Preparative Fractionation of Phenolic Compounds and Isolation of an Enriched Flavonol Fraction from Winemaking Industry By-Products by High-Performance Counter-Current Chromatography
Abstract
:1. Introduction
2. Results and Discussion
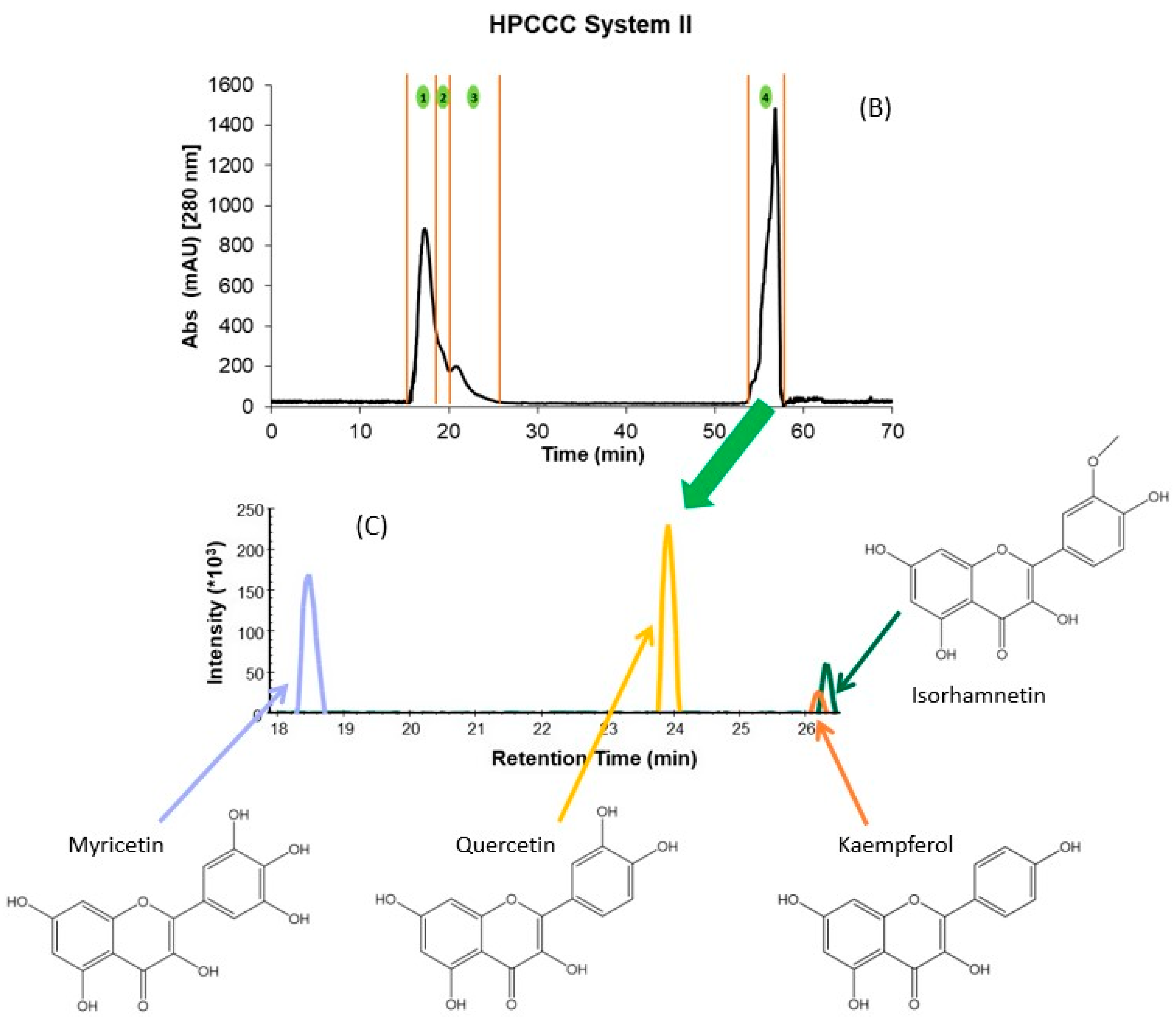
2.1. HPCCC Fractionation
2.2. Identification of PCs in the Different HPCCC Fractions
3. Materials and Methods
3.1. Standards, Solvents, and Materials
3.2. Sample Preparation and PCs Extraction
3.3. HPCCC Separation
3.4. Characterization of Fractions by UHPLC-ESI-MS/MS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- OIV 2021 Statistical Report on World Vitiviniculture. 2021. Available online: https://www.oiv.int/ (accessed on 23 April 2023).
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [Green Version]
- Schieber, A. Side Streams of Plant Food Processing As a Source of Valuable Compounds: Selected Examples. Annu. Rev. Food Sci. Technol. 2017, 8, 97–112. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape Pomace as a Sustainable Source of Bioactive Compounds: Extraction, Characterization, and Biotechnological Applications of Phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- De Iseppi, A.; Lomolino, G.; Marangon, M.; Curioni, A. Current and Future Strategies for Wine Yeast Lees Valorization. Food Res. Int. 2020, 137, 109352. [Google Scholar] [CrossRef]
- Contreras, M.; Romero-García, J.M. Residues from Grapevine and Wine Production as Feedstock for a Biorefinery. Food Bioprod. Process. 2022, 134, 56–79. [Google Scholar] [CrossRef]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable Wineries through Waste Valorisation: A Review of Grape Marc Utilisation for Value-Added Products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green Processing and Biotechnological Potential of Grape Pomace: Current Trends and Opportunities for Sustainable Biorefinery. Bioresour. Technol. 2020, 314, 123771. [Google Scholar] [CrossRef]
- Kopsahelis, N.; Dimou, C.; Papadaki, A.; Xenopoulos, E.; Kyraleou, M.; Kallithraka, S.; Kotseridis, Y.; Koutinas, A.A. Refining of Wine Lees and Cheese Whey for the Production of Microbial Oil, Polyphenol-Rich Extracts and Value-Added Co-Products. J. Chem. Technol. Biotechnol. 2018, 93, 257–268. [Google Scholar] [CrossRef]
- Romero-Díez, R.; Matos, M.; Rodrigues, L.; Bronze, M.R.; Rodríguez-Rojo, S.; Cocero, M.J.; Matias, A.A. Microwave and Ultrasound Pre-Treatments to Enhance Anthocyanins Extraction from Different Wine Lees. Food Chem. 2019, 272, 258–266. [Google Scholar] [CrossRef] [Green Version]
- López-Fernández-Sobrino, R.; Soliz-Rueda, J.R.; Margalef, M.; Arola-Arnal, A.; Suárez, M.; Bravo, F.I.; Muguerza, B. ACE Inhibitory and Antihypertensive Activities of Wine Lees and Relationship among Bioactivity and Phenolic Profile. Nutrients 2021, 13, 679. [Google Scholar] [CrossRef] [PubMed]
- Delgado De La Torre, M.P.; Priego-Capote, F.; Luque De Castro, M.D. Characterization and Comparison of Wine Lees by Liquid Chromatography-Mass Spectrometry in High-Resolution Mode. J. Agric. Food Chem. 2015, 63, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Romero-Díez, R.; Rodríguez-Rojo, S.; Cocero, M.J.; Duarte, C.M.M.; Matias, A.A.; Bronze, M.R. Phenolic Characterization of Aging Wine Lees: Correlation with Antioxidant Activities. Food Chem. 2018, 259, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Ye, Z.; Qin, Y.; Harrison, R.; Hider, R.; Bekhit, A.E.D.A. Characterization of Bioactive Compounds in Lees from New Zealand Wines with Different Vinification Backgrounds. Antioxidants 2022, 11, 2335. [Google Scholar] [CrossRef] [PubMed]
- Barcia, M.T.; Pertuzatti, P.B.; Gómez-Alonso, S.; Godoy, H.T.; Hermosín-Gutiérrez, I. Phenolic Composition of Grape and Winemaking By-Products of Brazilian Hybrid Cultivars BRS Violeta and BRS Lorena. Food Chem. 2014, 159, 95–105. [Google Scholar] [CrossRef]
- Delgado De La Torre, M.P.; Ferreiro-Vera, C.; Priego-Capote, F.; Luque De Castro, M.D. Anthocyanidins, Proanthocyanidins, and Anthocyanins Profiling in Wine Lees by Solid-Phase Extraction-Liquid Chromatography Coupled to Electrospray Ionization Tandem Mass Spectrometry with Data-Dependent Methods. J. Agric. Food Chem. 2013, 61, 12539–12548. [Google Scholar] [CrossRef]
- Li, L.; Zhao, J.; Yang, T.; Sun, B. High-Speed Countercurrent Chromatography as an Efficient Technique for Large Separation of Plant Polyphenols: A Review. Food Res. Int. 2022, 153, 110956. [Google Scholar] [CrossRef]
- Di, D.L.; Zheng, Y.Y.; Chen, X.F.; Huang, X.Y.; Feng, S.L. Advances in Application of High-Speed Countercurrent Chromatography in Separation and Purification of Flavonoids. Chin. J. Anal. Chem. 2011, 39, 269–275. [Google Scholar] [CrossRef]
- Xie, Q.; Wei, Y.; Zhang, G. Separation of Flavonol Glycosides from Flaveria bidentis (L.) Kuntze by High-Speed Counter-Current Chromatography. Sep. Purif. Technol. 2010, 72, 229–233. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Cui, Y.; Zhang, S.; Sun, B. Separation and Purification of Polyphenols from Red Wine Extracts Using High Speed Counter Current Chromatography. J. Chromatogr. B 2017, 1054, 105–113. [Google Scholar] [CrossRef]
- Yang, M.X.; Liang, Y.G.; Chen, H.R.; Huang, Y.F.; Gong, H.G.; Zhang, T.Y.; Ito, Y. Isolation of Flavonoids From Wild Aquilaria Sinensis Leaves by an Improved Preparative High-Speed Counter-Current Chromatography Apparatus. J. Chromatogr. Sci. 2018, 56, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Lv, X.; Yang, G.; Zhan, J.; Li, M.; Long, T.; Ho, C.T.; Li, S. Simultaneous Separation of Six Pure Polymethoxyflavones from Sweet Orange Peel Extract by High Performance Counter Current Chromatography. Food Chem. 2019, 292, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Cui, Y.; Zhang, S.; Li, L.; Li, Y.; Zhou, P.; Sun, B. Preparative Separation of Grape Skin Polyphenols by High-Speed Counter-Current Chromatography. Food Chem. 2016, 212, 712–721. [Google Scholar] [CrossRef]
- Alas, E.R.S.; Uen, M.O.D.; Heynier, Ä.R.C.; Le, Ä. Characterization of Pigments from Different High Speed Countercurrent Chromatography Wine Fractions. J. Agric. Food Chem. 2005, 53, 4536–4546. [Google Scholar] [CrossRef]
- Wray, V.; Winterhalter, P. Preparative Isolation of Procyanidins from Grape Seed Extracts by High-Speed Counter-Current Chromatography. J. Chromatogr. A 2008, 1177, 114–125. [Google Scholar] [CrossRef]
- Kong, Q.; Ren, X.; Hu, R.; Yin, X.; Jiang, G.; Pan, Y. Isolation and Purification of Two Antioxidant Isomers of Resveratrol Dimer from the Wine Grape by Counter-Current Chromatography. J. Sep. Sci. 2016, 39, 2374–2379. [Google Scholar] [CrossRef]
- Maier, T.; Sanzenbacher, S.; Kammerer, D.R.; Berardini, N.; Conrad, J.; Beifuss, U.; Carle, R.; Schieber, A. Isolation of Hydroxycinnamoyltartaric Acids from Grape Pomace by High-Speed Counter-Current Chromatography. J. Chromatogr. A 2006, 1128, 61–67. [Google Scholar] [CrossRef]
- Wang, W.G.; Bhunia, A.K. Isolation of Anthocyanins by High-Speed Countercurrent Chromatography and Application of the Color Activity Concept to Different Varieties of Red Grape Pomace from Macedonia. J. Nutr. Sci. 2013, 3, 1000243. [Google Scholar] [CrossRef] [Green Version]
- Mbeunkui, F.; Grace, M.H.; Yousef, G.G.; Ann Lila, M. Isolation and Characterization of Flavonols from Blackcurrant by High-Performance Counter-Current Chromatography and Electrospray Ionization Tandem Mass Spectrometry. J. Sep. Sci. 2012, 35, 1682–1689. [Google Scholar] [CrossRef]
- Pittol, V.; Doneda, E.; Bianchi, S.E.; Koetz, M.; Alegre, P. Box-Behnken Design for Extraction Optimization Followed by High Performance Countercurrent Chromatography: Production of a Flavonoid-Enriched Fraction from Achyrocline Satureioides. Planta Med. 2020, 86, 151–159. [Google Scholar] [CrossRef]
- Wang, L.X.; Hu, C.; Zhang, J.D.; Gong, P.S.; Zhang, H.; Zhao, S.H. Identification of Five Flavonoid Compounds from the Remaining Ginger Powder Purified by Using High-Speed Counter-Current Chromatography and Their Bioactivity. Food Anal. Methods 2022, 15, 485–497. [Google Scholar] [CrossRef]
- Zhang, T.-Y.; Xiao, R.; Xiao, Z.-Y.; Pannel, L.; Ito, Y. Rapid Separation of Flavonoids by Analytical High-Speed Counter Current Chromatography. J. Chromatogr. A 1988, 445, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xie, Q.; Fisher, D.; Sutherland, I.A. Separation of Patuletin-3-o-Glucoside, Astragalin, Quercetin, Kaempferol and Isorhamnetin from Flaveria bidentis (L.) Kuntze by Elution-Pump-out High-Performance Counter-Current Chromatography. J. Chromatogr. A 2011, 1218, 6206–6211. [Google Scholar] [CrossRef] [PubMed]
- Pekkarinen, S.S.; Heinonen, I.M.; Hopia, A.I. Flavonoids Quercetin, Myricetin, Kaemferol and (+)-Catechin as Antioxidants in Methyl Linoleate. J. Sci. Food Agric. 1999, 79, 499–506. [Google Scholar] [CrossRef]
- Wanasundara, U.N.; Shahidi, F. Stabilization of Canola Oil with Flavonoids. Food Chem. 1994, 50, 393–396. [Google Scholar] [CrossRef]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and Their Variation in Red Wines II. Anthocyanin Derived Pigments and Their Color Evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef] [Green Version]
- Amico, V.; Napoli, E.M.; Renda, A.; Ruberto, G.; Spatafora, C.; Tringali, C. Constituents of Grape Pomace from the Sicilian Cultivar “Nerello Mascalese”. Food Chem. 2004, 88, 599–607. [Google Scholar] [CrossRef]
- Monagas, M.; Garrido, I.; Bartolomé, B.; Gómez-Cordovés, C. Chemical Characterization of Commercial Dietary Ingredients from Vitis vinifera L. Anal. Chim. Acta 2006, 563, 401–410. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Jungfer, E.; Ritter, C.; Santiago-Schübel, B.; Thiele, B.; Fett, R.; Galensa, R. Characterization of Flavan-3-Ols in Seeds of Grape Pomace by CE, HPLC-DAD-MS n and LC-ESI-FTICR-MS. Food Res. Int. 2012, 48, 848–855. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Huang, X.-Y.; Pei, D.; Liu, J.-F.; Di, D.-L. A Model for Continuous Sample Feed and Separation with Counter-Current Chromatography Based on Elution-Extrusion Mode and Its Application. J. Sep. Sci. 2022, 45, 4364–4374. [Google Scholar] [CrossRef]

| System I | System II | ||||
|---|---|---|---|---|---|
| Fractions | Weight GP (mg) | Weight WL (mg) | Fractions | Weight GP (mg) | Weight WL (mg) |
| F1 | 101 | 134 | F1 | 150 | 90 |
| F2 | 78 | 45 | F2 | 16 | 48 |
| F3 | 55 | 11 | F3 | 16 | 27 |
| F4 | 11 | 20 | F4 | 113 | 106 |
| F5 | 2 | 2 | Total | 295 | 271 |
| F6 | 33 | 8 | |||
| F7 | - | 15 | |||
| Total | 280 | 235 | |||
| PCs | Fraction/s a | Retention Time (min) | [M + H]+ b or [M − H]− c | MS/MS Confirmation Fragment | |
|---|---|---|---|---|---|
| Monomeric Anthocyanins | GP | WL | |||
| delphinidin 3-O-glucoside | F2-F4, S-I | F3-F4, S-I; F1-F3, S-II | 7.6 | 465 a | 303 |
| cyanidin 3-O-glucoside | F4, S-I | F4, S-I; F1, S-II | 9.6 | 449 a | 287 |
| petunidin 3-O-glucoside | F1-F4, S-I | F1-F4, S-I; F1, S-II | 10.9 | 479 a | 317 |
| peonidin 3-O-glucoside | F3-F4, S-I; F1-F2, S-II | F1-F4, S-I; F1, S-II | 12.6 | 463 a | 301 |
| malvidin 3-O-glucoside | F1-F4, S-I; F1-F2, S-II | F1-F4, S-I; F1, S-II | 13.5 | 493 a | 331 |
| delphinidin 3-O-acetylglucoside | F2, S-I | F1, S-II | 14.9 | 507 a | 303 |
| malvidin 3-O-acetylglucoside | F1, S-II | F1-F4, S-I; F1-F2, S-II | 20.1 | 535 a | 531 |
| peonidin 3-O-p-coumaroylglucoside | F2, S-I; F1, S-II | F3, S-I; F1-F3, S-II | 23.9 | 609 a | 301 |
| Pyranoanthocyanins derivatives | |||||
| petunidin 3-O-glucoside-pyruvic acid | n.f. | F2, S-I | 12.2 | 547 a | 297 |
| malvidin 3-O-glucoside-pyruvic acid | n.f. | F2-F3, S-I; F1, S-II | 15.3 | 561 a | 399 |
| malvidin-3-O-glucoside-acetaldehyde | n.f. | F1-F3, S-II | 16.6 | 517 a | 355 |
| malvidin-3-O-glucoside-ethyl-epicatechin | F4, S-I; F1, S-II | F4, S-I; F1, S-II | 18.4 | 809 a | 357 |
| malvidin-3-O-acetylglucoside-pyruvic acid | n.f. | F4, S-I; F1, S-II | 16.9 | 603 a | 399 |
| malvidin 3-O-coumaroyl-pyruvic acid | n.f. | F1, S-II | 20.5 | 707 a | 535 |
| malvidin-6-(caffeoyl)-3-O-glucoside | n.f. | F3-F5, S-I; F1-F2, S-II | 21.6 | 655 a | 331 |
| malvidin-3-O-glucoside-vinylcatechol | n.f. | F3-F5, S-I; F1-F3, S-II | 21.9 | 625 a | 463 |
| malvidin-3-O-glucoside-vinyl-catechin | n.f. | F1, S-II | 18.9 | 805 a | 593 |
| malvidin-3-O-glucoside-vinylguaiacol | F1-F2, S-II | F1-F4, S-I; F1-F3, S-II | 23.8 | 639 a | 331 |
| catechin-ethyl-malvidin-3-O-coumaroylglucoside dimer | n.f. | F5, S-I; F1, S-II | 23.4 | 955 a | 609 |
| malvidin-3-O-glucoside-acetyl-vinylcatechol | n.f. | F4, S-I; F1-F2, S-II | 17.9 | 667 a | 521 |
| catechin-ethyl-malvidin-3-O-coumaroylglucoside dimer | n.f. | F1-F2, S-II | 26 | 955 a | 609 |
| delphinidin 3-O-acetylglucoside-piruvic acid | n.f. | F1, S-II | 15.9 | 575 a | 273 |
| delphinidin 3-O-coumarylglucoside-piruvic acid | n.f. | F3-F5, S-I; F1-F3, S-II | 20.4 | 679 a | 371 |
| delphinidin 3-O-acetylglucoside-4-vinylphenol | n.f. | F3, S-I; F1-F2, S-II | 18.7 | 623 a | 419 |
| peonidin 3-O-glucoside-pyruvic acid | F1, S-II | F1-F2, S-II | 13.2 | 531 a | 369 |
| peonidin 3-O-acetylglucoside-4-vinylphenol | n.f. | F1, S-II | 18.9 | 621 a | 535 |
| petunidin 3-O-glucoside 4-vinylphenol | n.f. | F3, S-I; F1-F3, S-II | 21.4 | 595 a | 533 |
| delphinidin 3-O-glucoside-4-vinylguaicol | n.f. | F3-F5, S-I; F1-F2, S-II | 19.7 | 611 a | 535 |
| peonidin 2-O-acetylglucoside-4-vinylepicatechin | n.f. | F1, S-II | 23 | 817 a | 613 |
| Phenolic acids | |||||
| gallic acid | F3-F4, S-I; F1-F3, SII | F1-F3, S-II | 1.5 | 169 c | 125 |
| protocatechuic acid | F5-F6, S-I; F2, S-II | F1, S-II | 7.3 | 153 c | 109 |
| caftaric acid | F1-F3, S-II | F5, S-I; F1-F3, S-II | 3.9 | 311 c | 179 |
| coutaric acid | F3, S-II | F5, S-I; F3, S-II | 5.9 | 295 c | 163 |
| caffeic acid | n.f. | F1-F3, S-II | 6.3 | 179 c | 135 |
| syringic acid | n.f. | F5-F6, S-I; F3, S-II | 9.7 | 197 c | 125 |
| ferulic acid | n.f. | F5, S-I | 7.5 | 193 c | 149 |
| Flavanols | |||||
| procyanidin B1 | F4-F6, S-I; F1-F2, SII | F1-F4, S-I; F1, S-II | 4.02 | 577 c | 425 |
| procyanidin dimer iso1 | F4, S-I; F1-F2, SII | F1-F4, S-II | 4.4 | 577 c | 425 |
| procyanidin dimer iso2 | F1, SII | F1, S-II | 4.7 | 577 c | 425 |
| catechin | F4-F6, S-I; F1-F3, SII | F1-F4, S-I; F1, S-II | 5.2 | 289 c | 245 |
| procyanidin trimer iso2 | F4, S-I; F1, SII | F2-F3, S-I; F1, S-II | 5.5 | 865 c | 695 |
| procyanidin trimer iso3 | F1, SII | F2-F4, S-I; F1, S-II | 5.8 | 865 c | 695 |
| procyanidin dimer iso4 | F4, S-I; F1-F2, SII | F2, S-I; F1, S-II | 6.1 | 577 c | 425 |
| procyanidin B2 | F4-F6, S-I; F1-F2, SII | F1, S-I; F1, S-II | 7.1 | 577 c | 425 |
| epicatechin | F4-F6, S-I; F1-F3, SII | F1-F4, S-I; F1, S-II | 8.66 | 289 c | 245 |
| procyanidin trimer iso4 | F1, SII | F1, S-II | 9.4 | 865 c | 695 |
| procyanidin trimer iso5 | F1, SII | F1, S-II | 10.23 | 865 c | 695 |
| procyanidin dimer iso5 | F4-F5, S-I; F1-F2, SII | F1, S-II | 10.1 | 577 c | 289 |
| Glycosilated flavonols | |||||
| quercetin 3-O-galactoside | F2, S-II | F1, S-I; F1-F3, S-II | 10.9 | 465 c | 301 |
| quercetin 3-O-glucuronide | F1-F2, SII | F1, S-I; F1-F3, S-II | 15.5 | 477 c | 301 |
| quercetin 3-O-glucoside | F1-F2, SII | F1-F2, S-II | 15.9 | 463 c | 301 |
| kaempferol 3-O-glucoside | F1, SII | F1-F2, S-II | 13.3 | 449 c | 317 |
| isorhamnetin 3-O-glucoside | F4, S-I; F1, SII | F1-F2, S-II | 12.7 | 479 c | 316 |
| Simple flavonols | |||||
| myricetin | F6, S-I; F4, S-II | F6-F7, S-I; F4, S-II | 18.3 | 319 b | 273 |
| quercetin | F6, S-I; F4, S-II | F5-F7, S-I; F4, S-II | 23.8 | 303 b | 257 |
| kaempferol | F6, S-I; F4, S-II | F7, S-I; F4, S-II | 26.3 | 287 b | 165 |
| isorhamnetin | F6, S-I; F4, S-II | F7, S-I; F4, S-II | 26.4 | 317 b | 302 |
| System I | System II | |||||||
|---|---|---|---|---|---|---|---|---|
| GP (mg) | GP (%) | WL (mg) | WL (%) | GP (mg) | GP (%) | WL (mg) | WL (%) | |
| myricetin | 2.2 | 6.6 | 11.1 | 37.1 | 10.7 | 9.5 | 48.4 | 45.7 |
| quercetin | 30.1 | 91.3 | 17.5 | 58.4 | 97.9 | 86.6 | 50.6 | 47.7 |
| kaempferol | 0.3 | 0.9 | 0.5 | 1.6 | 1.6 | 1.4 | 3.1 | 2.9 |
| isorhamnetin | 0.4 | 1.2 | 0.8 | 2.8 | 2.7 | 2.4 | 3.8 | 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontana, A.; Schieber, A. Preparative Fractionation of Phenolic Compounds and Isolation of an Enriched Flavonol Fraction from Winemaking Industry By-Products by High-Performance Counter-Current Chromatography. Plants 2023, 12, 2242. https://doi.org/10.3390/plants12122242
Fontana A, Schieber A. Preparative Fractionation of Phenolic Compounds and Isolation of an Enriched Flavonol Fraction from Winemaking Industry By-Products by High-Performance Counter-Current Chromatography. Plants. 2023; 12(12):2242. https://doi.org/10.3390/plants12122242
Chicago/Turabian StyleFontana, Ariel, and Andreas Schieber. 2023. "Preparative Fractionation of Phenolic Compounds and Isolation of an Enriched Flavonol Fraction from Winemaking Industry By-Products by High-Performance Counter-Current Chromatography" Plants 12, no. 12: 2242. https://doi.org/10.3390/plants12122242
APA StyleFontana, A., & Schieber, A. (2023). Preparative Fractionation of Phenolic Compounds and Isolation of an Enriched Flavonol Fraction from Winemaking Industry By-Products by High-Performance Counter-Current Chromatography. Plants, 12(12), 2242. https://doi.org/10.3390/plants12122242







