Comparative Amino Acid Profile and Antioxidant Activity in Sixteen Plant Extracts from Transylvania, Romania
Abstract
:1. Introduction
2. Results and Discussion
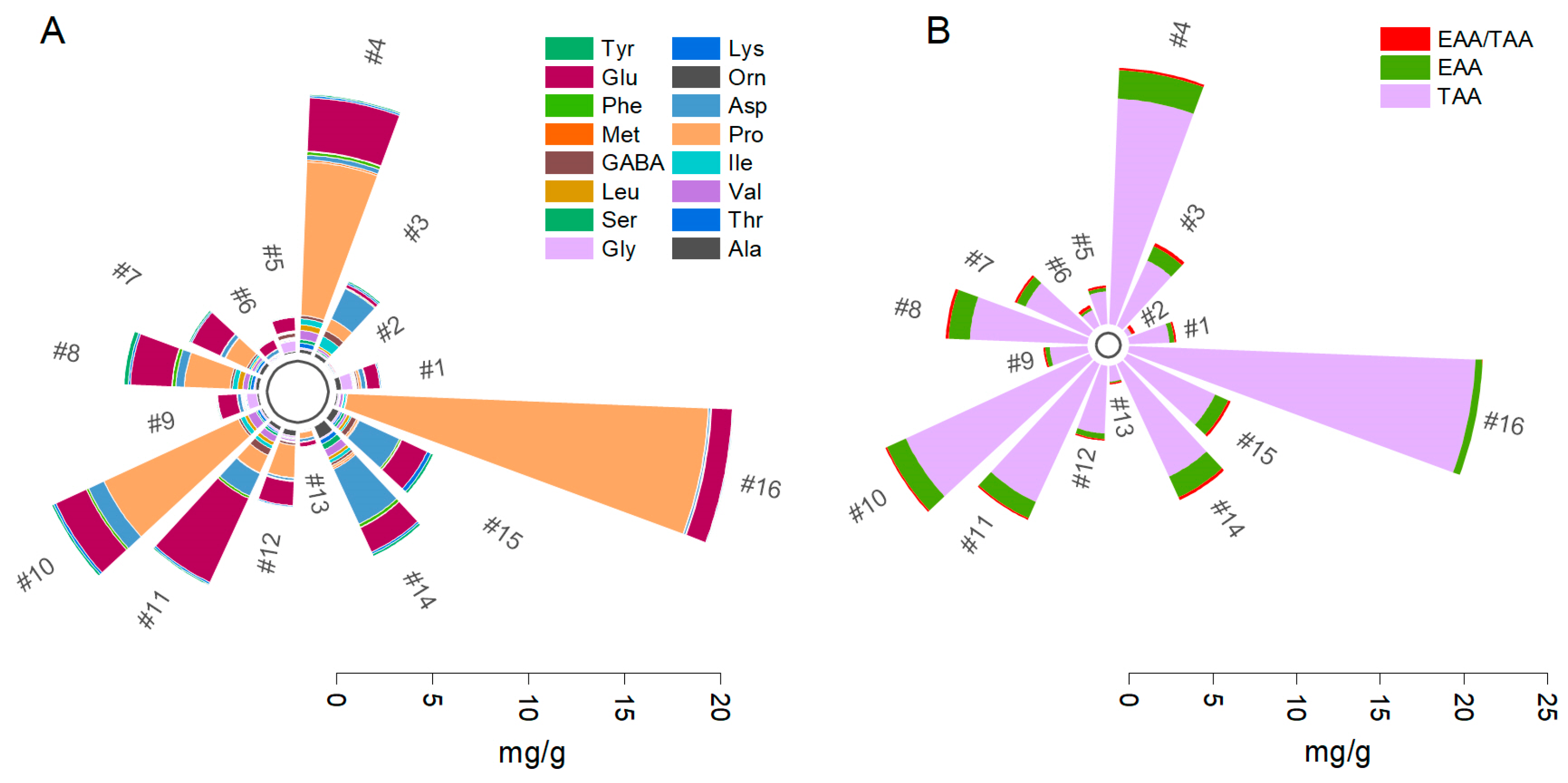
2.1. Amino Acid Quantification and Interspecies Comparison
2.2. Antioxidant Activity
2.3. Compounds, Active Principles, and Industry Importance of the Local Plant Extracts—Literature Synthesis
3. Materials and Methods
3.1. Plant Material
3.2. Apparatus and Reagents—Sample Preparation
3.3. Extraction Procedures
3.4. DPPH Assay
3.5. Analytical Performance of the Method
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuzimoto, A.D. An overview of the anti-SARS-CoV-2 properties of Artemisia annua, its antiviral action, protein-associated mechanisms, and repurposing for COVID-19 treatment. J. Integr. Med. 2021, 19, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Bechlem, H.; Benayache, F.; Benouchenne, D.; Labed, A. DNA intercalators alkaloids as Potential candidates to fight COVID-19 disease: Systematic review. J. Res. Pharm. 2022, 26, 1102–1111. [Google Scholar] [CrossRef]
- Yassein, A.S. Incidence of Fungi Contaminating Some Medicinal Plants and Their Antimicrobial and Anticancer Properties at Qena Governorate, Egypt. Egypt. J. Food Sci. 2021, 49, 157–171. [Google Scholar] [CrossRef]
- Makaremi, S.; Ganji, A.; Ghazavi, A.; Mosayebi, G. Inhibition of tumor growth in CT-26 colorectal cancer-bearing mice with alcoholic extracts of Curcuma longa and Rosmarinus officinalis. Gene Rep. 2021, 22, 101006. [Google Scholar] [CrossRef]
- Zammel, N.; Jedli, O.; Rebai, T.; Hamadou, W.S.; Elkahoui, S.; Jamal, A.; Alam, J.M.; Adnan, M.; Siddiqui, A.J.; Alreshidi, M.M.; et al. Kidney injury and oxidative damage alleviation by Zingiber officinale: Pharmacokinetics and protective approach in a combined murine model of osteoporosis. 3 Biotech 2022, 12, 112. [Google Scholar] [CrossRef]
- Shirzadi-Ahodashti, M.; Mortazavi-Derazkola, S.; Ebrahimzadeh, M.A. Biosynthesis of noble metal nanoparticles using crataegus monogyna leaf extract (CML@ X-NPs, X= Ag, Au): Antibacterial and cytotoxic activities against breast and gastric cancer cell lines. Surf. Interfaces 2020, 21, 100697. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Xu, H.B.; Zhang, S.J.; Li, X.Y. Identification of the active compounds and significant pathways of Artemisia annua in the treatment of non-small cell lung carcinoma based on network pharmacology. Med. Sci. Monit. 2020, 26, e923624-1. [Google Scholar] [CrossRef]
- Urbančič, S.; Kolar, M.H.; Dimitrijević, D.; Demšar, L.; Vidrih, R. Stabilisation of sunflower oil and reduction of acrylamide formation of potato with rosemary extract during deep-fat frying. LWT Food Sci. Technol. 2014, 57, 671–678. [Google Scholar] [CrossRef]
- Kefale, B.; Delele, M.A.; Fanta, S.W.; Mekonnen Abate, S. Nutritional, Physicochemical, Functional, and Textural Properties of Red Pepper (Capsicum annuum L.), Red Onion (Allium cepa), Ginger (Zingiber officinale), and Garlic (Allium sativum): Main Ingredients for the Preparation of Spicy Foods in Ethiopia. J. Food Qual. 2023, 2023, 3916692. [Google Scholar] [CrossRef]
- Beristain-Bauza, S.D.C.; Hernández-Carranza, P.; Cid-Pérez, T.S.; Ávila-Sosa, R.; Ruiz-López, I.I.; Ochoa-Velasco, C.E. Antimicrobial Activity of Ginger (Zingiber officinale) and Its Application in Food Products. Food Rev. Int. 2019, 35, 407–426. [Google Scholar] [CrossRef]
- Parzhanova, A.B.; Petkova, N.T.; Ivanov, I.G.; Ivanova, S.D. Evaluation of biologically active substance and antioxidant potential of medicinal plants extracts for food and cosmetic purposes. J. Pharm. Sci. Res. 2018, 10, 1804–1809. [Google Scholar]
- Anwar, F.; Abbas, A.; Mehmood, T.; Gilani, A.H.; Rehman, N.U. Mentha: A genus rich in vital nutra-pharmaceuticals—A review. Phytother. Res. 2019, 33, 2548–2570. [Google Scholar] [CrossRef] [PubMed]
- Voica, C.; Nechita, C.; Iordache, A.M.; Roba, C.; Zgavarogea, R.; Ionete, R.E. ICP-MS Assessment of Essential and Toxic Trace Elements in Foodstuffs with Different Geographic Origins Available in Romanian Supermarkets. Molecules 2021, 26, 7081. [Google Scholar] [CrossRef]
- Iordache, A.M.; Nechita, C.; Voica, C.; Roba, C.; Botoran, O.R.; Ionete, R.E. Assessing the Health Risk and the Metal Content of Thirty-Four Plant Essential Oils Using the ICP-MS Technique. Nutrients 2022, 14, 2363. [Google Scholar] [CrossRef]
- Voica, C.; Iordache, A.M.; Roba, C.; Nechita, C. Determination of Toxic Elements in Facial Cosmetics from the Romanian Market and Their Health Risk Assessment. Anal. Lett. 2023, 56, 244–256. [Google Scholar] [CrossRef]
- Baran, A.; Nadaroğlu, H. The Production of Pestil (Fruit leather) from Different Hawthorn (Crataegus spp.) Fruits. Turk. J. Agric.—Food Sci. Technol. 2022, 10, 1854–1861. [Google Scholar] [CrossRef]
- Bensaci, N.; Abdi, A.; Ben Aziza, H.; Aouadi, S. Characterization and biological evaluation of Crataegus azarolus fruit polysaccharides. J. Mol. Struct. 2022, 1270, 133889. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.D.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Chen, Q.; Liu, M.Y.; Yu, H.Y.; Xu, J.Q.; Wu, J.Q.; Zhang, Y.; Wang, T. Regulation effects of rosemary (Rosmarinus officinalis L.) on hepatic lipid metabolism in OA induced NAFLD rats. Food Funct. 2019, 10, 7356–7365. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, K.G.C.; Waliwita, W.A.L.C.; Liyanage, R.P. A review on medicinal uses of Zingiber officinale (ginger). Int. J. Health Sci. Res. 2020, 10, 142–148. [Google Scholar]
- Ajali, J.J.O.; Ikezue, E.N.; Ezeugo, J.O. Kinetic Modelling of Biopreservation of Mango Fruit Juice Using Murraya koenigii Sprengel. World Sci. News 2022, 170, 46–68. [Google Scholar]
- Yesil-Celiktas, O.; Sevimli, C.; Bedir, E.; Vardar-Sukan, F. Inhibitory Effects of Rosemary Extracts, Carnosic Acid and Rosmarinic Acid on the Growth of Various Human Cancer Cell Lines. Plant Foods Hum. Nutr. 2010, 65, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Granja, S.; Neves, N.M.; Reis, R.L.; Baltazar, F.; Silva, T.H.; Martins, A. Fucoidan from Fucus vesiculosus inhibits new blood vessel formation and breast tumor growth in vivo. Carbohydr. Polym. 2019, 223, 115034. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Khaidakov, B.; Korewo, D.; Węsierska, M.; Cyplik, W.; Kujawa, J.; Ahrné, L.M.; Kujawski, W. The Chemical and Cytotoxic Properties of Sambucus nigra Extracts—A Natural Food Colorant. Sustainability 2021, 13, 12702. [Google Scholar] [CrossRef]
- Panyajai, P.; Chueahongthong, F.; Viriyaadhammaa, N.; Nirachonkul, W.; Tima, S.; Chiampanichayakul, S.; Anuchapreeda, S.; Okonogi, S. Anticancer activity of Zingiber ottensii essential oil and its nanoformulations. PLoS ONE 2022, 17, e0262335. [Google Scholar] [CrossRef]
- Wan, X.; Ahmad, H.; Zhang, L.; Wang, Z.; Wang, T. Dietary enzymatically treated Artemisia annua L. improves meat quality, antioxidant capacity and energy status of breast muscle in heat-stressed broilers. J. Sci. Food Agric. 2018, 98, 3715–3721. [Google Scholar] [CrossRef]
- Bhatt, S.; Singh, B.; Gupta, M. Antioxidant and prebiotic potential of Murraya koenigii and Brassica oleracea var. botrytis leaves as food ingredient. J. Agric. Food Res. 2020, 2, 100069. [Google Scholar] [CrossRef]
- Abate, G.; Zhang, L.; Pucci, M.; Morbini, G.; Mac Sweeney, E.; Maccarinelli, G.; Ribaudo, G.; Gianoncelli, A.; Uberti, D.; Memo, M.; et al. Phytochemical Analysis and Anti-Inflammatory Activity of Different Ethanolic Phyto-Extracts of Artemisia annua L. Biomolecules 2021, 11, 975. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Arraouadi, S.; Noumi, E.; Aouadi, K.; Adnan, M.; Khan, M.A.; Kadri, A.; Snoussi, M. Antimicrobial, Antioxidant, Anti-Acetylcholinesterase, Antidiabetic, and Pharmacokinetic Properties of Carum carvi L. and Coriandrum sativum L. Essential Oils Alone and in Combination. Molecules 2021, 26, 3625. [Google Scholar] [CrossRef]
- Macovei, I.; Luca, S.V.; Skalicka-Woźniak, K.; Sacarescu, L.; Pascariu, P.; Ghilan, A.; Doroftei, F.; Ursu, E.-L.; Rimbu, C.M.; Horhogea, C.E.; et al. Phyto-Functionalized Silver Nanoparticles Derived from Conifer Bark Extracts and Evaluation of Their Antimicrobial and Cytogenotoxic Effects. Molecules 2022, 27, 217. [Google Scholar] [CrossRef]
- Macovei, I.; Luca, S.V.; Skalicka-Woźniak, K.; Horhogea, C.E.; Rimbu, C.M.; Sacarescu, L.; Vochita, G.; Gherghel, D.; Ivanescu, B.L.; Panainte, A.D.; et al. Silver Nanoparticles Synthesized from Abies alba and Pinus sylvestris Bark Extracts: Characterization, Antioxidant, Cytotoxic, and Antibacterial Effects. Antioxidants 2023, 12, 797. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Habtemariam, S.; Ahmed, T.; Sureda, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.M. Polyphenolic composition of Crataegus monogyna Jacq.: From chemistry to medical applications. Nutrients 2015, 7, 7708–7728. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Saeed, M.S.; Sattar, S.; Sajad, S.; Sajjad, M.; Adnan, M.; Iqbal, M.; Sharif, M.T. Specific role of proline against heavy metals toxicity in plants. Int. J. Pure Appl. Biosci. 2017, 5, 27–34. [Google Scholar] [CrossRef]
- Czarnocka, W.; Karpiński, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free. Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xue, Z.; Li, H.; Zhang, X.; Wang, X.; Lu, X. Cultivation of dandelion (Taraxacum erythropodium) on coastal saline land based on the control of salinity and fertilizer. Folia Hortic. 2019, 31, 277–284. [Google Scholar] [CrossRef]
- Arora, M.; Saxena, P.; Abdin, M.Z.; Varma, A. Interaction between Piriformospora indica and Azotobacter chroococcum diminish the effect of salt stress in Artemisia annua L. by enhancing enzymatic and non-enzymatic antioxidants. Symbiosis 2020, 80, 61–73. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Laftouhi, A.; Eloutassi, N.; Ech-Chihbi, E.; Rais, Z.; Abdellaoui, A.; Taleb, A.; Beniken, M.; Nafidi, H.-A.; Salamatullah, A.M.; Bourhia, M.; et al. The Impact of Environmental Stress on the Secondary Metabolites and the Chemical Compositions of the Essential Oils from Some Medicinal Plants Used as Food Supplements. Sustainability 2023, 15, 7842. [Google Scholar] [CrossRef]
- Hartley, I.E.; Liem, D.G.; Keast, R. Umami as an ‘Alimentary’ Taste. A New Perspective on Taste Classification. Nutrients 2019, 11, 182. [Google Scholar] [CrossRef]
- Shiga, K.; Yamamoto, S.; Nakajima, A.; Kodama, Y.; Imamura, M.; Sato, T.; Uchida, R.; Obata, A.; Bamba, T.; Fukusaki, E. Metabolic Profiling Approach to Explore Compounds Related to the Umami of Soy Sauce. J. Agric. Food Chem. 2014, 62, 7317–7322. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, E.-Y.; Son, H.J.; Lee, J.-J.; Choi, Y.-H.; Rhyu, M.-R. Identification of a key umami-active fraction in modernized Korean soy sauce and the impact thereof on bitter-masking. Food Chem. 2017, 233, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Asthir, B.J.B.P. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Raza, M.F.; Wang, Y.; Cai, Z.; Bai, S.; Yao, Z.; Awan, U.A.; Zhang, Z.; Zheng, W.; Zhang, H. Gut microbiota promotes host resistance to low-temperature stress by stimulating its arginine and proline metabolism pathway in adult Bactrocera dorsalis. PLoS Pathog. 2020, 16, e1008441. [Google Scholar] [CrossRef]
- Dou, M.; Lu, C.; Sun, Z.; Rao, W. Natural cryoprotectants combinations of l-proline and trehalose for red blood cells cryopreservation. Cryobiology 2019, 91, 23–29. [Google Scholar] [CrossRef]
- Kumari, L.; Mazumder, P.M.; Lal, U.R. Activity of proline and its analogs isolated from Murraya koenigii against hyperglycemia, oxidative stress and renal insufficiency in diabetic nephropathy. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 71–79. [Google Scholar]
- Ghosh, S.; Bheri, M.; Bisht, D.; Pandey, G.K. Calcium signaling and transport machinery: Potential for development of stress tolerance in plants. Curr. Plant Biol. 2022, 29, 100235. [Google Scholar] [CrossRef]
- Lynch, D.R.; Anegawa, N.J.; Verdoorn, T.; Pritchett, D.B. N-methyl-D-aspartate receptors: Different subunit requirements for binding of glutamate antagonists, glycine antagonists, and channel-blocking agents. Mol. Pharmacol. 1994, 45, 540–545. [Google Scholar]
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino acids in cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar] [CrossRef]
- Moe-Lange, J.; Gappel, N.M.; Machado, M.; Wudick, M.M.; Sies, C.S.A.; Schott-Verdugo, S.N.; Bonus, M.; Mishra, S.; Hartwig, T.; Bezrutczyk, M.; et al. Interdependence of a mechanosensitive anion channel and glutamate receptors in distal wound signaling. Sci. Adv. 2021, 7, abg4298. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, J.; Yong, J.W.; Liu, Y.; Cao, D.; He, X. Glutamate over-accumulation may serve as an endogenous indicator of tricarboxylic acid (TCA) cycle suppression under NH4+ nutrition in wheat (Triticum aestivum L.) seedlings. Environ. Exp. Bot. 2020, 177, 104130. [Google Scholar] [CrossRef]
- Yang, T.; Li, H.; Tai, Y.; Dong, C.; Cheng, X.; Xia, E.; Chen, Z.; Li, F.; Wan, X.; Zhang, Z. Transcriptional regulation of amino acid metabolism in response to nitrogen deficiency and nitrogen forms in tea plant root (Camellia sinensis L.). Sci. Rep. 2020, 10, 6868. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Ghosh, A.; Li, Z.-G.; Siddiqui, M.N.; Fujita, M.; Tran, L.-S.P. Methylglyoxal—A signaling molecule in plant abiotic stress responses. Free Radic. Biol. Med. 2018, 122, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jia, X.; Zhang, Z.; Chen, K.; Wang, L.; Chen, H.; Yang, Z.; Li, C.; Zhao, L. AgNPs seed priming accelerated germination speed and altered nutritional profile of Chinese cabbage. Sci. Total Environ. 2022, 808, 151896. [Google Scholar] [CrossRef]
- Li, M.; Guo, R.; Jiao, Y.; Jin, X.; Zhang, H.; Shi, L. Comparison of Salt Tolerance in Soja Based on Metabolomics of Seedling Roots. Front. Plant Sci. 2017, 8, 1101. [Google Scholar] [CrossRef] [PubMed]
- Stoilova, I.; Krastanov, A.; Stoyanova, A.; Denev, P.; Gargova, S. Antioxidant activity of a ginger extract (Zingiber officinale). Food Chem. 2007, 102, 764–770. [Google Scholar] [CrossRef]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef]
- Nicolai, M.; Pereira, P.; Vitor, R.F.; Reis, C.P.; Roberto, A.; Rijo, P. Antioxidant activity and rosmarinic acid content of ultrasound-assisted ethanolic extracts of medicinal plants. Measurement 2016, 89, 328–332. [Google Scholar] [CrossRef]
- Tepe, B.; Eminagaoglu, O.; Akpulat, H.A.; Aydin, E. Antioxidant potentials and rosmarinic acid levels of the methanolic extracts of Salvia verticillata (L.) subsp. verticillata and S. verticillata (L.) subsp. amasiaca (Freyn & Bornm.) Bornm. Food Chem. 2007, 100, 985–989. [Google Scholar] [CrossRef]
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep. 2021, 11, 10041. [Google Scholar] [CrossRef]
- Lamponi, S.; Baratto, M.C.; Miraldi, E.; Baini, G.; Biagi, M. Chemical profile, antioxidant, anti-proliferative, anticoagulant and mutagenic effects of a hydroalcoholic extract of Tuscan Rosmarinus officinalis. Plants 2021, 10, 97. [Google Scholar] [CrossRef]
- Sharma, Y.; Velamuri, R.; Fagan, J.; Schaefer, J. Full-spectrum analysis of bioactive compounds in rosemary (Rosmarinus officinalis L.) as influenced by different extraction methods. Molecules 2020, 25, 4599. [Google Scholar] [CrossRef]
- Lahouel, Z.; Kharoubi, O.; Boussadia, A.; Bekkouche, Z.; Aoues, A. Effect of Aluminium and Aqueous extract of Rosmarinus officinalis on rat Brain: Impact on Neurobehavioral and Histological study. J. Drug Deliv. Ther. 2020, 10, 179–187. [Google Scholar] [CrossRef]
- Kakouri, E.; Nikola, O.; Kanakis, C.; Hatziagapiou, K.; Lambrou, G.I.; Trigas, P.; Kanaka-Gantenbein, C.; Tarantilis, P.A. Cytotoxic Effect of Rosmarinus officinalis Extract on Glioblastoma and Rhabdomyosarcoma Cell Lines. Molecules 2022, 27, 6348. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, J.; Uthirapathy, S.; Mohammed Ameen, M.S.; Anwer, E.T. Essential oil composition and antidiabetic, anticancer activity of Rosmarinus officinalis L. leaves from Erbil (Iraq). J. Essent. Oil Bear. Plants 2019, 22, 1544–1553. [Google Scholar] [CrossRef]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef]
- Petiwala, S.M.; Johnson, J.J. Diterpenes from rosemary (Rosmarinus officinalis): Defining their potential for anti-cancer activity. Cancer Lett. 2015, 367, 93–102. [Google Scholar] [CrossRef]
- Petersen, M.; Abdullah, Y.; Benner, J.; Eberle, D.; Gehlen, K.; Hücherig, S.; Janiak, V.; Kim, K.H.; Sander, M.; Weitzel, C.; et al. Evolution of rosmarinic acid biosynthesis. Phytochemistry 2009, 70, 1663–1679. [Google Scholar] [CrossRef]
- Baba, S.; Osakabe, N.; Natsume, M.; Yasuda, A.; Muto, Y.; Hiyoshi, T.; Takano, H.; Yoshikawa, T.; Terao, J. Absorption, metabolism, degradation and urinary excretion of rosmarinic acid after intake of Perilla frutescens extract in humans. Eur. J. Nutr. 2005, 44, 1–9. [Google Scholar] [CrossRef]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef]
- Li, P.; Yang, X.; Lee, W.J.; Huang, F.; Wang, Y.; Li, Y. Comparison between synthetic and rosemary-based antioxidants for the deep frying of French fries in refined soybean oils evaluated by chemical and non-destructive rapid methods. Food Chem. 2021, 335, 127638. [Google Scholar] [CrossRef]
- Cedeño-Pinos, C.; Martínez-Tomé, M.; Mercatante, D.; Rodríguez-Estrada, M.T.; Bañón, S. Assessment of a Diterpene-Rich Rosemary (Rosmarinus officinalis L.) Extract as a Natural Antioxidant for Salmon Pâté Formulated with Linseed. Antioxidants 2022, 11, 1057. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Areco, S.; Guz, L.; Candal, R.; Goyanes, S. Release kinetics of rosemary (Rosmarinus officinalis) polyphenols from polyvinyl alcohol (PVA) electrospun nanofibers in several food simulants. Food Packag. Shelf Life 2018, 18, 42–50, Erratum in Food Packag. Shelf Life 2021, 29, 100630. [Google Scholar] [CrossRef]
- Bao, L.; Deng, A.; Li, Z.; Du, G.; Qin, H. Chemical constituents of rhizomes of Zingiber officinale. Zhongguo Zhong Yao Za Zhi 2010, 35, 598–601. [Google Scholar]
- Liao, Y.-R.; Leu, Y.-L.; Chan, Y.-Y.; Kuo, P.-C.; Wu, T.-S. Anti-Platelet Aggregation and Vasorelaxing Effects of the Constituents of the Rhizomes of Zingiber officinale. Molecules 2012, 17, 8928–8937. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Variation of the phytochemical constituents and antioxidant activities of Zingiber officinale var. rubrum Theilade associated with different drying methods and polyphenol oxidase activity. Molecules 2016, 21, 780. [Google Scholar] [CrossRef]
- Nishidono, Y.; Saifudin, A.; Nishizawa, M.; Fujita, T.; Nakamoto, M.; Tanaka, K. Identification of the Chemical Constituents in Ginger (Zingiber officinale) Responsible for Thermogenesis. Nat. Prod. Commun. 2018, 13, 1934578x1801300722. [Google Scholar] [CrossRef]
- Crichton, M.; Marshall, S.; Marx, W.; McCarthy, A.L.; Isenring, E. Efficacy of Ginger (Zingiber officinale) in Ameliorating Chemotherapy-Induced Nausea and Vomiting and Chemotherapy-Related Outcomes: A Systematic Review Update and Meta-Analysis. J. Acad. Nutr. Diet. 2019, 119, 2055–2068. [Google Scholar] [CrossRef]
- Nikkhah Bodagh, M.; Maleki, I.; Hekmatdoost, A. Ginger in gastrointestinal disorders: A systematic review of clinical trials. Food Sci. Nutr. 2018, 7, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Venkatadri, B.; Shanparvish, E.; Rameshkumar, M.; Arasu, M.V.; Al-Dhabi, N.A.; Ponnusamy, V.K.; Agastian, P. Green synthesis of silver nanoparticles using aqueous rhizome extract of Zingiber officinale and Curcuma longa: In-vitro anti-cancer potential on human colon carcinoma HT-29 cells. Saudi J. Biol. Sci. 2020, 27, 2980–2986. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, H.; Cheng, Z.; Wang, H. Regulation of the PI3K/AKT/mTOR signaling pathway with synthesized bismuth oxide nanoparticles from Ginger (Zingiber officinale) extract: Mitigating the proliferation of colorectal cancer cells. Arab. J. Chem. 2022, 15, 103607. [Google Scholar] [CrossRef]
- Yeh, H.-Y.; Chuang, C.-H.; Chen, H.-C.; Wan, C.-J.; Chen, T.-L.; Lin, L.-Y. Bioactive components analysis of two various gingers (Zingiber officinale Roscoe) and antioxidant effect of ginger extracts. LWT Food Sci. Technol. 2014, 55, 329–334. [Google Scholar] [CrossRef]
- Srinivasan, K. Ginger rhizomes (Zingiber officinale): A spice with multiple health beneficial potentials. PharmaNutrition 2017, 5, 18–28. [Google Scholar] [CrossRef]
- Shortle, E.; O’Grady, M.; Gilroy, D.; Furey, A.; Quinn, N.; Kerry, J. Influence of extraction technique on the anti-oxidative potential of hawthorn (Crataegus monogyna) extracts in bovine muscle homogenates. Meat Sci. 2014, 98, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Wyspiańska, D.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kolniak-Ostek, J. Physico-chemical, antioxidant, and anti-inflammatory properties and stability of hawthorn (Crataegus monogyna Jacq.) procyanidins microcapsules with inulin and maltodextrin. J. Sci. Food Agric. 2017, 97, 669–678. [Google Scholar] [CrossRef]
- Bekbolatova, E.; Kukula-Koch, W.; Baj, T.; Stasiak, N.; Ibadullayeva, G.; Koch, W.; Głowniak, K.; Tulemissov, S.; Sakipova, Z.; Boylan, F. Phenolic composition and antioxidant potential of different organs of Kazakh Crataegus almaatensis Pojark: A comparison with the European Crataegus oxyacantha L. flowers. Open Chem. 2018, 16, 415–426. [Google Scholar] [CrossRef]
- Rodrigues, S.; Calhelha, R.C.; Barreira, J.C.; Dueñas, M.; Carvalho, A.M.; Abreu, R.M.; Santos-Buelga, C.; Ferreira, I.C. Crataegus monogyna buds and fruits phenolic extracts: Growth inhibitory activity on human tumor cell lines and chemical characterization by HPLC–DAD–ESI/MS. Food Res. Int. 2012, 49, 516–523. [Google Scholar] [CrossRef]
- Moldovan, C.; Frumuzachi, O.; Babotă, M.; Menghini, L.; Cesa, S.; Gavan, A.; Sisea, C.R.; Tanase, C.; Dias, M.I.; Pereira, C.; et al. Development of an Optimized Drying Process for the Recovery of Bioactive Compounds from the Autumn Fruits of Berberis vulgaris L. and Crataegus monogyna Jacq. Antioxidants 2021, 10, 1579. [Google Scholar] [CrossRef]
- Coimbra, A.T.; Luís, Â.F.; Batista, M.T.; Ferreira, S.M.; Duarte, A.P.C. Phytochemical characterization, bioactivities evaluation and synergistic effect of Arbutus unedo and Crataegus monogyna extracts with Amphotericin B. Curr. Microbiol. 2020, 77, 2143–2154. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C. Comparing the composition and bioactivity of Crataegus Monogyna flowers and fruits used in folk medicine. Phytochem. Anal. 2011, 22, 181–188. [Google Scholar] [CrossRef]
- Alirezalu, A.; Ahmadi, N.; Salehi, P.; Sonboli, A.; Alirezalu, K.; Mousavi Khaneghah, A.; Barba, F.J.; Munekata, P.E.; Lorenzo, J.M. Physicochemical Characterization, Antioxidant Activity, and Phenolic Compounds of Hawthorn (Crataegus spp.) Fruits Species for Potential Use in Food Applications. Foods 2020, 9, 436. [Google Scholar] [CrossRef]
- Klecáková, J.; Chobot, V.; Jahodár, L.; Laakso, I.; Víchová, P. Antiradical activity of petals of Philadelphus coronarius L. Cent. Eur. J. Public Health 2004, 12, S39–S40. [Google Scholar] [PubMed]
- Song, X.; Wang, Y.; Gao, L. Mechanism of antioxidant properties of quercetin and quercetin-DNA complex. J. Mol. Model. 2020, 26, 133. [Google Scholar] [CrossRef] [PubMed]
- Pető, Á.; Kósa, D.; Haimhoffer, Á.; Nemes, D.; Fehér, P.; Ujhelyi, Z.; Vecsernyés, M.; Váradi, J.; Fenyvesi, F.; Frum, A.; et al. Topical Dosage Formulation of Lyophilized Philadelphus coronarius L. Leaf and Flower: Antimicrobial, Antioxidant and Anti-Inflammatory Assessment of the Plant. Molecules 2022, 27, 2652. [Google Scholar] [CrossRef]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crop. Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Aziz, S. Studies on the chemical constituents of Thymus serpyllum. Turk. J. Chem. 2008, 32, 605–614. [Google Scholar]
- Sonmezdag, A.S.; Kelebek, H.; Selli, S. Characterization of aroma-active and phenolic profiles of wild thyme (Thymus serpyllum) by GC-MS-Olfactometry and LC-ESI-MS/MS. J. Food Sci. Technol. 2016, 53, 1957–1965. [Google Scholar] [CrossRef]
- Nieto, G. A Review on Applications and Uses of Thymus in the Food Industry. Plants 2020, 9, 961. [Google Scholar] [CrossRef]
- Bozkurt, E.; Atmaca, H.; Kisim, A.; Uzunoglu, S.; Uslu, R.; Karaca, B. Effects of Thymus serpyllum Extract on Cell Proliferation, Apoptosis and Epigenetic Events in Human Breast Cancer Cells. Nutr. Cancer 2012, 64, 1245–1250. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Cardoso, S.M. Health-promoting effects of Thymus phenolic-rich extracts: Antioxidant, anti-inflammatory and antitumoral properties. Antioxidants 2020, 9, 814. [Google Scholar] [CrossRef]
- Pandey, A.K.; Chávez-González, M.L.; Silva, A.S.; Singh, P. Essential oils from the genus Thymus as antimicrobial food preservatives: Progress in their use as nanoemulsions—A new paradigm. Trends Food Sci. Technol. 2021, 111, 426–441. [Google Scholar] [CrossRef]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Terentjeva, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.; Kačániová, M. Thymus serpyllum Essential Oil and Its Biological Activity as a Modern Food Preserver. Plants 2021, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, J.B.; Naveen, G. Phytochemical analysis and antibacterial activity of different leaf extracts of Murraya koenigii. IJBB 2016, 1, 5. [Google Scholar]
- Kusuma, I.W.; Kuspradini, H.; Arung, E.T.; Aryani, F.; Min, Y.-H.; Kim, J.-S.; Kim, Y.-U. Biological Activity and Phytochemical Analysis of Three Indonesian Medicinal Plants, Murraya koenigii, Syzygium polyanthum and Zingiber purpurea. J. Acupunct. Meridian Stud. 2011, 4, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, J.U.; Bhuiyan, M.N.I.; Yusuf, M. Chemical composition of the leaf essential oils of Murraya koenigii (L.) Spreng and Murraya paniculata (L.) Jack. Bangladesh J. Pharmacol. 2008, 3, 59–63. [Google Scholar] [CrossRef]
- Nishan, M.; Subramanian, P. Murraya koenigii (curry leave)—A review on its potential. Int. J. Pharm. Tech. Res 2015, 7, 566–572. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A.; Devarajan, T. Evaluation of bioactive compounds, pharmaceutical quality, and anticancer activity of curry leaf (Murraya koenigii L.). Evid. Based Complement. Altern. Med. 2014, 2014, 873803. [Google Scholar] [CrossRef]
- Saini, S.C.; Reddy, G.B.S. A review on curry leaves (Murraya koenigii): Versatile multi-potential medicinal plant. Am. J. Phytomed. Clin. Ther. 2015, 3, 363–368. [Google Scholar]
- Bhatt, S.; Dadwal, V.; Padwad, Y.; Gupta, M. Study of physicochemical, nutritional, and anticancer activity of Murraya Koenigii extract for its fermented beverage. J. Food Process. Preserv. 2022, 46, e16137. [Google Scholar] [CrossRef]
- Murugan, K.; Anandaraj, K.; Al-Sohaibani, S. Antiaflatoxigenic food additive potential of Murraya koenigii: An in vitro and molecular interaction study. Food Res. Int. 2013, 52, 8–16. [Google Scholar] [CrossRef]
- Das, A.K.; Rajkumar, V.; Dwivedi, D.K. Antioxidant effect of curry leaf (Murraya koenigii) powder on quality of ground and cooked goat meat. Int. Food Res. J. 2011, 18, 563–569. [Google Scholar]
- Drisya, C.R.; Swetha, B.G.; Velu, V.; Indrani, D.; Singh, R.P. Effect of dried Murraya koenigii leaves on nutritional, textural and organoleptic characeteristics of cookies. J. Food Sci. Technol. 2014, 52, 500–506. [Google Scholar] [CrossRef]
- Ferreira, J.F.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as Antioxidants and Their Potential Synergism with Artemisinin against Malaria and Cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef]
- Soni, R.; Shankar, G.; Mukhopadhyay, P.; Gupta, V. A concise review on Artemisia annua L.: A major source of diverse medicinal compounds. Ind. Crop. Prod. 2022, 184, 115072. [Google Scholar] [CrossRef]
- Towler, M.J.; Weathers, P.J. Variations in key artemisinic and other metabolites throughout plant development in Artemisia annua L. for potential therapeutic use. Ind. Crop. Prod. 2015, 67, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.J.; Schmiech, M.; Hafner, S.; Paetz, C.; Steinborn, C.; Huber, R.; El Gaafary, M.; Werner, K.; Schmidt, C.Q.; Syrovets, T.; et al. Antitumor activity of an Artemisia annua herbal preparation and identification of active ingredients. Phytomedicine 2019, 62, 152962. [Google Scholar] [CrossRef]
- El-Askary, H.; Salem, H.H.; Abdel Motaal, A. Potential mechanisms involved in the protective effect of dicaffeoylquinic acids from Artemisia annua L. leaves against diabetes and its complications. Molecules 2022, 27, 857. [Google Scholar] [CrossRef]
- Sharma, V.; Hussain, S.; Gupta, M.; Saxena, A.K. In vitro anticancer activity of extracts of Mentha Spp. against human cancer cells. Indian J. Biochem. Biophys. 2014, 51, 416–419. [Google Scholar]
- Arumugam, P.; Ramamurthy, P.; Santhiya, S.T.; Ramesh, A. Antioxidant activity measured in different solvent fractions obtained from Mentha spicata Linn.: An analysis by ABTS + decolorization assay. Asia Pac. J. Clin. Nutr. 2006, 15, 119–124. [Google Scholar]
- avar Zeljković, S.; Šišková, J.; Komzáková, K.; De Diego, N.; Kaffková, K.; Tarkowski, P. Phenolic compounds and biological activity of selected Mentha species. Plants 2021, 10, 550. [Google Scholar] [CrossRef]
- Zhang, L.L.; Chen, Y.; Li, Z.J.; Li, X.; Fan, G. Bioactive properties of the aromatic molecules of spearmint (Mentha spicata L.) essential oil: A review. Food Funct. 2022, 13, 3110–3132. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). LWT Food Sci. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Przybylska-Balcerek, A.; Szablewski, T.; Szwajkowska-Michałek, L.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z.; Suchowilska, E.; Tomczyk, Ł.; Stuper-Szablewska, K. Sambucus Nigra Extracts–Natural Antioxidants and Antimicrobial Compounds. Molecules 2021, 26, 2910. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Martins-Gomes, C.; Nunes, F.M.; Silva, A.M. Elderberry (Sambucus nigra L.) extracts promote anti-inflammatory and cellular antioxidant activity. Food Chem. 2022, 15, 100437. [Google Scholar] [CrossRef]
- Mocanu, M.L.; Amariei, S. Elderberries—A Source of Bioactive Compounds with Antiviral Action. Plants 2022, 11, 740. [Google Scholar] [CrossRef]
- Vujanović, M.; Majkić, T.; Zengin, G.; Beara, I.; Tomović, V.; Šojić, B.; Đurović, S.; Radojković, M. Elderberry (Sambucus nigra L.) juice as a novel functional product rich in health-promoting compounds. RSC Adv. 2020, 10, 44805–44814. [Google Scholar] [CrossRef]
- Terzić, M.; Majkić, T.; Beara, I.; Zengin, G.; Miljić, U.; Đurović, S.; Mollica, A.; Radojković, M. Elderberry (Sambucus nigra L.) wine as a novel potential functional food product. Food Biosci. 2022, 50, 102047. [Google Scholar] [CrossRef]
- Nile, S.H.; Wang, H.; Nile, A.; Lin, X.; Dong, H.; Venkidasamy, B.; Sieniawska, E.; Enkhtaivan, G.; Kai, G. Comparative analysis of metabolic variations, antioxidant potential and cytotoxic effects in different parts of Chelidonium majus L. Food Chem. Toxicol. 2021, 156, 112483. [Google Scholar] [CrossRef]
- Stefanowski, N.; Tkachenko, H.; Kurhaluk, N. Antibacterial Activity of Ethanolic Extracts Obtained from Roots and Stems of Chelidonium majus L. Against Enterococcus Faecalis Strains. Agrobiodiversity Improv. Nutr. Health Life Qual. 2021, 5, 296–303. [Google Scholar] [CrossRef]
- Krzyżek, P.; Junka, A.; Słupski, W.; Dołowacka-Jóźwiak, A.; Płachno, B.J.; Sobiecka, A.; Matkowski, A.; Chodaczek, G.; Płusa, T.; Gościniak, G.; et al. Antibiofilm and Antimicrobial-Enhancing Activity of Chelidonium majus and Corydalis cheilanthifolia Extracts against Multidrug-Resistant Helicobacter pylori. Pathogens 2021, 10, 1033. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zuo, G.; Hao, X.; Wang, G.; Xiao, H.; Zhang, J.; Xu, G. Antifungal activity of the benzo[c]phenanthridine alkaloids from Chelidonium majus Linn against resistant clinical yeast isolates. J. Ethnopharmacol. 2009, 125, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Warowicka, A.; Popenda, Ł.; Bartkowiak, G.; Musidlak, O.; Litowczenko-Cybulska, J.; Kuźma, D.; Nawrot, R.; Jurga, S.; Goździcka-Józefiak, A. Protoberberine compounds extracted from Chelidonium majus L. as novel natural photosensitizers for cancer therapy. Phytomedicine 2019, 64, 152919. [Google Scholar] [CrossRef]
- Deljanin, M.; Nikolic, M.; Baskic, D.; Todorovic, D.; Djurdjevic, P.; Zaric, M.; Stankovic, M.; Todorovic, M.; Avramovic, D.; Popovic, S. Chelidonium majus crude extract inhibits migration and induces cell cycle arrest and apoptosis in tumor cell lines. J. Ethnopharmacol. 2016, 190, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, A.; Zucchetti, P. A comprehensive review of the benefits of Taraxacum officinale on human health. Bull. Natl. Res. Cent. 2021, 45, 110. [Google Scholar] [CrossRef]
- Lis, B.; Olas, B. Pro-health activity of dandelion (Taraxacum officinale L.) and its food products—History and present. J. Funct. Foods 2019, 59, 40–48. [Google Scholar] [CrossRef]
- Astafieva, A.A.; Enyenihi, A.A.; Rogozhin, E.A.; Kozlov, S.A.; Grishin, E.V.; Odintsova, T.I.; Zubarev, R.A.; Egorov, T.A. Novel proline-hydroxyproline glycopeptides from the dandelion (Taraxacum officinale Wigg.) flowers: De novo sequencing and biological activity. Plant Sci. 2015, 238, 323–329. [Google Scholar] [CrossRef]
- Jeon, H.J.; Kang, H.J.; Jung, H.J.; Kang, Y.S.; Lim, C.J.; Kim, Y.M.; Park, E.H. Anti-inflammatory activity of Taraxacum officinale. J. Ethnopharmacol. 2008, 115, 82–88. [Google Scholar] [CrossRef]
- Menke, K.; Schwermer, M.; Falke, K.; Felenda, J.; Beckmann, C.; Stintzing, F.; Voigt, A.; Schramm, A.; Zuzak, T.J. Taraxacum officinale extract induces antitumorigenic effects in ovarian carcinoma cell lines. Eur. J. Gynaecol Oncol. 2019, 40, 106–112. [Google Scholar]
- Bhosale, P.B.; Abusaliya, A.; Kim, H.H.; Ha, S.E.; Park, M.Y.; Jeong, S.H.; Vetrivel, P.; Heo, J.D.; Kim, J.-A.; kil Won, C.; et al. Apigetrin Promotes TNFα-Induced Apoptosis, Necroptosis, G2/M Phase Cell Cycle Arrest, and ROS Generation through Inhibition of NF-κB Pathway in Hep3B Liver Cancer Cells. Cells 2022, 11, 2734. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Golkar, P.; Tarkesh, M. Effects of methyl jasmonate elicitation on the carvone and limonene contents, phenolic compounds and antioxidant activity in caraway (Carum carvi L.) callus cultures. Nat. Prod. Res. 2023, 1–6, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.; Maraei, R.; Rezk, A.; Diab, A. Phytochemical constitutes and biological activities of essential oil extracted from irradiated caraway seeds (Carum carvi L.). Int. J. Radiat. Biol. 2023, 99, 318–328. [Google Scholar] [CrossRef]
- Khatamian, N.; Tabrizi, M.H.; Ardalan, P. Effect of Carum carvi essential oil nanoemulsion on tubo cancer cells and L929 normal cells and evaluation of antioxidant activity. Urmia Med. J. 2019, 30, 315–321. [Google Scholar]
- Es-Safi, I.; Mechchate, H.; Amaghnouje, A.; Jawhari, F.Z.; Al Kamaly, O.M.; Imtara, H.; Grafov, A.; Bari, A.; Bousta, D. An Insight into the Anxiolytic and Antidepressant-Like Proprieties of Carum carvi L. and Their Association with Its Antioxidant Activity. Life 2021, 11, 207. [Google Scholar] [CrossRef]
- Bourgou, S.; Bettaieb Rebey, I.; Dakhlaoui, S.; Msaada, K.; Saidani Tounsi, M.; Ksouri, R.; Fauconnier, M.; Hamrouni-Sellami, I. Green extraction of oil from Carum carvi seeds using bio-based solvent and supercritical fluid: Evaluation of its antioxidant and anti-inflammatory activities. Phytochem. Anal. 2019, 31, 37–45. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Tumer, B.; Ozleyen, A.; Pérez, R.; Ezzat, S.M.; Azzini, E.; Hosseinabadi, T.; Butnariu, M.; Sarac, I.; et al. Symphytum Species: A Comprehensive Review on Chemical Composition, Food Applications and Phytopharmacology. Molecules 2019, 24, 2272. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.R.; Costab, G.; Figueirinha, A.; Liberal, J.; Prior, J.A.V.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Urtica spp.: Phenolic composition, safety, antioxidant and anti-inflammatory activities. Food Res. Int. 2017, 99, 485–494. [Google Scholar] [CrossRef]
- Batool, R.; Salahuddin, H.; Mahmood, T.; Ismail, M. Study of anticancer and antibacterial activities of Foeniculum vulgare, Justicia adhatoda and Urtica dioica as natural curatives. Cell. Mol. Biol. 2017, 63, 109–114. [Google Scholar] [CrossRef]
- Otles, S.; Yalcin, B. Phenolic compounds analysis of root, stalk, and leaves of nettle. Sci. World J. 2012, 2012, 564367. [Google Scholar] [CrossRef]
- Bisht, R.; Joshi, B.C.; Kalia, A.N.; Prakash, A. Antioxidant-Rich Fraction of Urtica dioica Mediated Rescue of Striatal Mito-Oxidative Damage in MPTP-Induced Behavioral, Cellular, and Neurochemical Alterations in Rats. Mol. Neurobiol. 2017, 54, 5632–5645. [Google Scholar] [CrossRef] [PubMed]


| Sample ID | Plants | Family | Parts Investigated | TAA | AOA at 30 min |
|---|---|---|---|---|---|
| #1 | Carum carvi L. | Apiaceae | seeds | 2.42 | 12.30 |
| #2 | Ocimum basilicum L. | Lamiaceae | leaf | 0.26 | 8.04 |
| #3 | Sambucus nigra L. | Adoxaceae | fruits | 4.31 | 67.09 |
| #4 | Taraxacum officinale (L.) Weber ex F.H. Wigg. | Asteraceae | leaf | 13.50 | 25.75 |
| #5 | Symphytum officinale L. | Boraginaceae | root | 1.94 | 19.67 |
| #6 | Crataegus monogyna Jacq. | Rosaceae | fruits | 1.04 | 92.95 |
| #7 | Philadelphus coronarius L. | Hydrangeaceae | aerial plant | 4.22 | 89.00 |
| #8 | Chelidonium majus L. | Papaverales | aerial plant | 7.04 | 35.19 |
| #9 | Thymus serpyllum L. | Lamiaceae | aerial plant | 2.26 | 93.76 |
| #10 | Artemisia annua L. | Asteraceae | aerial plant | 12.09 | 55.81 |
| #11 | Mentha spicata L. | Lamiaceae | aerial plant | 9.08 | 56.72 |
| #12 | Salvia officinalis L. | Lamiaceae | aerial plant | 4.05 | 92.36 |
| #13 | Rosmarinus officinalis L. | Lamiaceae | aerial plant | 0.95 | 95.16 |
| #14 | Urtica dioica L. | Urticaceae | aerial plant | 7.41 | 10.50 |
| #15 | Zingiber officinale Roscoe | Zingiberaceae | root | 5.82 | 86.86 |
| #16 | Murraya koenigii L. | Rutaceae | leaf | 20.71 | 91.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iordache, A.M.; Nechita, C.; Podea, P.; Șuvar, N.S.; Mesaroṣ, C.; Voica, C.; Bleiziffer, R.; Culea, M. Comparative Amino Acid Profile and Antioxidant Activity in Sixteen Plant Extracts from Transylvania, Romania. Plants 2023, 12, 2183. https://doi.org/10.3390/plants12112183
Iordache AM, Nechita C, Podea P, Șuvar NS, Mesaroṣ C, Voica C, Bleiziffer R, Culea M. Comparative Amino Acid Profile and Antioxidant Activity in Sixteen Plant Extracts from Transylvania, Romania. Plants. 2023; 12(11):2183. https://doi.org/10.3390/plants12112183
Chicago/Turabian StyleIordache, Andreea Maria, Constantin Nechita, Paula Podea, Niculina Sonia Șuvar, Cornelia Mesaroṣ, Cezara Voica, Ramona Bleiziffer, and Monica Culea. 2023. "Comparative Amino Acid Profile and Antioxidant Activity in Sixteen Plant Extracts from Transylvania, Romania" Plants 12, no. 11: 2183. https://doi.org/10.3390/plants12112183
APA StyleIordache, A. M., Nechita, C., Podea, P., Șuvar, N. S., Mesaroṣ, C., Voica, C., Bleiziffer, R., & Culea, M. (2023). Comparative Amino Acid Profile and Antioxidant Activity in Sixteen Plant Extracts from Transylvania, Romania. Plants, 12(11), 2183. https://doi.org/10.3390/plants12112183






