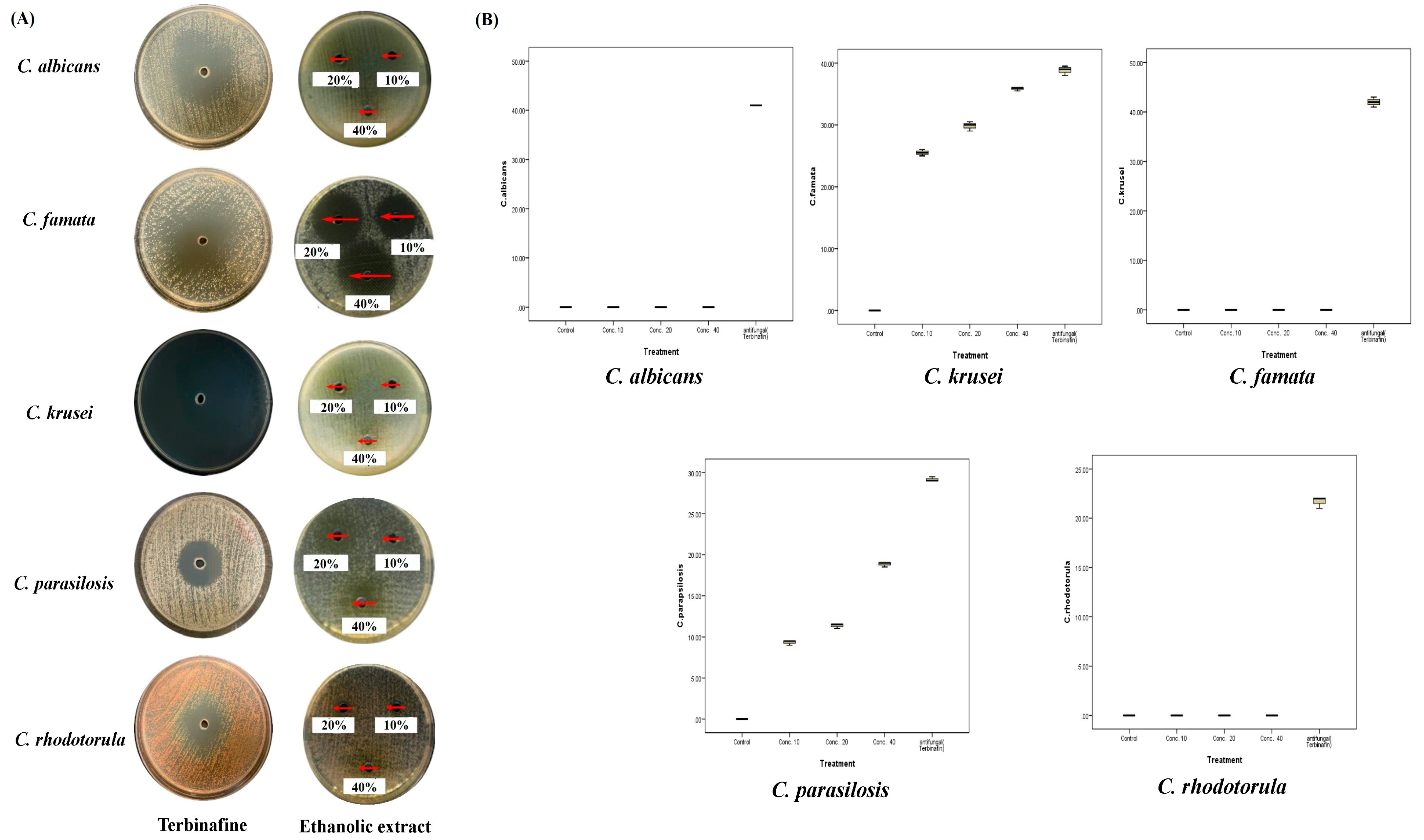
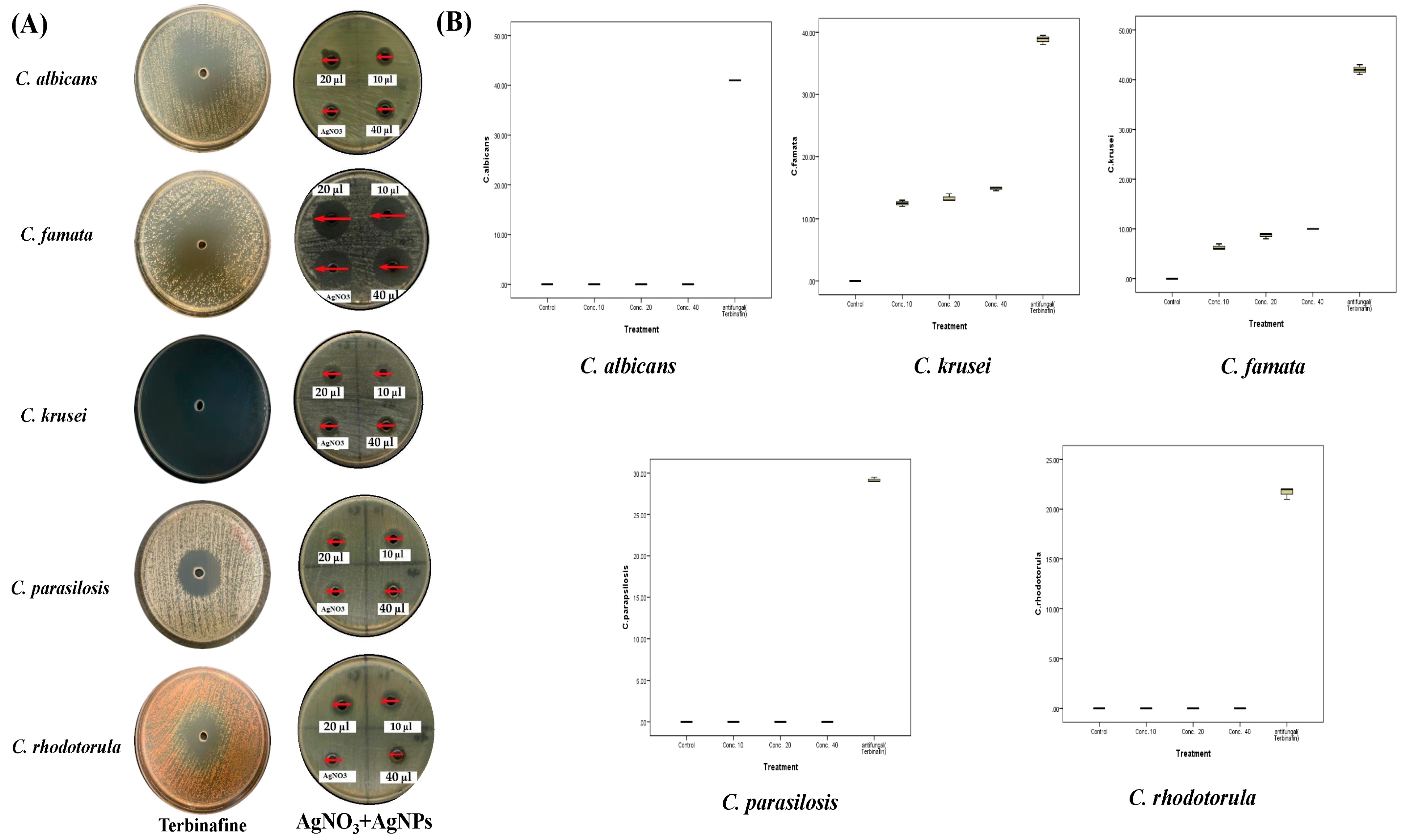
2.4. Antifungal Activities of A. sieberi
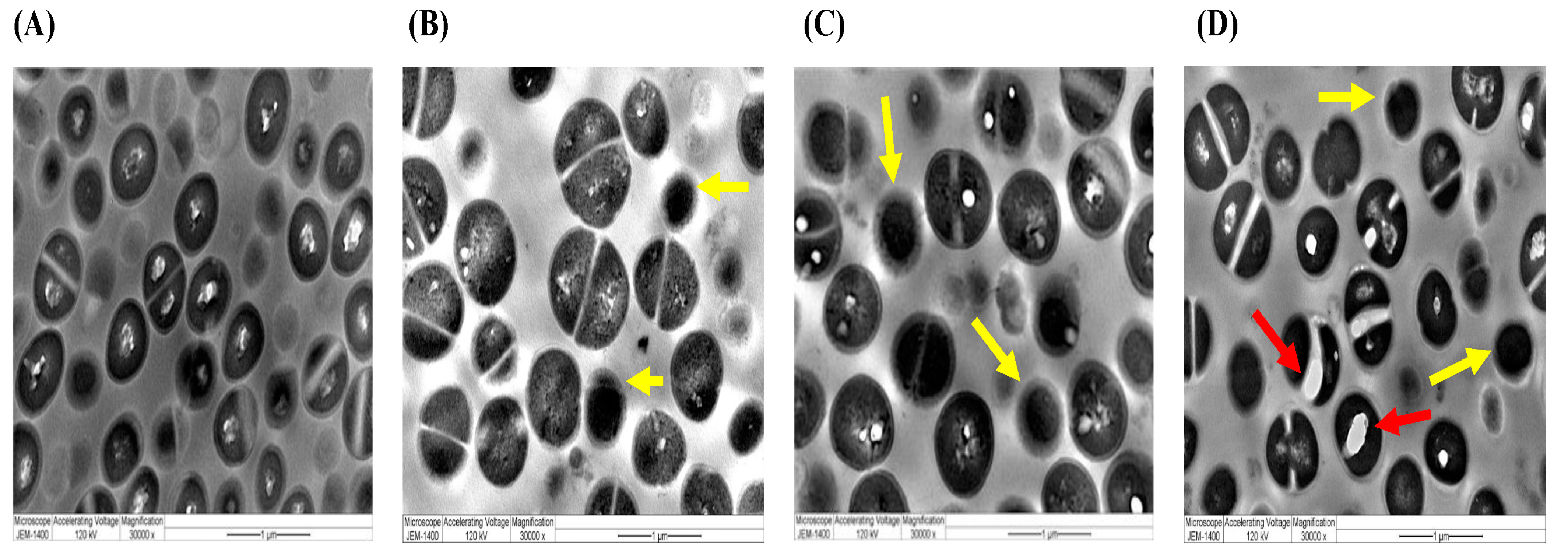
2.5. Ultrastructural Characterizations by TEM of Selected Species Treated with Different Preparations of A. sieberi
3. Discussion
4. Materials and Methods
4.1. Plant Material and Preparation of Extracts
4.2. Biosynthesis and Characterization of Silver Nanoparticles
4.3. Microbial Strains
4.4. Gas Chromatography–Mass Spectrometry (GC-MS)
4.5. Fourier-Transfigure Infrared Spectroscopy (FTIR)
4.6. Growth Inhibition Assay
4.7. Determination of the Effect of Plant Extracts on the Morphology and Ultrastructure of the Organisms under Study
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batiha, G.E.-S.; Beshbishy, A.M.; Tayebwa, D.S.; Adeyemi, O.S.; Yokoyama, N.; Igarashi, I. Anti-piroplasmic potential of the methanolic Peganum harmala seeds and ethanolic Artemisia absinthium leaf extracts. J. Protozool. Res. 2019, 29, 8–25. [Google Scholar]
- Batiha, G.E.-S.; Olatunde, A.; El-Mleeh, A.; Hetta, H.F.; Al-Rejaie, S.; Alghamdi, S.; Zahoor, M.; Magdy Beshbishy, A.; Murata, T.; Zaragoza-Bastida, A.; et al. Bioactive Compounds, Pharmacological Actions, and Pharmacokinetics of Wormwood (Artemisia absinthium). Antibiotics 2020, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Aati, H.Y.; Perveen, S.; Orfali, R.; Al-Taweel, A.M.; Aati, S.; Wanner, J.; Khan, A.; Mehmood, R. Chemical composition and antimicrobial activity of the essential oils of Artemisia absinthium, Artemisia scoparia, and Artemisia sieberi grown in Saudi Arabia. Arab. J. Chem. 2020, 13, 8209–8217. [Google Scholar] [CrossRef]
- Bunse, M.; Daniels, R.; Gründemann, C.; Heilmann, J.; Kammerer, D.R.; Keusgen, M.; Lindequist, U.; Melzig, M.F.; Morlock, G.E.; Schulz, H.; et al. Essential oils as multicomponent mixtures and their potential for human health and well-being. Front. Pharmacol. 2022, 13, 956541. [Google Scholar] [CrossRef] [PubMed]
- Bisht, D.; Kumar, D.; Kumar, D.; Dua, K.; Chellappan, D.K. Phytochemistry and pharmacological activity of the genus artemisia. Arch. Pharm. Res. 2021, 44, 439–474. [Google Scholar] [CrossRef]
- Szopa, A.; Pajor, J.; Klin, P.; Rzepiela, A.; Elansary, H.O.; Al-Mana, F.A.; Mattar, M.A.; Ekiert, H. Artemisia absinthium L.-importance in the history of medicine, the latest advances in phytochemistry and therapeutical, cosmetological and culinary uses. Plants 2020, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shan, N.N.; Sui, X.H. Research Progress on Artemisinin and Its Derivatives against Hematological Malignancies. Chin. J. Integr. Med. 2020, 26, 947–955. [Google Scholar] [CrossRef]
- Lam, N.S.; Long, X.; Wong, J.W.; Griffin, R.C.; Doery, J.C.G. Artemisinin and its derivatives: A potential treatment for leukemia. Anti-Cancer Drugs 2019, 30, 1–18. [Google Scholar] [CrossRef]
- Sahu, N.; Meena, S.; Shukla, V.; Chaturvedi, P.; Kumar, B.; Datta, D.; Arya, K.R. Extraction, fractionation and re-fractionation of Artemisia nilagirica for anticancer activity and HPLC-ESI-QTOF-MS/MS determination. J. Ethnopharmacol. 2018, 213, 72–80. [Google Scholar] [CrossRef]
- Parham, S.; Wicaksono, D.H.; Bagherbaigi, S.; Lee, S.L.; Nur, H. Antimicrobial treatment of different metal oxide nanoparticles: A critical review. J. Chin. Chem. Soc. 2016, 63, 385–393. [Google Scholar] [CrossRef]
- Abbas, Z.K.; Saggu, S.; Rehman, H.; Al Thbiani, A.; Ansari, A.A. Ecological variations and role of heat shock protein in Artemisia judaica L. in response to temperature regimes of Tabuk, Saudi Arabia. Saudi J. Biol. Sci. 2017, 24, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef] [PubMed]
- Pokrajac, L.A.; Abbas, A.; Chrzanowski, W.; Dias, G.M.; Eggleton, B.J.; Maguire, S.; Maine, E.; Malloy, T.F.; Nathwani, J.; Nazar, L.; et al. Nanotechnology for a sustainable future: Addressing global challenges with the international network for sustainable nanotechnology. ACS Nano 2021, 15, 18608–18623. [Google Scholar] [CrossRef] [PubMed]
- Almatroudi, A. Silver nanoparticles: Synthesis, characterisation and biomedical applications. Open Life Sci. 2020, 15, 819–839. [Google Scholar] [CrossRef]
- Dewi, M.K.; Chaerunisaa, A.Y.; Muhaimin, M.; Joni, I.M. Improved Activity of Herbal Medicines through Nanotechnology. Nanomaterials 2022, 12, 4073. [Google Scholar] [CrossRef] [PubMed]
- Huq, M.A. Paenibacillus anseongense sp. nov. a Silver Nanoparticle Producing Bacterium Isolated from Rhizospheric Soil. Curr. Microbiol. 2020, 77, 2023–2030. [Google Scholar] [CrossRef]
- Huq, M.A. Biogenic silver nanoparticles synthesized by Lysinibacillus xylanilyticus MAHUQ-40 to control antibiotic-resistant human pathogens Vibrio parahaemolyticus and Salmonella Typhimurium. Front. Bioeng. Biotechnol. 2020, 8, 597502. [Google Scholar] [CrossRef]
- Bonifácio, B.V.; Silva, P.B.; Ramos, M.A.; Negri, K.M.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar] [CrossRef]
- Sinico, C.; De Logu, A.; Lai, F.; Valenti, D.; Manconi, M.; Loy, G.; Bonsignore, L.; Fadda, A.M. Liposomal incorporation of Artemisia arborescens L. essential oil and in vitro antiviral activity. Eur. J. Pharm. Biopharm. 2005, 59, 161–168. [Google Scholar] [CrossRef]
- Malapermal, V.; Botha, I.; Krishna, S.B.N.; Mbatha, J.N. Enhancing antidiabetic and antimicrobial performance of Ocimum basilicum, and Ocimum sanctum (L.) using silver nanoparticles. Saudi J. Biol. Sci. 2017, 24, 1294–1305. [Google Scholar] [CrossRef]
- Afzal, O.; Altamimi, A.S.A.; Nadeem, M.S.; Alzarea, S.I.; Almalki, W.H.; Tariq, A.; Mubeen, B.; Murtaza, B.N.; Iftikhar, S.; Riaz, N.; et al. Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials 2022, 12, 4494. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Wirth, S.; Behrendt, U.; Ahmad, P.; Berg, G. Antimicrobial Activity of Medicinal Plants Correlates with the Proportion of Antagonistic Endophytes. Front. Microbiol. 2017, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Rousta, M.H.; Ghasemi, N. Green synthesis of silver nanoparticles using a mountain plant extract. Rev. Roum. Chim. 2019, 64, 143–152. [Google Scholar] [CrossRef]
- Salmen, S.H.; Alkammash, N.M.; Alahmadi, T.A.; Alharbi, S.A. Characterization and antibacterial activity of silver nanoparticles biosynthesized using leaves extract of Artemisia sieberi and Calotropis procera. Rev. Chim. 2021, 72, 76–82. [Google Scholar] [CrossRef]
- Alotibi, F.O.; Rizwana, H. Chemical composition, FTIR studies, morphological alterations, and antifungal activity of leaf extracts of Artemisia sieberi from Saudi Arabia. Int. J. Agric. Biol. 2019, 21, 1241–1248. [Google Scholar] [CrossRef]
- Jamroży, M.; Głąb, M.; Kudłacik-Kramarczyk, S.; Drabczyk, A.; Gajda, P.; Tyliszczak, B. The Impact of the Matricaria chamomilla L. Extract, Starch Solution and the Photoinitiator on Physiochemical Properties of Acrylic Hydrogels. Materials 2022, 15, 2837. [Google Scholar] [CrossRef]
- Rostami-Vartooni, A.; Moradi-Saadatmand, A.; Bagherzadeh, M.; Mahdavi, M. Green synthesis of Ag/Fe3O4/ZrO2 nanocomposite using aqueous Centaurea cyanus flower extract and its catalytic application for reduction of organic pollutants. Iran. J. Catal. 2019, 9, 27–35. [Google Scholar]
- Zaman, S.; Barkatulllah; Zahoor, M.; Wadood Ali Shah, S.; Ullah, Z.; Ullah, R.; Alotaibi, A. Pharmacognostic evaluation of Artemisia maritima L. a highly medicinal specie of genus Artemisia. Saudi J. Biol. Sci. 2022, 29, 103419. [Google Scholar] [CrossRef]
- Rather, M.A.; Dar, B.A.; Shah, W.A.; Prabhakar, A.; Bindu, K.; Banday, J.A.; Qurishia, M.A. spectroscopic analysis of the volatile aroma constituents of Artemisia indica and Artemisia vestita essential oils. Arab. J. Chem. 2017, 10, S3798–S3803. [Google Scholar] [CrossRef]
- Agustina, T.E.; Handayani, W.; Imawan, C. The UV-VIS spectrum analysis from silver nanoparticles synthesized using Diospyros maritima Blume leaves extract. In Proceedings of the 3rd KOBI Congress, International and National Conferences (KOBICINC 2020), Virtual, 24–25 November 2020; Atlantis Press: Amsterdam, The Netherlands, 2021; pp. 411–419. [Google Scholar] [CrossRef]
- Alkammash, N.M. Synthesis of silver nanoparticles from Artemisia Sieberi and Calotropis Procera medical plant extracts and their characterization using SEM analysis. Biosci. Biotech. Res. Asia 2017, 14, 521–526. [Google Scholar] [CrossRef]
- Ali, E.M.; Abdallah, B.M. Effective inhibition of invasive pulmonary aspergillosis by silver nanoparticles biosynthesized with Artemisia sieberi leaf extract. Nanomaterials 2021, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Aghajanyan, A.; Gabrielyan, L.; Schubert, R.; Trchounian, A. Silver ion bioreduction in nanoparticles using Artemisia annua L. extract: Characterization and application as antibacterial agents. AMB Express 2020, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Kisiriko, M.; Anastasiadi, M.; Terry, L.A.; Yasri, A.; Beale, M.H.; Ward, J.L. Phenolics from medicinal and aromatic plants: Characterisation and potential as biostimulants and bioprotectants. Molecules 2021, 26, 6343. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Miri, R.; Baldwin, I.T. Comparative hydrodistillation and headspace SPME-GC-MS analysis of volatile constituents of roots and shoots of Artemisia annua and Artemisia sieberi. Chem. Nat. Compd. 2014, 49, 1148–1153. [Google Scholar] [CrossRef]
- Ansari, S.; Shamshi, Y.; Khan, Q.A. A review of Artemisia absinthium, Linn. (afsanteen) with special reference of Unani medicine. J. Pharm. Sci. Innov. 2019, 8, 11–18. [Google Scholar] [CrossRef]
- Jaleel, G.A.R.A.; Abdallah, H.M.I.; Gomaa, N.E.S. Pharmacological effects of ethanol extract of Egyptian Artemisia herba-alba in rats and mice. Asian Pac. J. Trop. Biomed. 2016, 6, 44–49. [Google Scholar] [CrossRef]
- Simpson, B.W.; Trent, M.S. Pushing the envelope: LPS modifications and their consequences. Nat. Rev. Microbiol. 2019, 17, 403–416. [Google Scholar] [CrossRef]
- Khanam, Z.; Wen, C.S.; Bhat, I.U.H. Phytochemical screening and antimicrobial activity of root and stem extracts of wild Eurycoma longifolia Jack (Tongkat Ali). J. King Saud Univ. Sci. 2015, 27, 23–30. [Google Scholar] [CrossRef]
- Mohamed, T.A.; Hegazy, M.F.; Abd El Aty, A.A.; Ghabbour, H.A.; Alsaid, M.S.; Shahat, A.A.; Paré, P.W. Antimicrobial sesquiterpene lactones from Artemisia sieberi. J. Asian Nat. Prod. Res. 2017, 19, 1093–1101. [Google Scholar] [CrossRef]
- Ghasemi, G.; Alirezalu, A.; Ishkeh, S.R.; Ghosta, Y. Phytochemical properties of essential oil from Artemisia sieberi Besser (Iranian accession) and its antioxidant and antifungal activities. Nat. Prod. Res. 2021, 35, 4154–4158. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M.; Valian, M.; Kazempour, N. Chemical composition, antioxidant and antimicrobial activity of Artemisia sieberi oils from different parts of Iran and France. J. Essent. Oil Res. 2015, 27, 140–147. [Google Scholar] [CrossRef]
- Chokkareddy, R.; Redhi, G.G. Green synthesis of metal nanoparticles and its reaction mechanisms. In Green Metal Nanoparticles: Synthesis, Characterization and Their Applications; Kanchi, S., Ahmed, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 113–139. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Bulut, O.; Some, S.; Mndal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticle: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef]
- Prasad, R. Synthesis of silver nanoparticles in photosynthetic plants. J. Nanopart. 2014, 2014, 963961. [Google Scholar] [CrossRef]
- Chandran, K.; Song, S.; Yun, S. Effect of size and shape controlled biogenic synthesis of gold nanoparticles and their mode of interactions against food borne bacterial pathogens. Arab. J. Chem. 2014, 12, 1994–2006. [Google Scholar] [CrossRef]
- Gabrielyan, L.; Trchounian, A. Antibacterial activities of transient metals nanoparticles and membranous mechanisms of action. World J. Microbiol. Biotechnol. 2019, 35, 162. [Google Scholar] [CrossRef]
- Vega-Jiménez, A.L.; Vázquez-Olmos, A.R.; Acosta-Gío, E.; Antonio Álvarez-Pérez, M. In vitro antimicrobial activity evaluation of metal oxide nanoparticles. In Nanoemulsions—Properties, Fabrications and Applications; Koh, K.S., Wong, V.L., Eds.; IntechOpen: London, UK, 2019; pp. 13–30. [Google Scholar] [CrossRef]
- Huq, M.A. Green synthesis of silver nanoparticles using Pseudoduganella eburnea mahuq-39 and their antimicrobial mechanisms investigation against drug resistant human pathogens. Int. J. Mol. Sci. 2020, 21, 1510. [Google Scholar] [CrossRef]
- Huq, M.A.; Akter, S. Bacterial mediated rapid and facile synthesis of silver nanoparticles and their antimicrobial efficacy against pathogenic microorganisms. Materials 2021, 14, 2615. [Google Scholar] [CrossRef]
- Akter, S.; Huq, M.A. Biologically rapid synthesis of silver nanoparticles by Sphingobium sp. MAH-11T and their antibacterial activity and mechanisms investigation against drug-resistant pathogenic microbes. Artif. Cells Nanomed. Biotechnol. 2020, 48, 672–682. [Google Scholar] [CrossRef]
- Wacławek, S.; Gončuková, Z.; Adach, K.; Fijałkowski, M.; Černík, M. Green synthesis of gold nanoparticles using Artemisia dracunculus extract: Control of the shape and size by varying synthesis conditions. Environ. Sci. Pollut. Res. Int. 2018, 25, 24210–24219. [Google Scholar] [CrossRef] [PubMed]
- Balciunaitiene, A.; Viskelis, P.; Viskelis, J.; Streimikyte, P.; Liaudanskas, M.; Bartkiene, E.; Zavistanaviciute, P.; Zokaityte, E.; Starkute, V.; Ruzauskas, M.; et al. Green Synthesis of Silver Nanoparticles Using Extract of Artemisia absinthium L., Humulus lupulus L. and Thymus vulgaris L., Physico-Chemical Characterization, Antimicrobial and Antioxidant Activity. Processes 2021, 9, 1304. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef]
- Dinh, N.X.; Quy, N.V.; Huy, T.Q.; Le, A.T. Decoration of silver nanoparticles on multiwalled carbon nanotubes: Antibacterial mechanism and ultrastructural analysis. J. Nanomater. 2015, 2015, 814379. [Google Scholar] [CrossRef]
- Gopinath, V.; Priyadarshini, S.; Loke, M.F.; Arunkumar, J.; Marsili, E.; MubarakAli, D.; Velusamy, P.; Vadivelu, J. Biogenic synthesis, characterization of antibacterial silver nanoparticles and its cell cytotoxicity. Arab. J. Chem. 2017, 10, 1107–1117. [Google Scholar] [CrossRef]
- Gaspar, M.E.D.F.A.; Peixoto, C.A.; da Silva Amorim, R.V. Ultrastructural analysis of chitosan antibacterial activity against clinical isolates of Staphylococcus aureus and Escherichia coli. Adv. Microbiol. 2019, 9, 893. [Google Scholar] [CrossRef]
- Shirazi, M.A.; Shariati, F.; Ramezanpour, Z. Toxic effect of aluminum oxide nanoparticles on green micro-algae Dunaliella salina. Int. J. Environ. Res. 2015, 9, 585–594. [Google Scholar]
- Duong, T.T.; Le, T.S.; Tran, T.T.H.; Nguyen, T.K.; Ho, C.T.; Dao, T.H.; Le, T.P.Q.; Nguyen, H.C.; Dang, D.K.; Le, T.T.H.; et al. Inhibition effect of engineered silver nanoparticles to bloom forming cyanobacteria. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035018. [Google Scholar] [CrossRef]
- de Abreu Costa, L.; Henrique Fernandes Ottoni, M.; Dos Santos, M.G.; Meireles, A.B.; Gomes de Almeida, V.; de Fátima Pereira, W.; Alves de Avelar-Freitas, B.; Eustáquio Alvim Brito-Melo, G. Dimethyl Sulfoxide (DMSO) Decreases Cell Proliferation and TNF-α, IFN-γ, and IL-2 Cytokines Production in Cultures of Peripheral Blood Lymphocytes. Molecules 2017, 22, 1789. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, D.K.; Mohan, S.; Hasan, S.H. Photo-induced biosynthesis of silver nanoparticles using aqueous extract of Erigeron bonariensis and its catalytic activity against Acridine Orange. J. Photochem. Photobiol. B 2016, 155, 39–50. [Google Scholar] [CrossRef]
- Al-Otibi, F.; Al-Ahaidib, R.A.; Alharbi, R.I.; Al-Otaibi, R.M.; Albasher, G. Antimicrobial potential of biosynthesized silver nanoparticles by Aaronsohnia factorovskyi extract. Molecules 2021, 26, 130. [Google Scholar] [CrossRef]
- Swanner, J.; Singh, R. Synthesis, purification, characterization, and imaging of Cy3-functionalized fluorescent silver nanoparticles in 2D and 3D tumor models. Methods Mol. Biol. 2018, 1790, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Pingale, S.S.; Rupanar, S.V.; Chaskar, M.G. Plant-mediated biosynthesis of silver nanoparticles from Gymnema sylvestre and their use in photodegradation of Methyl orange dye. J. Water Environ. Nanotechnol. 2018, 3, 106–115. [Google Scholar] [CrossRef]
- Rizwana, H.; AlOtibi, F.; Al-Malki, N. Chemical composition, FTIR studies and antibacterial activity of Passiflora edulis f. edulis (Fruit). J. Pure Appl. Microbiol. 2019, 13, 2489–2498. [Google Scholar] [CrossRef]
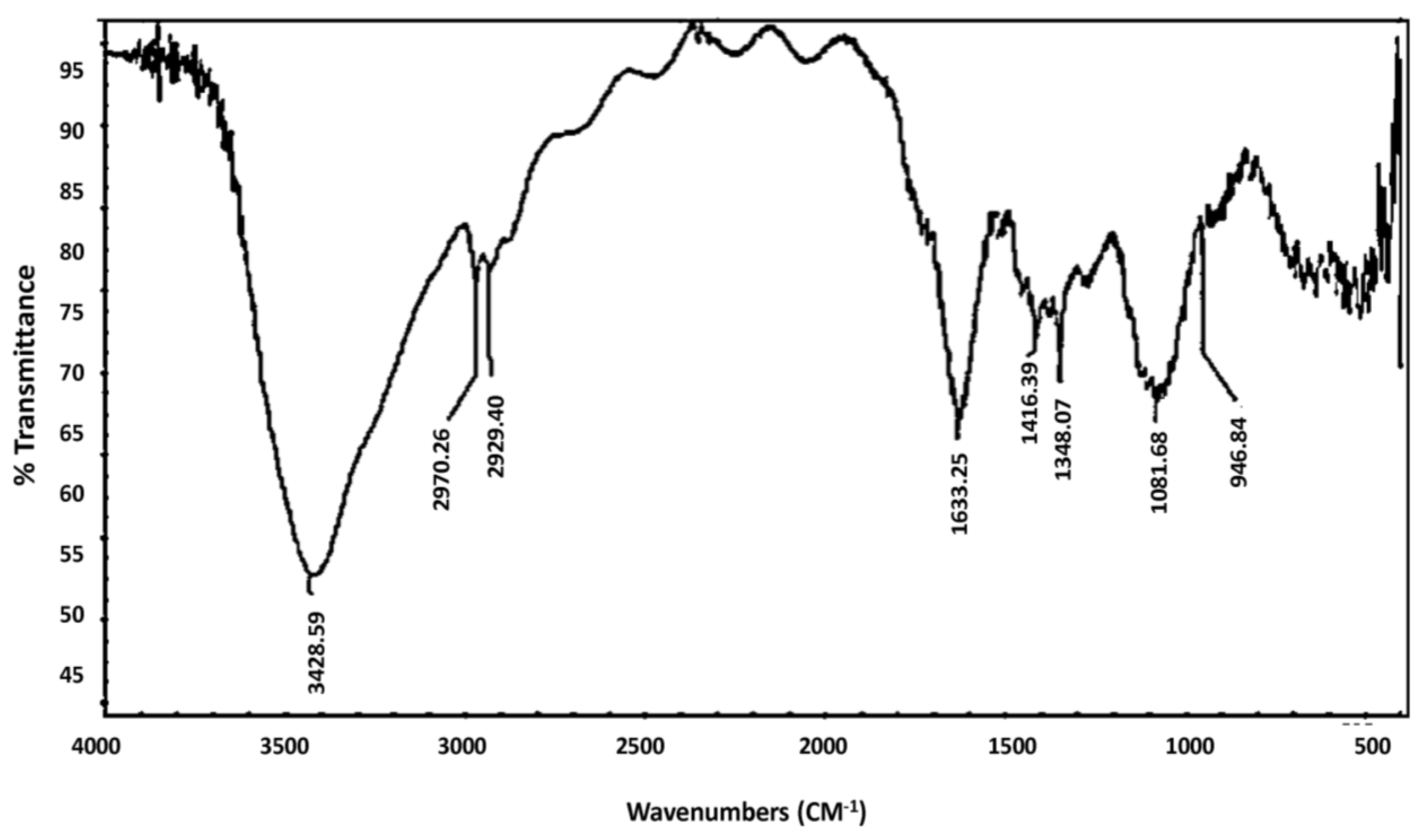
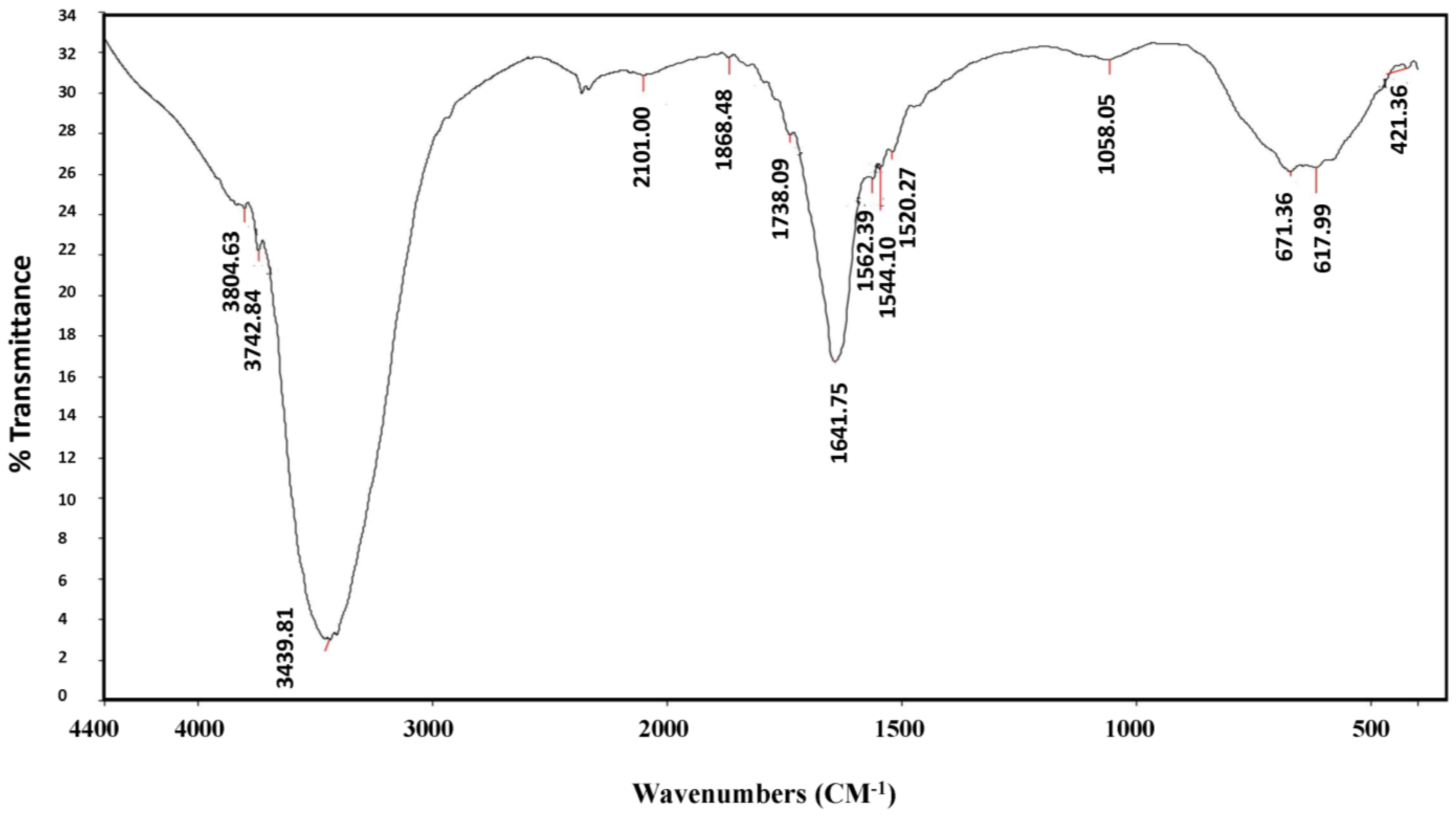





| Material | Absorption (cm−1) | Appearance | Group | Compound Class |
|---|---|---|---|---|
| Aqueous extract | 3428 | Strong, Broad | O-H Stretching | Alcohol |
| 2970, 2929 | Weak, Broad | N-H Stretching | Amine Salt | |
| 1633 | Medium | C=C Stretching | Alkene | |
| 1416, 1348 | Medium | O-H Bending | Alcohol | |
| 1081 | Medium | C-N Stretching | Amine | |
| 946 | Strong | C=C bending | Alkene | |
| AgNPs | 3804, 3742 | Medium, sharp | O-H Stretching | Alcohol |
| 3439 | Strong, Broad | O-H Stretching | Alcohol | |
| 2101 | Weak | CΞC stretching | Alkyne | |
| 1868, 1738 | Weak | C-H bending | Aromatic compound | |
| 1641 | Medium | C=C Stretching | Alkene | |
| 1562, 1544, 1520 | Strong | N-O stretching | Nitro compound | |
| 1058 | Strong | C-O stretching | Primary alcohol | |
| 671 | Strong | C=C bending | Alkene | |
| 617, 421 | Strong | C-X | Halogen compound |
| Phenolic Compound | Chemical Structure | Formula | Molecular Weight | Peak Area (%) |
|---|---|---|---|---|
| 2-Hexoxyethanol |  | C8H18O2 | 148 | 30.5 |
| Dihexyl ether |  | C12H26O | 186 | 7.31 |
| Dichloroacetic acid | 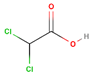 | C2H2CI2O2 | 128 | 7.31 |
| Piperidine |  | C5H11N | 85 | 18.5 |
| Chloral | 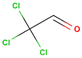 | C2HCl3O | 146 | 11.6 |
| N, N-Bis(2-hydroxyethyl)-2-aminoethanesulfonic acid | 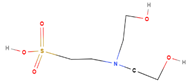 | C6H15NO5S | 213 | 9.34 |
| Phenprobamate | 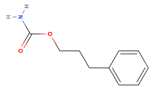 | C10H13NO2 | 179 | 9.80 |
| Cumene Hydroperoxide | 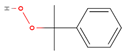 | C9H12O2 | 152 | 7.70 |
| 4-Ethyl-o-xylene |  | C10H14 | 134 | 4.97 |
| Species | DMSO | AgNO3 | Cephalexin | 10 µg/mL | 20 µg/mL | 40 µg/mL | MIC | |
|---|---|---|---|---|---|---|---|---|
| Aqueous Extract | ||||||||
| E. coli | Mean ± SD | 0 | - | 18 ± 0 S | 0 R | 10 ± 0.5 R | 11.7 ± 0.3 R | 15 µg/mL R |
| Median (Min–Max) | 0 | - | 18 (18–18) | 0 | 10 (9.5–10.5) | 11.5 (11.5–12) | ||
| p value | 1 | <0.001 * | 1 | <0.001 * | <0.001 * | |||
| B. subtilis | Mean ± SD | 0 | - | 25.8 ± 0.8 S | 9.7 ± 1.5 R | 10 ± 0.5 R | 13.2 ± 1.3 I | <10 µg/mL R |
| Median (Min–Max) | 0 | - | 26 (25–26.5) | 10 (9.5–10.5) | 10 (9.5–10.5) | 13 (12–14.5) | ||
| p value | 1 | <0.001 * | <0.001 * | <0.001 * | <0.001 * | |||
| P. aeruginosa | Mean ± SD | 0 | - | 19.7 ± 0.6 S | 0 R | 0 R | 0 R | >40 µg/mL R |
| Median (Min–Max) | 0 | - | 20 (19–20) | 0 | 0 | 0 | ||
| p value | 1 | <0.001 * | 1 | 1 | 1 | |||
| S. aureus | Mean ± SD | 0 | - | 26.8 ± 0.8 S | 12 ± 1 I | 14.7 ± 1.5 I | 16.7 ± 0.6 I | <10 µg/mL I |
| Median (Min–Max) | 0 | - | 27 (26–27.5) | 12 (11–13) | 15 (13–16) | 17 (16–17) | ||
| p value | 1 | <0.001 * | <0.001 * | <0.001 * | <0.001 * | |||
| Ethanolic extract | ||||||||
| E. coli | Mean ± SD | 0 | - | 18 ± 0 S | 0 R | 8.3 ± 0.6 R | 10.2 ± 0.3 R | 15 µg/mL R |
| Median (Min–Max) | 0 | - | 18 (18–18) | 0 | 8 (8–9) | 10 (10–10.5) | ||
| p value | 1 | <0.001 * | 1 | <0.001 * | <0.001 * | |||
| B. subtilis | Mean ± SD | 0 | - | 25.8 ± 0.8 S | 12.3 ± 0.8 I | 14 ± 0 I | 16.5 ± 0.5 I | <10 µg/mL I |
| Median (Min–Max) | 0 | - | 26 (25–26.5) | 12.5 (11.5–13) | 14 (14) | 16.5 (16–17) | ||
| p value | 1 | <0.001 * | <0.001 * | <0.001 * | <0.001 * | |||
| P. aeruginosa | Mean ± SD | 0 | - | 19.7 ± 0.6 S | 0 R | 0 R | 0 R | >40 µg/mL R |
| Median (Min–Max) | 0 | - | 20 (19–20) | 0 | 0 | 0 | ||
| p value | 1 | <0.001 * | 1 | 1 | 1 | |||
| S. aureus | Mean ± SD | 0 | - | 26.8 ± 0.8 S | 9.7 ± 0.3 R | 13.8 ± 0.8 I | 18 ± 0.5 I | <10 µg/mL I |
| Median (Min–Max) | 0 | - | 27 (26–27.5) | 9.5 (9.5–10) | 14 (13–14) | 18 (17.5–18.5) | ||
| p value | 1 | <0.001 * | <0.001 * | <0.001 * | <0.001 * | |||
| A. sieberi AgNPs | ||||||||
| E. coli | Mean ± SD | 0 | 7.7 ± 0.6 R | 18 ± 0 5 | 12 ± 0 R | 13.7 ± 0.3 I | 14.8 ± 0.3 I | <10 µg/mL I |
| Median (Min–Max) | 0 | 8 (7–8) | 18 (18–18) | 12 (12) | 13.5 (13.5–14) | 15 (14.5–15) | ||
| p value | 1 | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | ||
| B. subtilis | Mean ± SD | 0 | 7.3 ± 0.3 R | 25.8 ± 0.8 S | 11 ± 0 R | 12.7 ± 0.6 I | 14 ± 0.5 I | <10 µg/mL I |
| Median (Min–Max) | 0 | 7.5 (7–7.5) | 26 (25–26.5) | 11 (11) | 13 (12–13) | 14 (13.5–14.5) | ||
| p value | 1 | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | ||
| P. aeruginosa | Mean ± SD | 0 | 8.2 ± 0.6 R | 19.7 ± 0.6 S | 11.7 ± 0.3 R | 15 ± 1 I | 17 ± 0 I | <10 µg/mL I |
| Median (Min–Max) | 0 | 8.5 (7.5–8.5) | 20 (19–20) | 11.5 (11.5–12) | 15 (14–16) | 17 (17) | ||
| p value | 1 | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | ||
| S. aureus | Mean ± SD | 0 | 8.7 ± 0.6 R | 26.8 ± 0.8 S | 12 ± 0 R | 14.7 ± 0.6 I | 19 ± 1 I | <10 µg/mL I |
| Median (Min–Max) | 0 | 9 (8.5–9) | 27 (26–27.5) | 12 (12) | 15 (14–15) | 19 (18–20) | ||
| p value | 1 | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | ||
| Summarized comparative data analysis (mean ± SD) of the highest effect at the highest dose (40 µg/mL). | ||||||||
| Organisms | Aqueous extract | Ethanolic extract | AgNPs | p-value | ||||
| Aqueous vs. Ethanolic | Aqueous vs. AgNPs | Ethanolic vs. AgNPs | ||||||
| E. coli | 11.7 ± 0.3 | 10.2 ± 0.3 | 14.8 ± 0.3 | 0.28 | 0.77 | 0.74 | ||
| B. subtilis | 13.2 ± 1.3 | 16.5 ± 0.5 | 14 ± 0.5 | 0.75 | 0.27 | 0.04 * | ||
| P. aeruginosa | 0 | 0 | 17 ± 0 | - | <0.001 * | <0.001 * | ||
| S. aureus | 16.7 ± 0.6 | 18 ± 0.5 | 19 ± 1 | 0.85 | 0.61 | 0.92 | ||
| Species | DMSO | AgNO3 | Terbinafine | 10 µg/mL | 20 µg/mL | 40 µg/mL | |
|---|---|---|---|---|---|---|---|
| Aqueous Extract | |||||||
| C. albicans | Mean ± SD | 0 | - | 41 ± 0 | 0 | 0 | 0 |
| Median (Min–Max) | 0 | - | 41 (41) | 0 | 0 | 0 | |
| p value | 1 | <0.001 * | 1 | 1 | 1 | ||
| C. famata | Mean ± SD | 0 | - | 38.8 ± 0.8 | 12.5 ± 0.5 | 13.3 ± 0.6 | 14.8 ± 0.3 |
| Median (Min–Max) | 0 | - | 39 (38–39.5) | 12.5 (12–13) | 13 (13–14) | 15 (14.5–15) | |
| p value | 1 | <0.001 * | <0.001 * | <0.001 * | <0.001 * | ||
| C. krusei | Mean ± SD | 0 | - | 42 ± 1 | 6.3 ± 0.6 | 8.7 ± 0.6 | 10 ± 0 |
| Median (Min–Max) | 0 | - | 42 (41–43) | 6 (6–7) | 9 (8–9) | 10 (10) | |
| p value | 1 | <0.001 * | <0.001 * | <0.001 * | <0.001 * | ||
| C. parapsilosis | Mean ± SD | 0 | - | 29.2 ± 0.3 | 0 | 0 | 0 |
| Median (Min–Max) | 0 | - | 29 (29–29.5) | 0 | 0 | 0 | |
| p value | 1 | <0.001 * | 1 | 1 | 1 | ||
| C. rhodotorula | Mean ± SD | 0 | - | 21.7 ± 0.6 | 0 | 0 | 0 |
| Median (Min–Max) | 0 | - | 22 (21–22) | 0 | 0 | 0 | |
| p value | 1 | <0.001 * | 1 | 1 | 1 | ||
| Ethanolic extract | |||||||
| C. albicans | Mean ± SD | 0 | - | 41 ± 0 | 0 | 0 | 0 |
| Median (Min–Max) | 0 | - | 41 (41) | 0 | 0 | 0 | |
| p value | 1 | <0.001 * | 1 | 1 | 1 | ||
| C. famata | Mean ± SD | 0 | - | 38.8 ± 0.8 | 25.5 ± 0.5 | 29.8 ± 0.8 | 35.8 ± 0.3 |
| Median (Min–Max) | 0 | - | 39 (38–39.5) | 25.5 (25–26) | 30 (29–30.5) | 36 (35.5–36) | |
| p value | 1 | <0.001 * | <0.001 * | <0.001 * | <0.001 * | ||
| C. krusei | Mean ± SD | 0 | - | 42 ± 1 | 0 | 0 | 0 |
| Median (Min–Max) | 0 | - | 42 (41–43) | 0 | 0 | 0 | |
| p value | 1 | <0.001 * | 1 | 1 | 1 | ||
| C. parapsilosis | Mean ± SD | 0 | - | 29.2 ± 0.3 | 9.3 ± 0.3 | 11.3 ± 0.3 | 18.8 ± 0.3 |
| Median (Min–Max) | 0 | - | 29 (29–29.5) | 9.5 (9–9.5) | 11.5 (11–11.5) | 19 (18.5–19) | |
| p value | 1 | <0.001 * | <0.001 * | <0.001 * | <0.001 * | ||
| C. rhodotorula | Mean ± SD | 0 | - | 21.7 ± 0.6 | 0 | 0 | 0 |
| Median (Min–Max) | 0 | - | 22 (21–22) | 0 | 0 | 0 | |
| p value | 1 | <0.001 * | 1 | 1 | 1 | ||
| A. sieberi AgNPs | |||||||
| C. albicans | Mean ± SD | 0 | 10 ± 0 | 41 ± 0 | 11 ± 0 | 12 ± 0 | 14.3 ± 0.8 |
| Median (Min–Max) | 0 | 10 (10) | 41 (41) | 11 (11) | 12 (12) | 13.5 (13.5–15) | |
| p value | 1 | >0.05 | <0.001 * | <0.001 * | <0.001 * | <0.001 * | |
| C. famata | Mean ± SD | 0 | 23.5 ± 0 | 38.8 ± 0.8 | 22.3 ± 0.6 | 24.7 ± 0.3 | 26.2 ± 0.8 |
| Median (Min–Max) | 0 | 13.5 (13.5) | 39 (38–39.5) | 22 (22–23) | 24.5 (24.5–25) | 26 (25.5–27) | |
| p value | 1 | >0.05 | <0.001 * | <0.001 * | <0.001 * | <0.001 * | |
| C. krusei | Mean ± SD | 0 | 10.5 ± 0 | 42 ± 1 | 10.7 ± 0.6 | 12 ± 0 | 14.7 ± 0.8 |
| Median (Min–Max) | 0 | 10.5 (10.5) | 42 (41–43) | 11 (10–11) | 12 (12) | 14 (13.5–15) | |
| p value | 1 | >0.05 | <0.001 * | <0.001 * | <0.001 * | <0.001 * | |
| C. parapsilosis | Mean ± SD | 0 | 6 ± 0 | 29.2 ± 0.3 | 6.3 ± 0.6 | 7 ± 0 | 8.3 ± 0.6 |
| Median (Min–Max) | 0 | 6 (6) | 29 (29–29.5) | 6 (6–7) | 7 (7) | 8 (8–9) | |
| p value | 1 | >0.05 | <0.001 * | <0.001 * | <0.001 * | <0.001 * | |
| C. rhodotorula | Mean ± SD | 0 | 8 ± 0 | 21.7 ± 0.6 | 7 ± 0 | 8.8 ± 0.3 | 10.3 ± 0.6 |
| Median (Min–Max) | 0 | 8 (8) | 22 (21–22) | 7 (7) | 9 (8.5–9) | 10 (10–11) | |
| p value | 1 | >0.05 | <0.001 * | <0.001 * | <0.001 * | <0.001 * | |
| Summarized comparative data analysis (mean ± SD) of the highest effect at the highest dose (40 µg/mL). | |||||||
| Organisms | Aqueous extract | Ethanolic extract | AgNPs | p-value | |||
| Aqueous vs. Ethanolic | Aqueous vs. AgNPs | Ethanolic vs. AgNPs | |||||
| C. albicans | 0 | 0 | 14.3 ± 0.8 | - | - | 0.29 | |
| C. famata | 14.8 ± 0.3 | 35.8 ± 0.3 | 26.2 ± 0.8 | <0.001 * | <0.001 * | - | |
| C. krusei | 10 ± 0 | 0 | 14.7 ± 0.8 | <0.001 * | <0.001 * | <0.001 * | |
| C. parapsilosis | 0 | 18.8 ± 0.3 | 8.3 ± 0.6 | - | - | <0.001 * | |
| C. rhodotorula | 0 | 0 | 10.3 ± 0.6 | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Otibi, F.; Alshammry, N.A.; Alharbi, R.I.; Bin-Jumah, M.N.; AlSubaie, M.M. Silver Nanoparticles of Artemisia sieberi Extracts: Chemical Composition and Antimicrobial Activities. Plants 2023, 12, 2093. https://doi.org/10.3390/plants12112093
Al-Otibi F, Alshammry NA, Alharbi RI, Bin-Jumah MN, AlSubaie MM. Silver Nanoparticles of Artemisia sieberi Extracts: Chemical Composition and Antimicrobial Activities. Plants. 2023; 12(11):2093. https://doi.org/10.3390/plants12112093
Chicago/Turabian StyleAl-Otibi, Fatimah, Nourah A. Alshammry, Raedah I. Alharbi, May N. Bin-Jumah, and Maha M. AlSubaie. 2023. "Silver Nanoparticles of Artemisia sieberi Extracts: Chemical Composition and Antimicrobial Activities" Plants 12, no. 11: 2093. https://doi.org/10.3390/plants12112093