Multitask Approach to Localize Rhizobial Type Three Secretion System Effector Proteins Inside Eukaryotic Cells
Abstract
1. Introduction
2. Results
2.1. The S. fredii HH103 NopL Is Phosphorylated by Soybean Kinases
2.2. Subcellular Localization of NopL in Tobacco Leaves
2.3. NopL Overexpression by Salmonella and Detection on Infected HeLa Cell Cultures
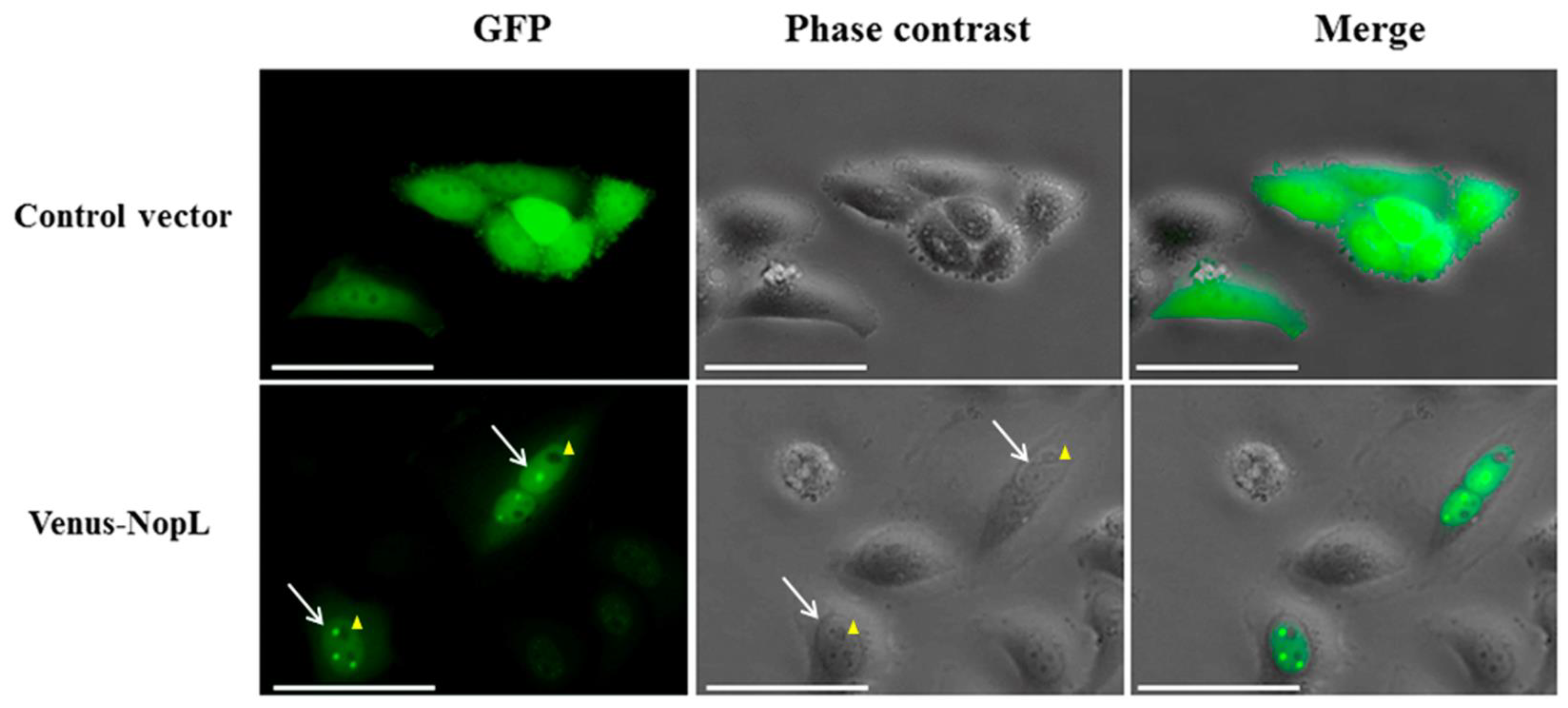
2.4. NopL Transient Expression and Detection on Transfected HeLa Cells
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Molecular Biology General Procedures
4.3. In Vitro Phosphorylation Assay
4.4. Transient Gene Expression in Nicotiana benthamiana Leaves and Confocal Imaging
4.5. In Vitro Bacterial Infection of HeLa Cells
4.6. Heterologous Protein and/or Lysis Induction in Cellular Infected Cultures
4.7. Fluorescence Immunostaining of Infected HeLa Cells
4.8. Transfection of Cell Cultures and Cell Cycle Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oldroyd, G.E. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Liu, W.; Nandety, R.S.; Crook, A.; Mysore, K.S.; Pislariu, C.I.; Frugoli, J.; Dickstein, R.; Udvardi, M.K. Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation. Plant Cell 2020, 32, 15–41. [Google Scholar] [CrossRef]
- Wang, D.; Dong, W.; Murray, J.; Wang, E. Innovation and appropriation in mycorrhizal and rhizobial symbioses. Plant Cell 2022, 34, 1573–1599. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Rachwał, K.; Marzec, A.; Grzadziel, J.; Palusinska-Szysz, M. Signal molecules and cell-surface components involved in early stages of the legume-rhizobium interactions. Appl. Soil Ecol. 2015, 85, 94–113. [Google Scholar] [CrossRef]
- Acosta-Jurado, S.; Fuentes-Romero, F.; Ruiz-Sainz, J.E.; Janczarek, M.; Vinardell, J.M. Rhizobial exopolysaccharides: Genetic regulation of their synthesis and relevance in symbiosis with legumes. Int. J. Mol. Sci. 2021, 22, 6233. [Google Scholar] [CrossRef]
- Jiménez-Guerrero, I.; Medina, C.; Vinardell, J.M.; Ollero, F.J.; López-Baena, F.J. The Rhizobial Type 3 Secretion System: The Dr. Jekyll and Mr. Hyde in the Rhizobium–Legume Symbiosis. Int. J. Mol. Sci. 2022, 23, 11089. [Google Scholar] [CrossRef]
- Deng, W.; Marshall, N.C.; Rowland, J.L.; Mccoy, J.M.; Worrall, L.J.; Santos, A.S.; Strynadka, N.C.J.; Finlay, B.B. Assembly, structure, function and regulation of type III secretion systems. Nat. Rev. Microbiol. 2017, 15, 323–337. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, H.; Cheng, X.; Shu, X.; White, A.P.; Stavrinides, J.; Köster, W.; Zhu, G.; Zhao, Z.; Wang, Y. A global survey of bacterial type III secretion systems and their effectors. Environ. Microbiol. 2017, 19, 3879–3895. [Google Scholar] [CrossRef] [PubMed]
- Rapisarda, C.; Fronzes, R. Secretion systems used by bacteria to subvert host functions. Curr. Issues Mol. Biol. 2018, 25, 1–42. [Google Scholar] [CrossRef]
- Alfano, J.R.; Collmer, A. Type III secretion system effector proteins: Double agents in bacterial disease and plant defense. Annu. Rev. Phytopathol. 2004, 42, 385–414. [Google Scholar] [CrossRef]
- Mansfield, J.W. From bacterial avirulence genes to effector functions via the hrp delivery system: An overview of 25 years of progress in our understanding of plant innate immunity. Mol. Plant. Pathol. 2009, 10, 721–734. [Google Scholar] [CrossRef]
- Nümberger, T.; Brunner, F.; Kemmerling, B.; Piater, L. Innate immunity in plants and animals: Striking similarities and obvious differences. Immunol. Rev. 2004, 198, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Teulet, A.; Camuel, A.; Perret, X.; Giraud, E. The versatile roles of type III secretion systems in rhizobium-legume symbioses. Annu. Rev. Microbiol. 2022, 76, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Guerrero, I.; Pérez-Montaño, F.; Medina, C.; Ollero, F.J.; López-Baena, F.J. NopC Is a Rhizobium-Specific Type 3 Secretion System Effector Secreted by Sinorhizobium (Ensifer) fredii HH103. PLoS ONE 2015, 16, e0142866. [Google Scholar] [CrossRef]
- Jiménez-Guerrero, I.; Pérez-Montaño, F.; Medina, C.; Ollero, F.J.; López-Baena, F.J. The Sinorhizobium (Ensifer) fredii HH103 Nodulation Outer Protein NopI Is a Determinant for Efficient Nodulation of Soybean and Cowpea Plants. Appl. Environ. Microbiol. 2017, 15, e02770-16. [Google Scholar] [CrossRef]
- Sory, M.P.; Cornelis, G.R. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol. Microbiol. 1994, 14, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Torruellas-Garcia, J.; Ferracci, F.; Jackson, M.W.; Joseph, S.S.; Pattis, I.; Plano, L.R.W.; Fischer, W.; Plano, G.V. Measurement of Effector Protein Injection by Type III and Type IV Secretion Systems by Using a 13-Residue Phosphorylatable Glycogen Synthase Kinase Tag. Infect. Immun. 2006, 74, 5645–5657. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.; Connolly, J.P.R.; Roe, A.J. Tracking elusive cargo: Illuminating spatio-temporal Type 3 effector protein dynamics using reporters. Cell. Microbiol. 2018, 20, e12797. [Google Scholar] [CrossRef]
- Bartsev, A.V.; Boukli, N.M.; Deakin, W.J.; Staehelin, C.; Broughton, W.J. Purification and phosphorylation of the effector protein NopL from Rhizobium sp. NGR234. FEBS Lett. 2003, 20, 271–274. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.J.; Lu, H.B.; Xie, Z.P.; Staehelin, C. Functional Analysis of the Type 3 Effector Nodulation Outer Protein L (NopL) from Rhizobium sp. NGR234: Symbiotic effects, phosphorylation, and interference with mitogen-activated protein kinase signaling. J. Biol. Chem. 2011, 16, 32178–32187. [Google Scholar] [CrossRef] [PubMed]
- Bartsev, A.V.; Deakin, W.J.; Boukli, N.M.; McAlvin, C.B.; Stacey, G.; Malnoë, P.; Broughton, W.J.; Staehelin, C. NopL, an Effector Protein of Rhizobium sp. NGR234, Thwarts Activation of Plant Defense Reactions. Plant Physiol. 2004, 134, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.Y.; Xiang, Q.W.; Wagner, C.; Zhang, D.; Xie, Z.P.; Staehelin, C. The type 3 effector NopL of Sinorhizobium sp. strain NGR234 is a mitogen-activated protein kinase substrate. J. Exp. Bot. 2016, 67, 2483–2494. [Google Scholar]
- Peng, S.; Zhang, Y.; Zhang, J.; Wang, H.; Ren, B. ERK in learning and memory: A review of recent research. Int. J. Mol. Sci. 2010, 13, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.F.; Breton, G.; Harmon, A. Decoding Ca2+ signals through plant protein kinases. Annu. Rev. Plant Biol. 2004, 55, 263–288. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, Y.; Han, L.; Yamazaki, T.; Suzuki, R.; Hayashi, M.; Imaizumi-Anraku, H. Rhizobial and fungal symbioses show different requirements for calmodulin binding to calcium calmodulin dependent protein kinase in Lotus japonicus. Plant Cell 2012, 24, 304–321. [Google Scholar] [CrossRef]
- Miller, J.B.; Pratap, A.; Miyahara, A.; Zhou, L.; Bornemann, S.; Morris, R.J.; Oldroyd, G.E. Calcium/calmodulin-dependent protein kinase is negatively and positively regulated by calcium, providing a mechanism for decoding calcium responses during simbiosis signaling. Plant Cell 2013, 25, 5053–5066. [Google Scholar] [CrossRef]
- Khare, T.; Srivastav, A.; Kumar, V. Calcium/calmodulin activated protein kinases in stress signaling in plants. In Protein Kinases and Stress Signaling in Plants. Functional Genomic Perspective; Pandei, G.K., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2020; pp. 266–280. [Google Scholar]
- Stephenson, M.J.; Reed, J.; Brouwer, B.; Osbourn, A. Transient Expression in Nicotiana Benthamiana Leaves for Triterpene Production at a Preparative Scale. J. Vis. Exp. 2018, 16, 58169. [Google Scholar]
- D’Aoust, M.A.; Lavoie, P.O.; Belles-Isles, J.; Bechtold, N.; Martel, M.; Vézina, L.P. Transient expression of antibodies in plants using syringe Agroinfiltration. In Recombinant Proteins From Plants. Methods in Molecular Biology; Faye, L., Gomord, V., Eds.; Humana Press: Totowa, NJ, USA, 2008; Volume 483, pp. 41–50. [Google Scholar]
- Medina, C.; Camacho, E.M.; Flores, A.; Mesa-Pereira, B.; Santero, E. Improved Expression Systems for Regulated Expression in Salmonella Infecting Eukaryotic Cells. PLoS ONE 2011, 6, e23055. [Google Scholar] [CrossRef]
- Medina, C.; Santero, E.; Gómez-Skarmeta, J.L.; Royo, J.L. Engineered Salmonella allows real-time heterologous gene expression monitoring within infected zebrafish embryos. J. Biotechnol. 2012, 10, 413–416. [Google Scholar] [CrossRef]
- Troisfontaines, P.; Cornelis, G. Type III Secretion: More Systems Than You Think. Physiology 2005, 20, 326–339. [Google Scholar] [CrossRef]
- Tampakaki, A.P. Commonalities and differences of T3SSs in rhizobia and plant pathogenic bacteria. Front. Plant Sci. 2014, 5, 114. [Google Scholar] [CrossRef]
- Subtil, A.; Parsot, C.; Dautry-Varsat, A. Secretion of predicted Inc proteins of Chlamydia pneumoniae by a heterologous type III machinery. Mol. Microbiol. 2001, 39, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Camacho, E.M.; Mesa-Pereira, B.; Medina, C.; Flores, A.; Santero, E. Engineering Salmonella as intracellular factory for effective killing of tumour cells. Sci. Rep. 2016, 6, 30591. [Google Scholar] [CrossRef] [PubMed]
- Young, R. Bacteriophage lysis: Mechanism and regulation. Microbiol. Rev. 1992, 56, 430–481. [Google Scholar] [CrossRef] [PubMed]
- Degenkolb, J.; Takahashi, M.; Ellestad, G.A.; Hillen, W. Structural requirements of tetracycline-Tet repressor interaction: Determination of equilibrium binding constants for tetracycline analogs with the Tet repressor. Antimicrob. Agents Chemother. 1991, 35, 1591–1595. [Google Scholar] [CrossRef] [PubMed]
- Beuzón, C.R.; Méresse, S.; Unsworth, K.E.; Ruíz-Albert, J.; Garvis, S.; Waterman, S.R.; Ryder, T.A.; Boucrot, E.; Holden, D.W. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 2000, 19, 3235–3249. [Google Scholar] [CrossRef]
- Sontag, R.L.; Mihai, C.; Orr, G.; Savchenko, A.; Skarina, T.; Cui, H.; Cort, J.R.; Adkins, J.N.; Brown, R.N. Electroporation of functional bacterial effectors into mammalian cells. J. Vis. Exp. 2015, 19, 52296. [Google Scholar]
- Dean, P. Functional domains and motifs of bacterial type III effector proteins and their roles in infection. FEMS Microbiol. Rev. 2011, 35, 1100–1125. [Google Scholar] [CrossRef]
- Hicks, S.W.; Galán, J.E. Exploitation of eukaryotic subcellular targeting mechanisms by bacterial effectors. Nat. Rev. Microbiol. 2013, 11, 316–326. [Google Scholar] [CrossRef]
- Zeng, C.; Zou, L. An account of in silico identification tools of secreted effector proteins in bacteria and future challenges. Brief Bioinform. 2019, 18, 110–129. [Google Scholar] [CrossRef]
- Jiménez-Guerrero, I.; Pérez-Montaño, F.; Da Silva, G.M.; Wagner, N.; Shkedy, D.; Zhao, M.; Pizarro, L.; Bar, M.; Walcott, R.; Sessa, G.; et al. Show me your secret(ed) weapons: A multifaceted approach reveals a wide arsenal of type III-secreted effectors in the cucurbit pathogenic bacterium Acidovorax citrulli and novel effectors in the Acidovorax genus. Mol. Plant Pathol. 2020, 21, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Braet, J.; Catteeuw, D.; Van Damme, P. Recent Advancements in Tracking Bacterial Effector Protein Translocation. Microorganisms 2022, 24, 260. [Google Scholar] [CrossRef]
- Maffei, B.; Francetic, O.; Subtil, A. Tracking Proteins Secreted by Bacteria: What’s in the Toolbox? Front. Cell Infect. Microbiol. 2017, 31, 221. [Google Scholar] [CrossRef]
- Xin, X.F.; Nomura, K.; Ding, X.; Chen, X.; Wang, K.; Aung, K.; Uribe, F.; Rosa, B.; Yao, J.; Chen, J.; et al. Pseudomonas syringae effector avirulence protein E localizes to the host plasma membrane and down-regulates the expression of the nonrace-specific disease resistance1/harpin-induced1-like13 gene required for antibacterial immunity in Arabidopsis. Plant Physiol. 2015, 169, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, P.; Shen, D.; Wei, Q.; He, J.; Lu, Y. The Ralstonia solanacearum effector RipN suppresses plant PAMP-triggered immunity, localizes to the endoplasmic reticulum and nucleus, and alters the NADH/NAD+ ratio in Arabidopsis. Mol. Plant Path. 2019, 20, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Montiel, J.; Reid, D.; Grønbæk, T.H.; Benfeldt, C.M.; James, E.K.; Ott, T.; Ditengou, A.; Nadzieja, M.; Kelly, S.; Stougaard, J. Distinct signaling routes mediate intercellular and intracellular rhizobial infection in Lotus japonicus. Plant Physiol. 2021, 185, 1131–1147. [Google Scholar] [CrossRef]
- Kapila, J.; De Rycke, R.; Van Montagu, M.; Angenon, G. An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci. 1997, 122, 101–108. [Google Scholar] [CrossRef]
- Choi, S.; Jayaraman, J.; Segonzac, C.; Park, H.J.; Park, H.; Han, S.W.; Sohn, K.H. Pseudomonas syringae pv. actinidiae type III effectors localized at multiple cellular compartments activate or suppress innate immune responses in Nicotiana benthamiana. Front. Plant. Sci. 2017, 8, 2157. [Google Scholar] [CrossRef]
- Xin, D.W.; Liao, S.; Xie, Z.P.; Hann, D.R.; Steinle, L.; Boller, T.; Staehelin, C. Functional analysis of NopM, a novel E3 ubiquitin ligase (NEL) domain effector of Rhizobium sp. strain NGR234. PLoS Pathog. 2012, 8, e1002707. [Google Scholar] [CrossRef]
- Marchetti, M.; Capela, D.; Poincloux, R.; Benmeradi, N.; Auriac, M.C.; Le Ru, A.; Maridonneau-Parini, I.; Batut, J.; Masson-Boivin, C. Queuosine biosynthesis is required for sinorhizobium meliloti-induced cytoskeletal modifications on HeLa Cells and symbiosis with Medicago truncatula. PLoS ONE 2013, 8, e56043. [Google Scholar] [CrossRef] [PubMed]
- LeVier, K.; Phillips, R.W.; Grippe, V.K.; Roop, R.M., II; Walker, G.C. Similar requirements of a plant symbiont and a mammalian pathogen for prolonged intracellular survival. Science 2000, 287, 2492–2493. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Pereira, B.; Medina, C.; Camacho, E.M.; Flores, A.; Santero, E. Novel tools to analyze the function of Salmonella effectors show that SvpB ectopic expression induces cell cycle arrest in tumor cells. PLoS ONE 2013, 21, e78458. [Google Scholar] [CrossRef] [PubMed]
- Behringer, J.E. R factor transfer in Rhizobium leguminosarum. J. Gen. Microbiol. 1974, 84, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D.W. Molecular Cloning. A laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Earley, K.W.; Haag, J.R.; Pontes, O.; Opper, K.; Juehne, T.; Song, K.; Pikaard, C.S. Gateway-compatible vectors for plant functional genomics and proteomics. Plant. J. 2006, 45, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Morgan, R.L.; Guttman, D.S.; Ma, W. Allelic variants of the Pseudomonas syringae type III effector HopZ1 are differentially recognized by plant resistance systems. Mol. Plant Microbe Interact. 2009, 22, 176–189. [Google Scholar] [CrossRef]
- Kapuscinski, J.; Skoczylas, B. Simple and rapid fluorimetric method for DNA microassay. Anal. Biochem. 1977, 83, 252–257. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Guerrero, I.; López-Baena, F.J.; Medina, C. Multitask Approach to Localize Rhizobial Type Three Secretion System Effector Proteins Inside Eukaryotic Cells. Plants 2023, 12, 2133. https://doi.org/10.3390/plants12112133
Jiménez-Guerrero I, López-Baena FJ, Medina C. Multitask Approach to Localize Rhizobial Type Three Secretion System Effector Proteins Inside Eukaryotic Cells. Plants. 2023; 12(11):2133. https://doi.org/10.3390/plants12112133
Chicago/Turabian StyleJiménez-Guerrero, Irene, Francisco Javier López-Baena, and Carlos Medina. 2023. "Multitask Approach to Localize Rhizobial Type Three Secretion System Effector Proteins Inside Eukaryotic Cells" Plants 12, no. 11: 2133. https://doi.org/10.3390/plants12112133
APA StyleJiménez-Guerrero, I., López-Baena, F. J., & Medina, C. (2023). Multitask Approach to Localize Rhizobial Type Three Secretion System Effector Proteins Inside Eukaryotic Cells. Plants, 12(11), 2133. https://doi.org/10.3390/plants12112133





