Effect of Biochar Type, Concentration and Washing Conditions on the Germination Parameters of Three Model Crops
Abstract
1. Introduction
2. Results
2.1. Germination Tests
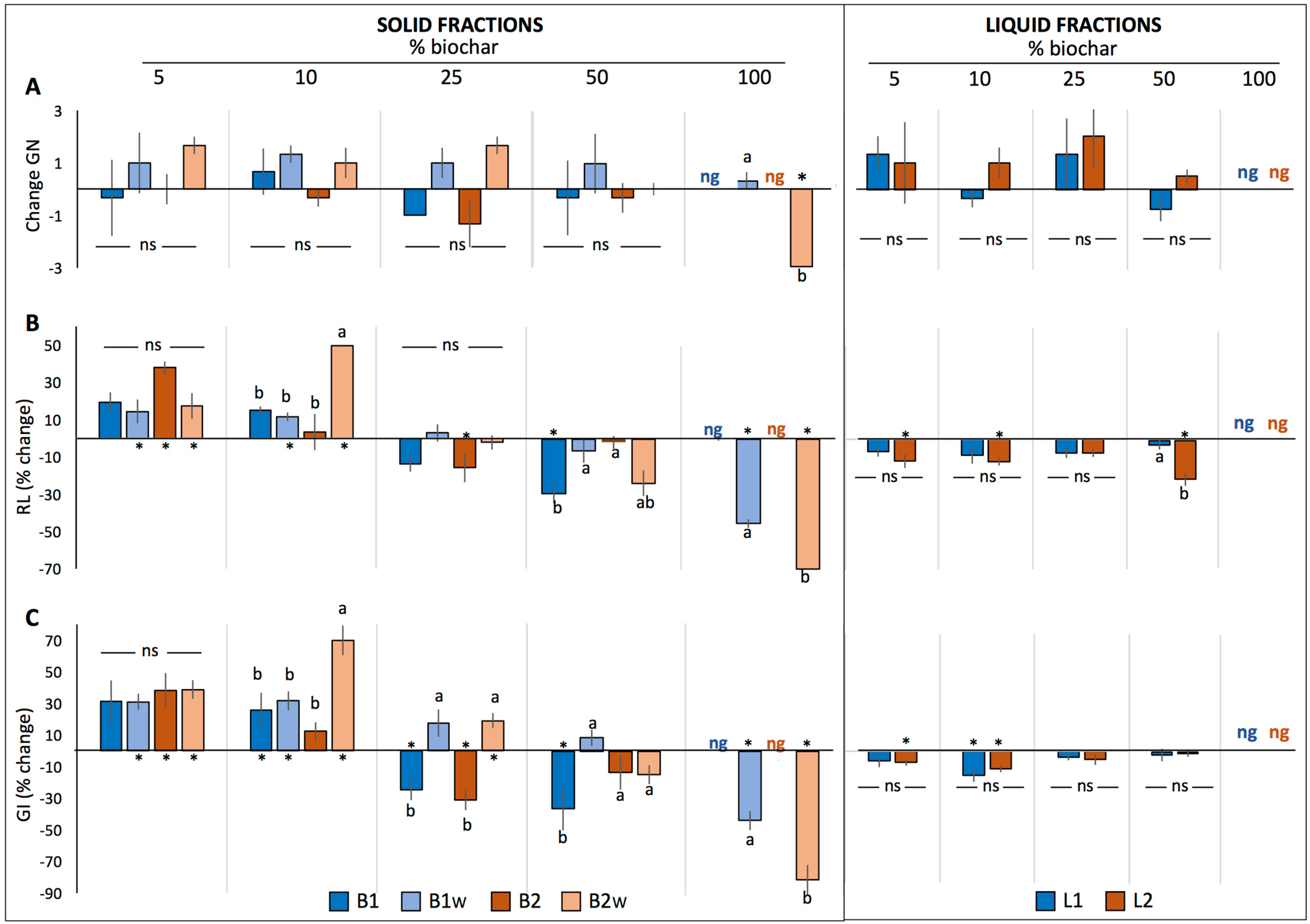
2.1.1. Basil
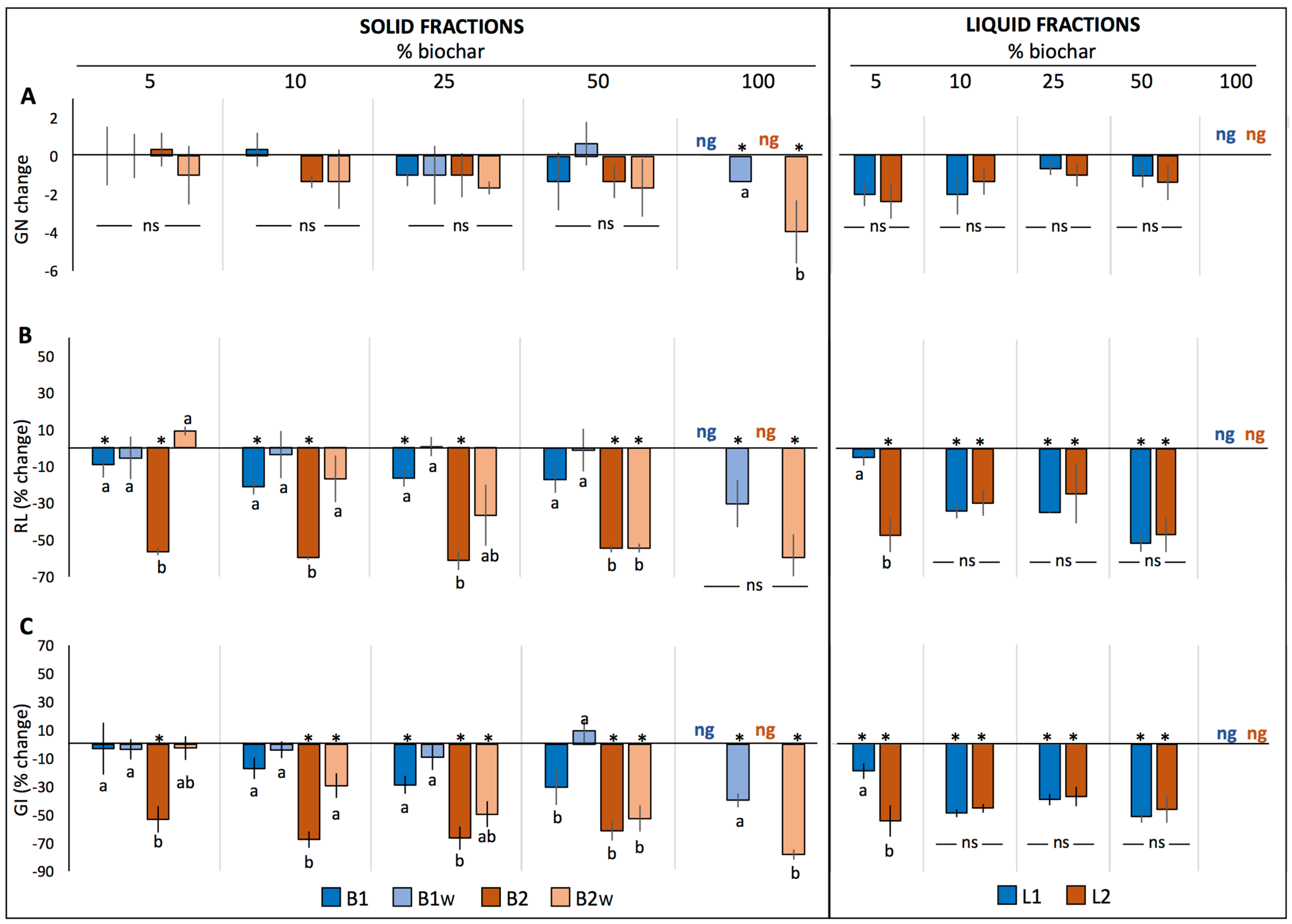
2.1.2. Lettuce
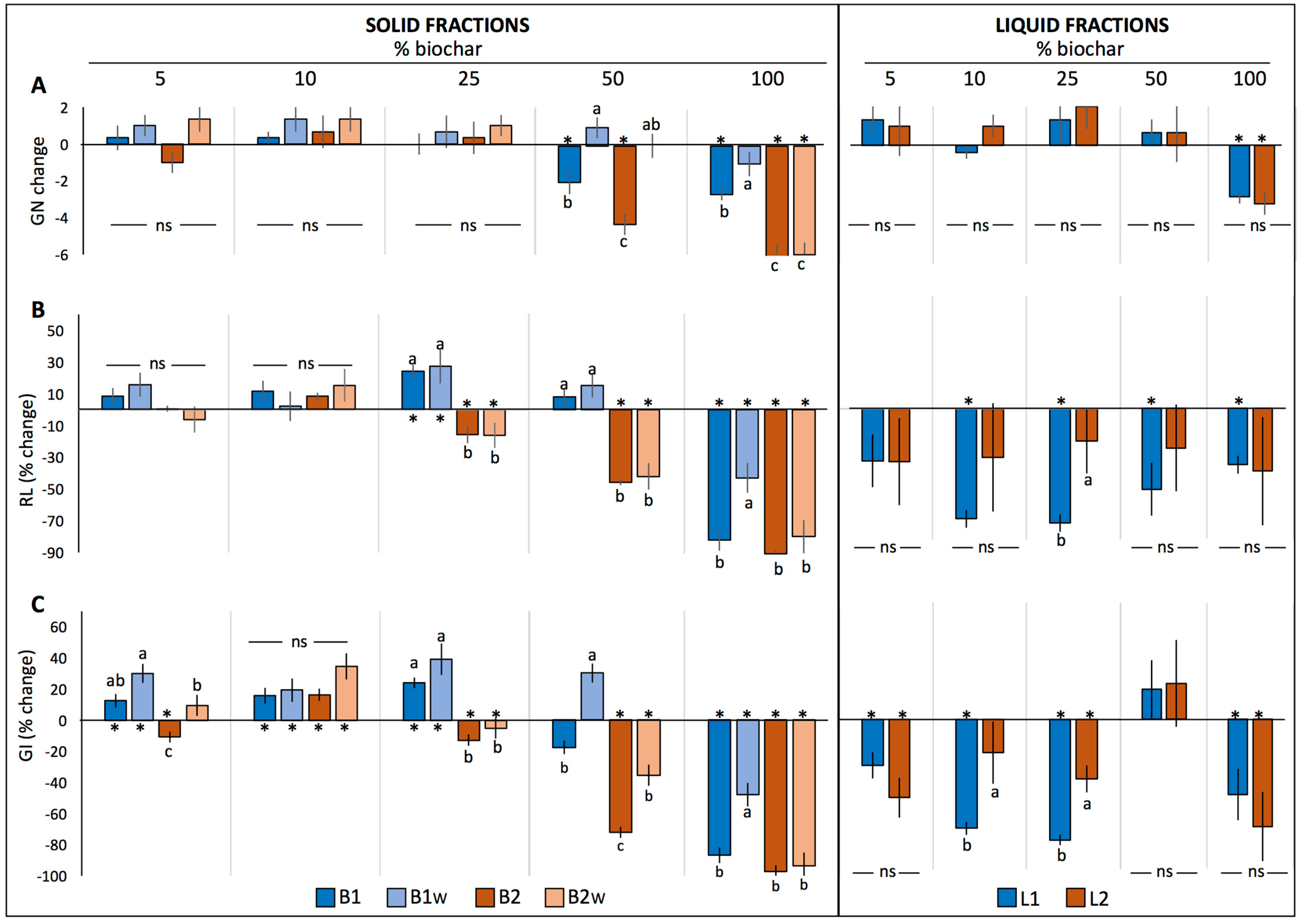
2.1.3. Tomato
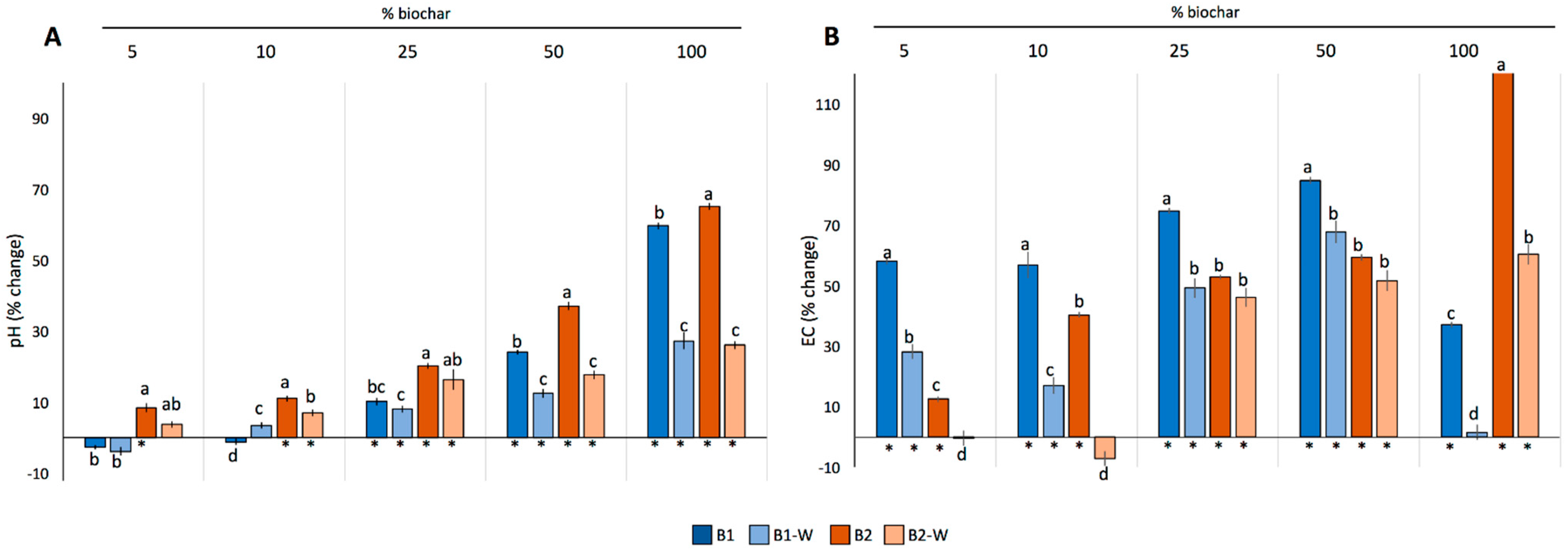
2.2. Effects of Biochar Addition on pH and EC
3. Discussion
4. Materials and Methods
4.1. Soil–Biochar Mixtures
4.2. Germination Test
4.3. Germination Test in the Washed Mixtures and in the Liquid Fractions
4.4. Measurement of pH and EC
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srivastava, L.M. Plant Growth and Development: Hormones and Environment; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Bever, J.D.; Mangan, S.A.; Alexander, H.M. Maintenance of plant species diversity by pathogens. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 305–325. [Google Scholar] [CrossRef]
- Nelson, E.B. The seed microbiome: Origins, interactions, and impacts. Plant Soil 2018, 422, 7–34. [Google Scholar] [CrossRef]
- Nonogaki, H. Seed germination—The biochemical and molecular mechanisms. Breed. Sci. 2006, 56, 93–105. [Google Scholar] [CrossRef]
- Huang, L.; Gu, M. Effects of biochar on container substrate properties and growth of plants—A review. Horticulturae 2019, 5, 14. [Google Scholar] [CrossRef]
- Rankin, T.; Strachan, I.B.; Strack, M. Carbon dioxide and methane exchange at a post-extraction, unrestored peatland. Ecol. Eng. 2018, 122, 241–251. [Google Scholar] [CrossRef]
- Leifeld, J.; Menichetti, L. The underappreciated potential of peatlands in global climate change mitigation strategies. Nat. Commun. 2018, 9, 1071. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Cui, L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K.; et al. Feedstock choice, pyrolysis temperature and type influence biochar characteristics: A comprehensive meta-data analysis review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Ghodke, P.K.; Sharma, A.K.; Pandey, J.K.; Chen, W.H.; Patel, A.; Ashokkumar, V. Pyrolysis of sewage sludge for sustainable biofuels and value-added biochar production. J. Environ. Manag. 2021, 298, 113450. [Google Scholar] [CrossRef]
- Anand, A.; Gautam, S.; Ram, L.C. Feedstock and pyrolysis conditions affect suitability of biochar for various sustainable energy and environmental applications. J. Anal. Appl. Pyrolysis 2023, 170, 105881. [Google Scholar] [CrossRef]
- Vannini, A.; Bianchi, E.; Avi, D.; Damaggio, N.; Di Lella, L.A.; Nannoni, F.; Protano, G.; Loppi, S. Biochar amendment reduces the availability of Pb in the soil and its uptake in lettuce. Toxics 2021, 9, 268. [Google Scholar] [CrossRef]
- Fedeli, R.; Alexandrov, D.; Celletti, S.; Nafikova, E.; Loppi, S. Biochar improves the performance of Avena sativa L. grown in gasoline-polluted soils. Environ. Sci. Pollut. Res. 2022, 30, 28791–28802. [Google Scholar] [CrossRef]
- Karhu, K.; Mattila, T.; Bergström, I.; Regina, K. Biochar addition to agricultural soil increased CH4 uptake and water holding capacity—Results from a short-term pilot field study. Agric. Ecosyst. Environ. 2011, 140, 309–313. [Google Scholar] [CrossRef]
- Qayyum, M.F.; Haider, G.; Iqbal, M.; Hameed, S.; Ahmad, N.; ur Rehman, M.Z.; Majeed, A.; Rizwan, M.; Ali, S. Effect of alkaline and chemically engineered biochar on soil properties and phosphorus bioavailability in maize. Chemosphere 2021, 266, 128980. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Bamberg, J.B.; Hanneman, R.E.; Towill, L.E. Use of activated charcoal to enhance the germination of botanical seeds of potato. Am. Potato J. 1986, 63, 181–189. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; Calero, J.M.; Barrón, V.; Torrent, J.; del Campillo, M.C.; Gallardo, A.; Villar, R. Effects of biochars produced from different feedstocks on soil properties and sunflower growth. J. Plant Nutr. Soil Sci. 2014, 177, 16–25. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Murphy, D.V.; Abbott, L.K. Biochars influence seed germination and early growth of seedlings. Plant Soil 2012, 353, 273–287. [Google Scholar] [CrossRef]
- Rogovska, N.; Laird, D.; Cruse, R.M.; Trabue, S.; Heaton, E. Germination tests for assessing biochar quality. J. Environ. Qual. 2012, 41, 1014–1022. [Google Scholar] [CrossRef]
- Turk, M.A.; Tawaha, A.M. Allelopathic effect of black mustard (Brassica nigra L.) on germination and growth of wild oat (Avena fatua L.). Crop Prot. 2003, 22, 673–677. [Google Scholar] [CrossRef]
- Xuan, T.D.; Tawata, S.; Khan, T.D.; Chung, I.M. Decomposition of allelopathic plants in soil. J. Agron. Crop Sci. 2005, 191, 162–171. [Google Scholar] [CrossRef]
- Fornes, F.; Belda, R.M.; Fernández de Córdova, P.; Cebolla-Cornejo, J. Assessment of biochar and hydrochar as minor to major constituents of growing media for containerized tomato production. J. Sci. Food Agric. 2017, 97, 3675–3684. [Google Scholar] [CrossRef] [PubMed]
- Bargmann, I.; Rillig, M.C.; Buss, W.; Kruse, A.; Kuecke, M. Hydrochar and biochar effects on germination of spring barley. J. Agron. Crop Sci. 2013, 199, 360–373. [Google Scholar] [CrossRef]
- Kolton, M.; Graber, E.R.; Tsehansky, L.; Elad, Y.; Cytryn, E. Biochar-stimulated plant performance is strongly linked to microbial diversity and metabolic potential in the rhizosphere. New Phytol. 2017, 213, 1393–1404. [Google Scholar] [CrossRef]
- Greco, G.; Videgain, M.; Di Stasi, C.; Pires, E.; Manyà, J. Importance of pyrolysis temperature and pressure in the concentration of polycyclic aromatic hydrocarbons in wood waste-derived biochars. J. Anal. Appl. Pyrolysis 2021, 159, 105337. [Google Scholar] [CrossRef]
- Meschewski, E.; Holm, N.; Sharma, B.K.; Spokas, K.; Minalt, N.; Kelly, J.J. Pyrolysis biochar has negligible effects on soil greenhouse gas production, microbial communities, plant germination, and initial seedling growth. Chemosphere 2019, 228, 565–576. [Google Scholar] [CrossRef]
- Free, H.F.; McGill, C.R.; Rowarth, J.S.; Hedley, M.J. The effect of biochars on maize (Zea mays) germination. N. Z. J. Agric. Res. 2010, 53, 1–4. [Google Scholar] [CrossRef]
- Vannini, A.; Carbognani, M.; Chiari, G.; Forte, T.A.; Lumiero, F.; Malcevschi, A.; Rodolfi, M.; Ganino, T.; Petraglia, A. Effects of Wood-Derived Biochar on Germination, Physiology, and Growth of European Beech (Fagus sylvatica L.) and Turkey Oak (Quercus cerris L.). Plants 2022, 11, 3254. [Google Scholar] [CrossRef]
- Hoover, B.K. Herbaceous perennial seed germination and seedling growth in biochar-amended propagation substrates. HortScience 2018, 53, 236–241. [Google Scholar] [CrossRef]
- Buss, W.; Graham, M.C.; Shepherd, J.G.; Mašek, O. Risks and benefits of 389 marginal biomass-derived biochars for plant growth. Sci. Total Environ. 2019, 569–570, 496–506. [Google Scholar] [CrossRef]
- Barber, S.A. Soil Nutrient Bioavailability: A Mechanistic Approach; John Wiley & Sons: Hoboken, NJ, USA, 1995. [Google Scholar]
- Bergamasco, M.A.M.; Braos, L.B.; Guidini Lopes, I.; Cruz, M.C.P. Nitrogen mineralization and nitrification in two soils with different pH levels. Commun. Soil Sci. Plant Anal. 2019, 50, 2873–2880. [Google Scholar] [CrossRef]
- Cui, H.J.; Wang, M.K.; Fu, M.L.; Ci, E. Enhancing phosphorus availability in phosphorus-fertilized zones by reducing phosphate adsorbed on ferrihydrite using rice straw-derived biochar. J. Soils Sediments 2011, 11, 1135–1141. [Google Scholar] [CrossRef]
- Glaser, B.; Lehr, V.I. Biochar effects on phosphorus availability in agricultural soils: A meta-analysis. Sci. Rep. 2019, 9, 9338. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Mandal, S.; Niazi, N.K.; Vithanage, M.; Parikh, S.J.; Mukome, F.N.; Rizwan, M.; Oleszczuk, P.; Al-Wabel, M.; Bolan, N.; et al. Advances and future directions of biochar characterization methods and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2275–2330. [Google Scholar] [CrossRef]
- Mumme, J.; Getz, J.; Prasad, M.; Lüder, U.; Kern, J.; Mašek, O.; Buss, W. Toxicity screening of biochar-mineral composites using germination tests. Chemosphere 2018, 207, 91–100. [Google Scholar] [CrossRef]
- Prapagdee, S.; Tawinteung, N. Effects of biochar on enhanced nutrient use efficiency of green bean, Vigna radiata L. Environ. Sci. Pollut. Res. 2017, 24, 9460–9467. [Google Scholar] [CrossRef]
- Nobile, C.; Denier, J.; Houben, D. Linking biochar properties to biomass of basil, lettuce and pansy cultivated in growing media. Sci. Hortic. 2020, 261, 109001. [Google Scholar] [CrossRef]
- Mtisi, M.; Gwenzi, W. Evaluation of the phytotoxicity of coal ash on lettuce (Lactuca sativa L.) germination, growth and metal uptake. Ecotoxicol. Environ. Saf. 2019, 170, 750–762. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, S.; Shao, J.; Chen, A.; Jiang, J.; Chen, A.; Luo, S. Exploring the negative effects of biochars on the germination, growth, and antioxidant system of rice and corn. J. Environ. Chem. Eng. 2022, 10, 107398. [Google Scholar] [CrossRef]
- Liao, S.; Pan, B.; Li, H.; Zhang, D.; Xing, B. Detecting free radicals in biochars and determining their ability to inhibit the germination and growth of corn, wheat and rice seedlings. Environ. Sci. Technol. 2014, 48, 8581–8587. [Google Scholar] [CrossRef]
- Buss, W.; Mašek, O. Mobile organic compounds in biochar—A potential source of contamination–phytotoxic effects on cress seed (Lepidium sativum) germination. J. Environ. Manag. 2014, 137, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.T.; Wirth, B.; Lüder, U.; Werner, M. Behavior of selected hydrolyzed and dehydrated products during hydrothermal carbonization of biomass. Bioresour. Technol. 2014, 169, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Busch, D.; Kammann, C.; Grünhage, L.; Müller, C. Simple biotoxicity tests for evaluation of carbonaceous soil additives: Establishment and reproducibility of four test procedures. J. Environ. Qual. 2012, 41, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zheng, H.; Wang, Z.Y. The formation of toxic compounds during biochar production. Appl. Mech. Mater. 2013, 361, 867–870. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Celletti, S.; Bergamo, A.; Benedetti, V.; Pecchi, M.; Patuzzi, F.; Basso, D.; Baratieri, M.; Cesco, S.; Mimmo, T. Phytotoxicity of hydrochars obtained by hydrothermal carbonization of manure-based digestate. J. Environ. Manag. 2021, 280, 111635. [Google Scholar] [CrossRef]
- Celletti, S.; Fedeli, R.; Ghorbani, M.; Loppi, S. Impact of starch-based bioplastic on growth and biochemical parameters of basil plants. Sci. Total Environ. 2023, 856, 159163. [Google Scholar] [CrossRef]
| O. basilicum | L. sativa | S. lycopersicum | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GN | RL | GI | GN | RL | GI | GN | RL | GI | |
| C | 1.81−12 *** | 2−16 *** | 2−16 *** | ns | 4.8−3 ** | 2.25−3 ** | ns | 2−16 *** | 2−16 *** |
| T | ns | ns | ns | ns | 2−16 *** | 2.5−16 *** | ns | 2−16 *** | 2−16 *** |
| W | 7.19−9 *** | 2.47−2 * | ns | ns | 7.1−4 *** | ns | ns | 3.5−3 ** | 1.4−15 *** |
| C*T | ns | 3.68−2 * | 0.012 * | ns | 9.4−4 *** | ns | 4.21−3 ** | 2−16 *** | 9.2−15 *** |
| C*W | 2.04−5 *** | 3.7−16 *** | 2−16 *** | ns | 2−16 *** | 9.2−14 *** | ns | 4.5−4 *** | 2.3−4 *** |
| T*W | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| C*T*W | ns | 4.95−6 *** | 7.26−5 *** | ns | 6−6 *** | 4.6−4*** | 7.21−3 ** | 0.016 * | 0.02 * |
| Characteristics | B1 | B2 | Soil |
|---|---|---|---|
| Particle diameter (mm) | <2 | <2 | |
| Particle diameter (ground, mm) | <0.5 | <0.5 | |
| Total nitrogen (%) | <0.4 | <0.4 | |
| Total potassium (mg kg−1) | 3020 | 3020 | |
| Total phosphorus (mg kg−1) | 340 | 340 | |
| Total calcium (mg kg−1) | 9920 | 9920 | |
| Total magnesium (mg kg−1) | 852 | 852 | |
| Total sodium (mg kg−1) | 291 | 291 | |
| Carbon from carbonate (%) | <0.1 | <0.1 | |
| Total carbon (%) | 68.7 | 68.7 | |
| Water holding capacity (%) | 23.5 | 23.5 | |
| Has content (%) | 4.6 | 4.6 | |
| Carbon/hydrogen ratio | 0.2 | 0.2 | |
| Available phosphorus (mg kg−1, this study *) | 50 ± 4 | 34 ± 2 | |
| EC (µS cm−1, this study **) | 552 ± 5.7 | 999 ± 3.2 | 400 ± 3.3. |
| pH (this study **) | 9.55 ± 0.03 | 9.88 ± 0.03 | 5.98 ± 0.07 |
| CEC (meq 100 kg−1, this study ***) | 90 ± 6 | 115 ± 3 | 553 ± 38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carril, P.; Ghorbani, M.; Loppi, S.; Celletti, S. Effect of Biochar Type, Concentration and Washing Conditions on the Germination Parameters of Three Model Crops. Plants 2023, 12, 2235. https://doi.org/10.3390/plants12122235
Carril P, Ghorbani M, Loppi S, Celletti S. Effect of Biochar Type, Concentration and Washing Conditions on the Germination Parameters of Three Model Crops. Plants. 2023; 12(12):2235. https://doi.org/10.3390/plants12122235
Chicago/Turabian StyleCarril, Pablo, Majid Ghorbani, Stefano Loppi, and Silvia Celletti. 2023. "Effect of Biochar Type, Concentration and Washing Conditions on the Germination Parameters of Three Model Crops" Plants 12, no. 12: 2235. https://doi.org/10.3390/plants12122235
APA StyleCarril, P., Ghorbani, M., Loppi, S., & Celletti, S. (2023). Effect of Biochar Type, Concentration and Washing Conditions on the Germination Parameters of Three Model Crops. Plants, 12(12), 2235. https://doi.org/10.3390/plants12122235









