Optimization of the Green Chemistry-like Extraction of Phenolic Compounds from Grape (Vitis labrusca L.) and Blackberry (Rubus fruticosus L.) Seeds with Concomitant Biological and Antioxidant Activity Assessments
Abstract
:1. Introduction
2. Results and Discussion
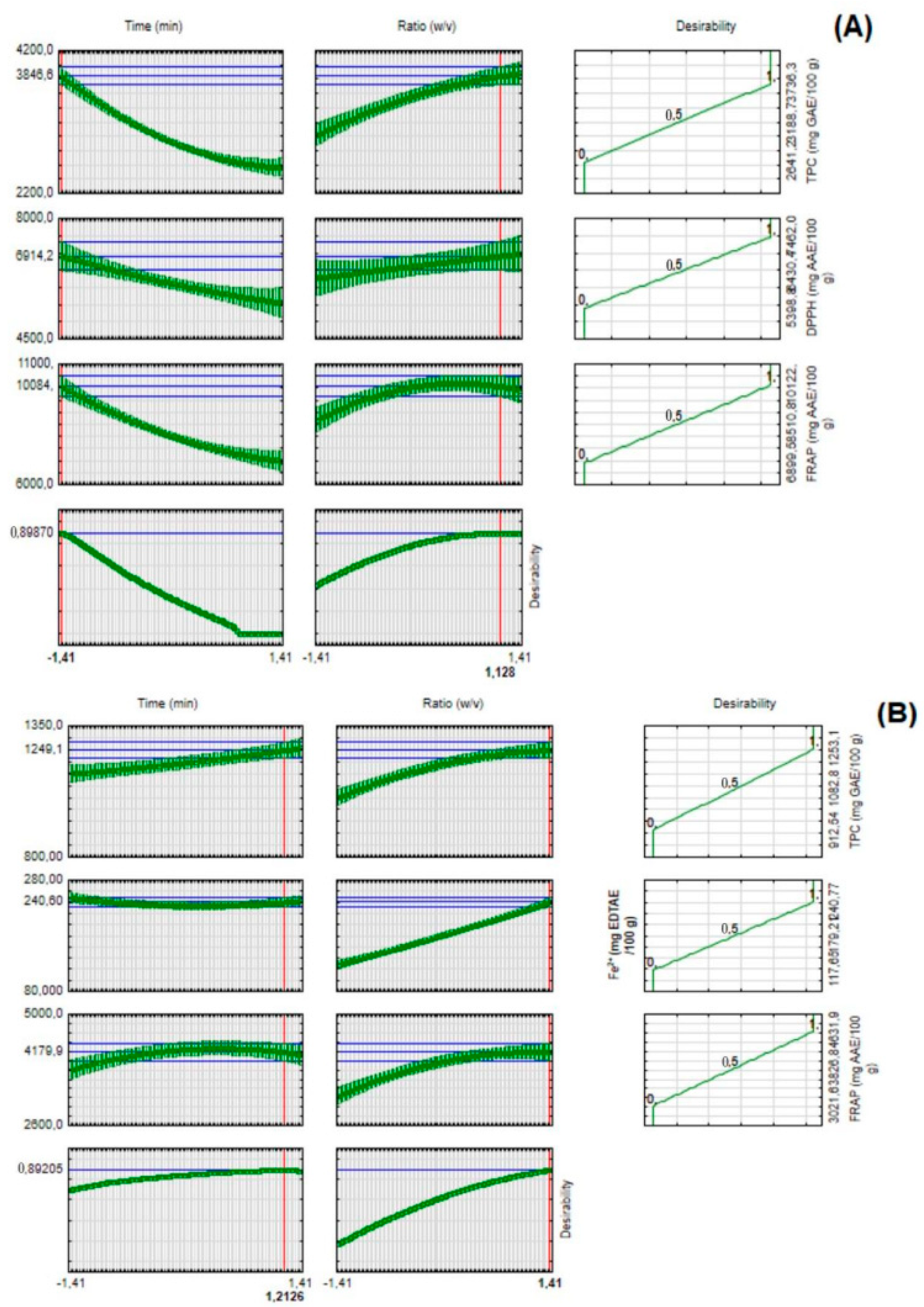
2.1. Effect of Extraction Time and Solid-to-Solvent Ratio on the Total Phenolic Composition and Antioxidant Activity
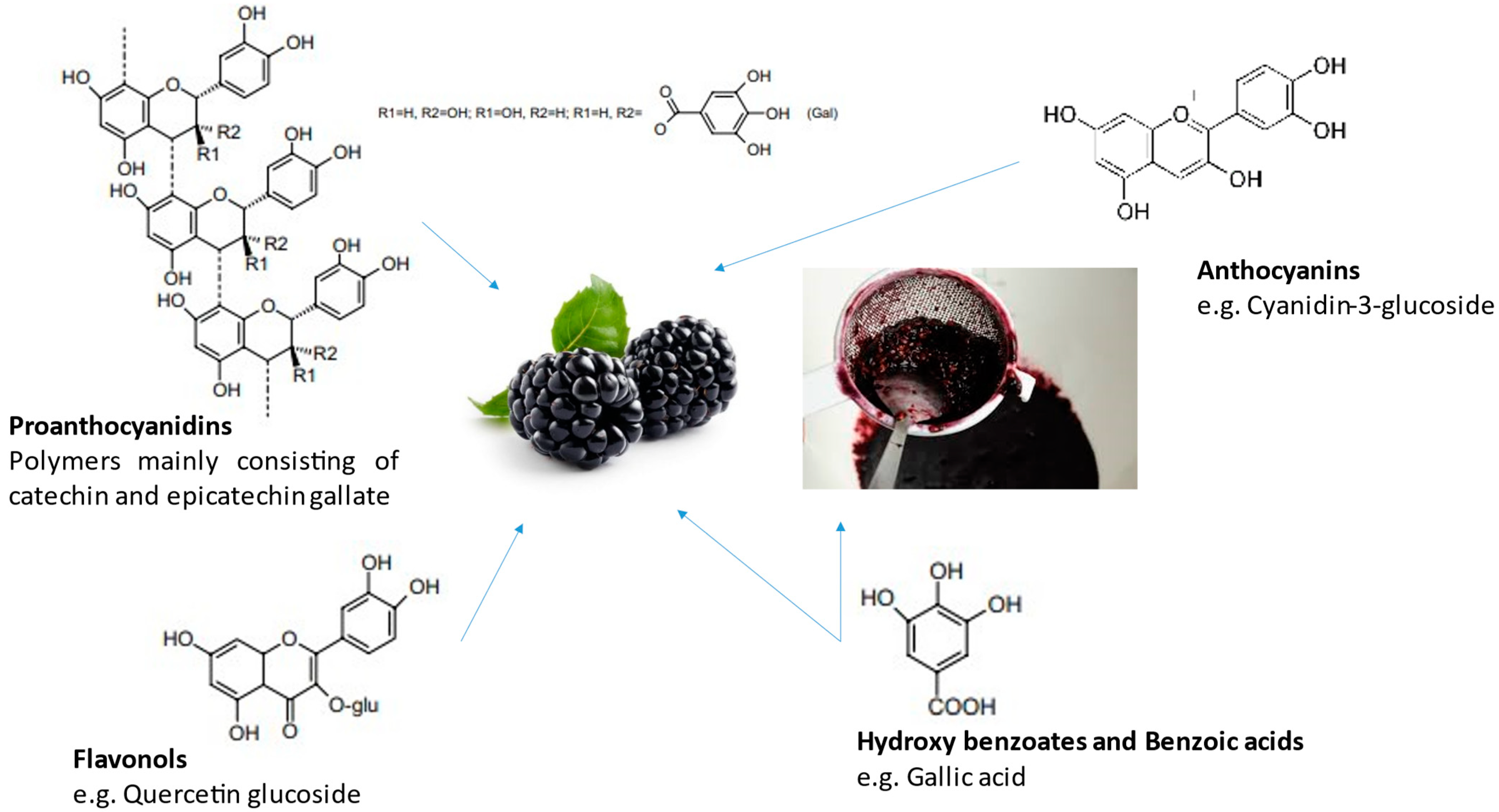
2.2. Chemical Composition of the Optimized and Lyophilized Extracts
2.3. Phenolic Composition by LC-MS
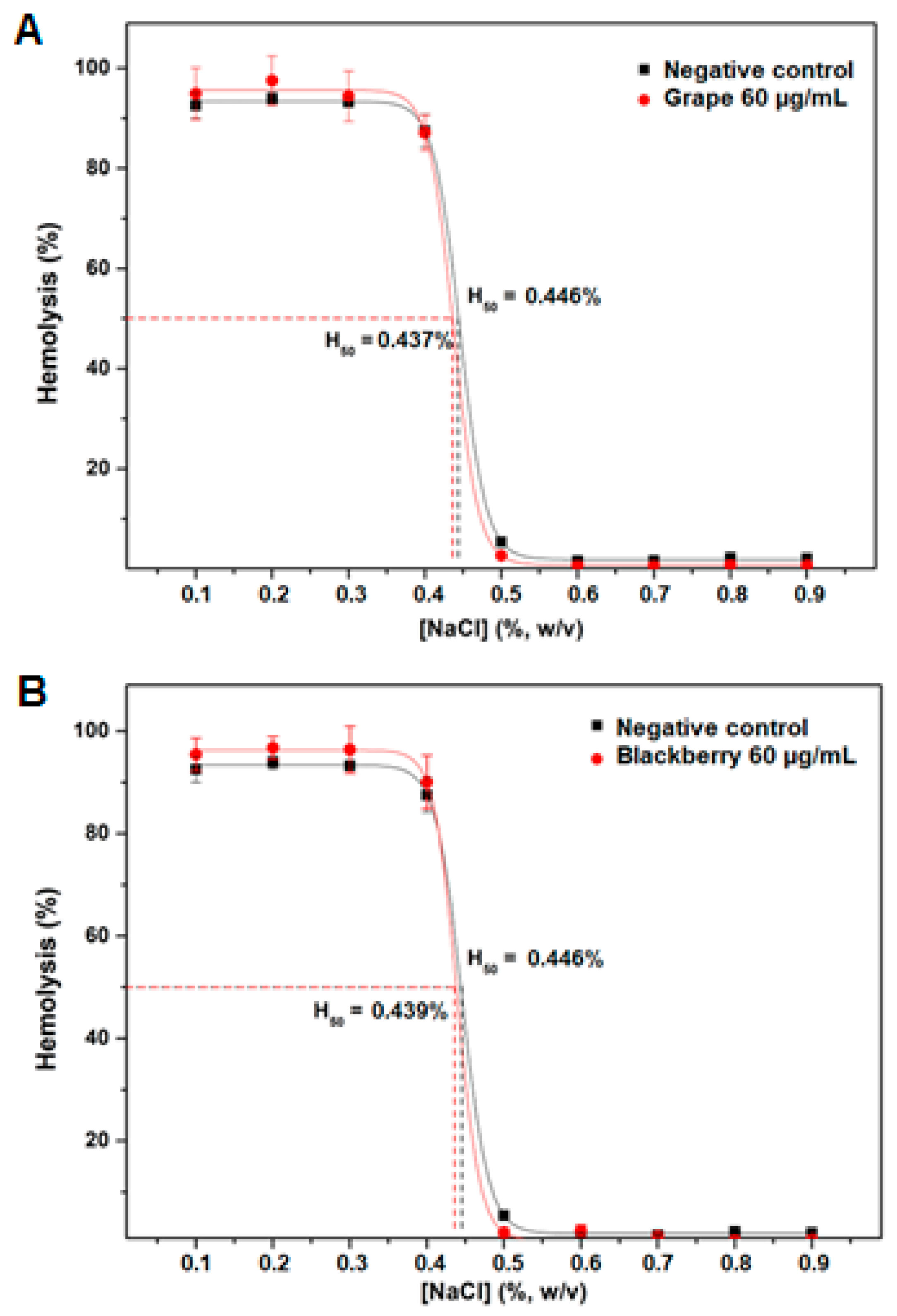
2.4. Antihemolytic Activity
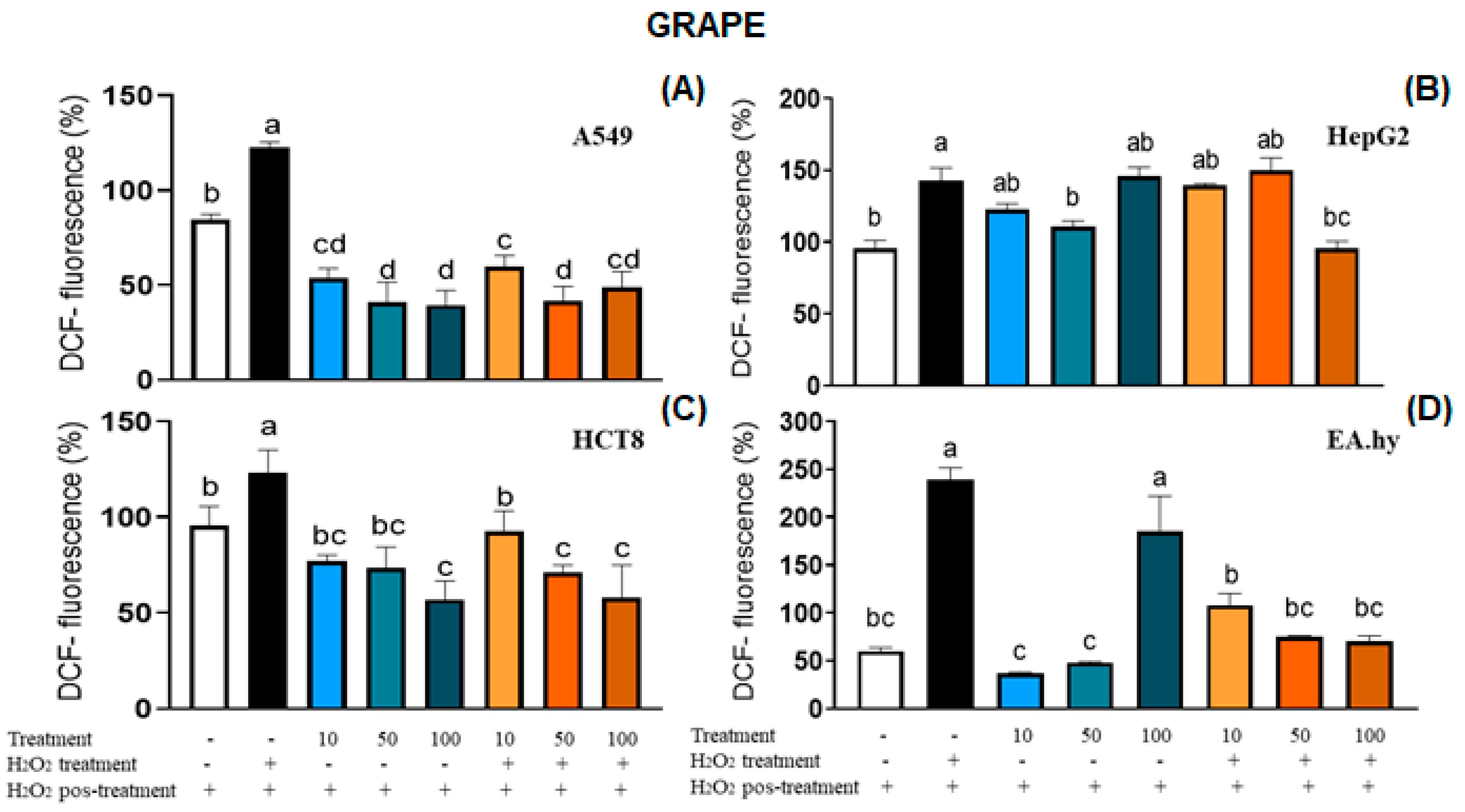
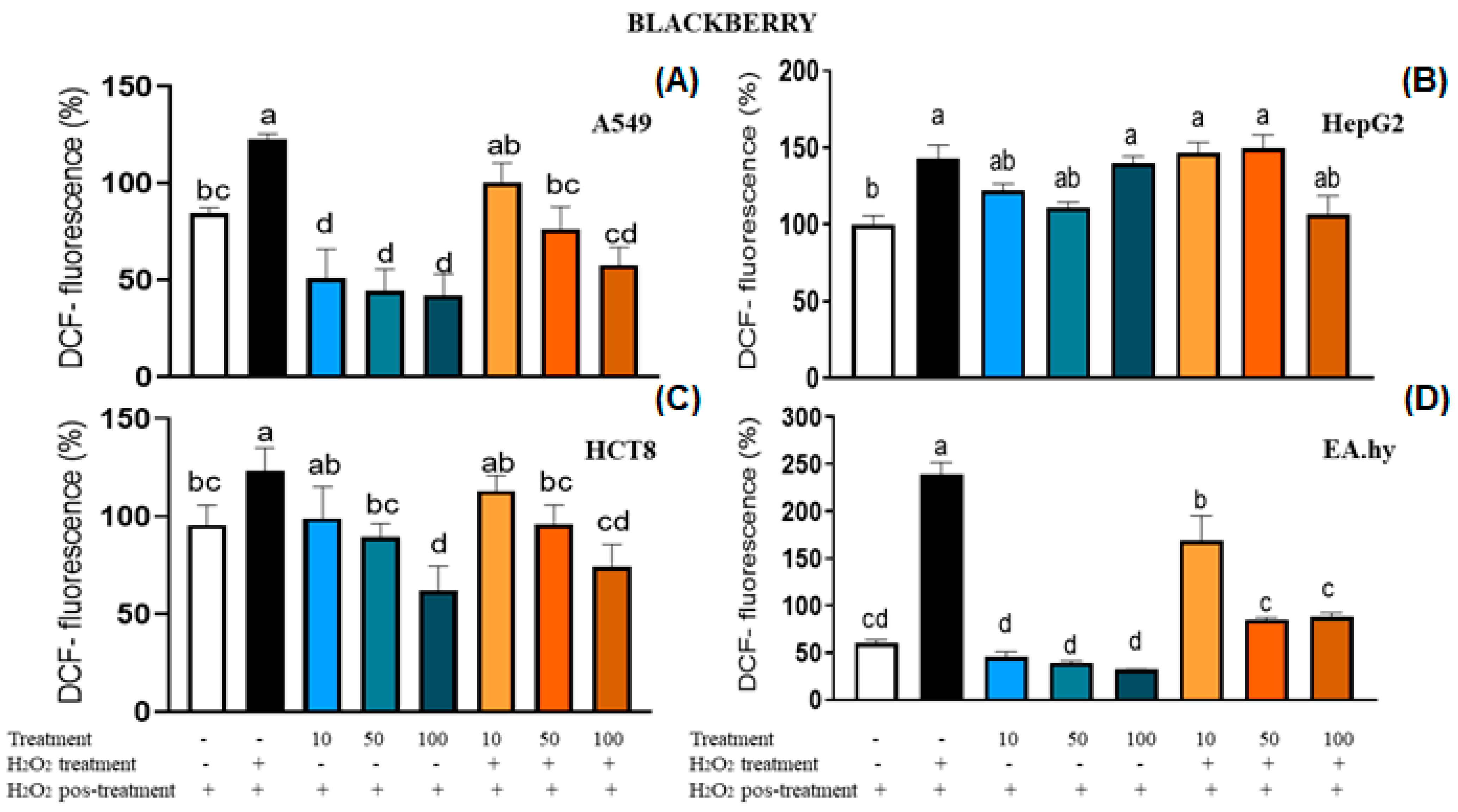
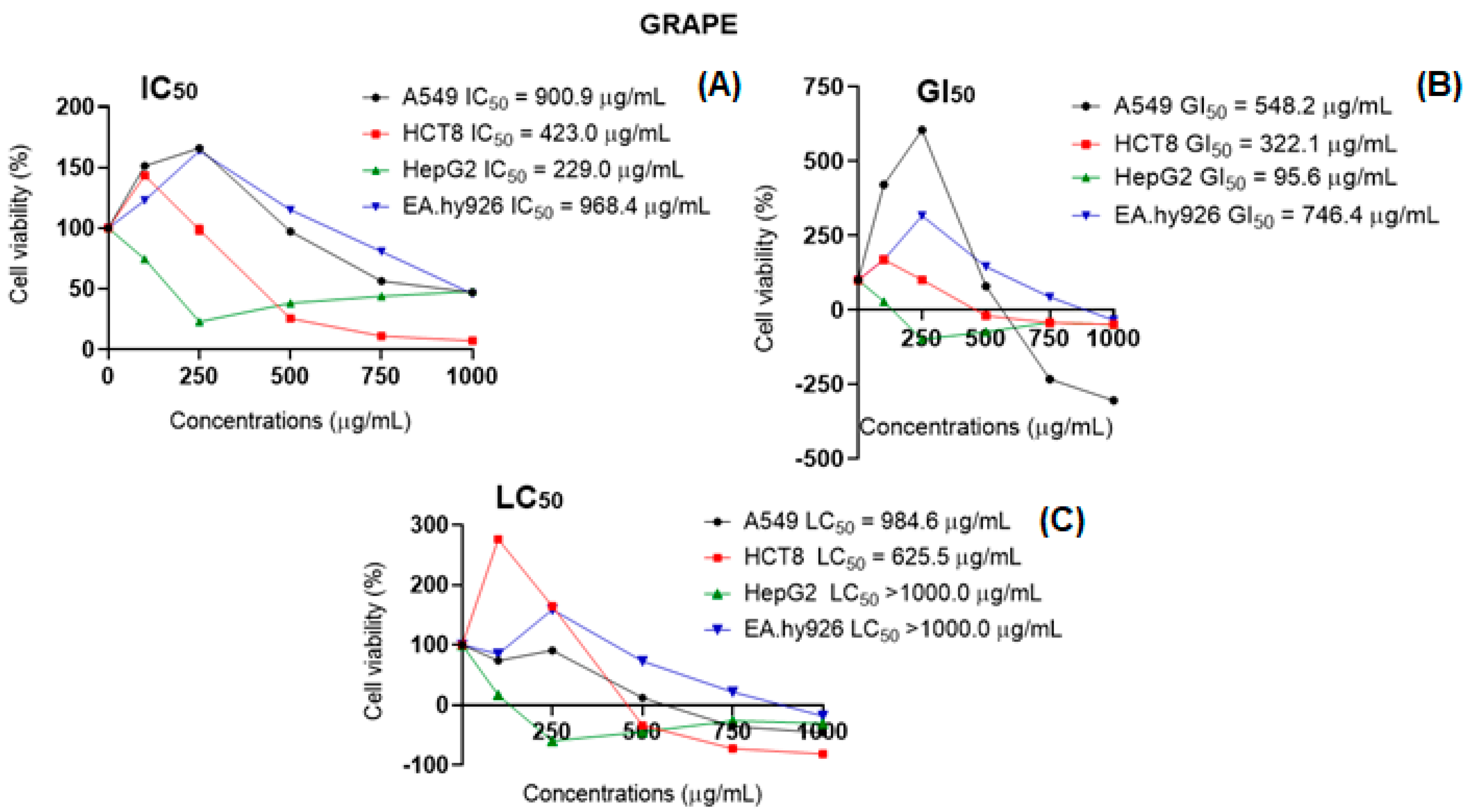
2.5. Intracellular ROS Generation and Cell Viability
3. Material and Methods
3.1. Chemical Reagents and Cells
3.2. Vegetable Material
3.3. Obtaining Hydroalcoholic Extracts and Extraction Optimization
3.4. Phenolic Composition by LC-MS/MS and UV-VIS Spectrophotometry
3.5. Antioxidant Activity
3.6. Toxicity Profiling and Cellular Antioxidant Activity
3.7. In Vitro Antihemolytic Effect
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
Appendix B
| Coded Values | Actual Values | ||
|---|---|---|---|
| Time (min) | Mass/Volume Ratio (w/v) | Time (min) | Mass/Volume Ratio (w/v) |
| −1 | −1 | 40 | 1:40 |
| −1 | 1 | 40 | 1:60 |
| 1 | −1 | 60 | 1:40 |
| 1 | 1 | 60 | 1:60 |
| −1.41 | 0 | 35.9 | 1:50 |
| 1.41 | 0 | 64.1 | 1:50 |
| 0 | −1.41 | 50 | 1:35.9 |
| 0 | 1.41 | 50 | 1:64.1 |
| 0 | 0 | 50 | 1:50 |
| 0 | 0 | 50 | 1:50 |
| 0 | 0 | 50 | 1:50 |
References
- Zacharof, M.-P. Grape winery waste as feedstock for bioconversions: Applying the biorefinery concept. Waste Biomass Valorization 2017, 8, 1011–1025. [Google Scholar] [CrossRef] [Green Version]
- Prasad, C.; Imrhan, V.; Juma, S.; Maziarz, M.; Prasad, A.; Tiernan, C.; Vijayagopal, P. Bioactive plant metabolites in the management of non-communicable metabolic diseases: Looking at opportunities beyond the horizon. Metabolites 2015, 5, 733–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippi, K.; Georgaka, N.; Alexandri, M.; Papapostolou, H.; Koutinas, A. Valorisation of grape stalks and pomace for the production of bio-based succinic acid by Actinobacillus succinogenes. Ind. Crops Prod. 2021, 168, 113578. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Mikkelsen, L.H.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. A combined approach based on matrix solid-phase dispersion extraction assisted by titanium dioxide nanoparticles and liquid chromatography to determine polyphenols from grape residues. J. Chromatogr. A 2021, 1644, 462128. [Google Scholar] [CrossRef]
- İşçimen, E.M.; Hayta, M. Microwave-assisted aqueous two-phase system based extraction of phenolics from pulses: Antioxidant properties, characterization and encapsulation. Ind. Crops Prod. 2021, 173, 114144. [Google Scholar] [CrossRef]
- Silva, R.F.M.; Pogačnik, L. Polyphenols from food and natural products: Neuroprotection and safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Mejía, E.; Vicente-Zurdo, D.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Screening the extraction process of phenolic compounds from pressed grape seed residue: Towards an integrated and sustainable management of viticultural waste. LWT Food Sci. Technol. 2022, 169, 113988. [Google Scholar] [CrossRef]
- Monteiro, G.C.; Minatel, I.O.; Junior, A.P.; Gomez-Gomez, H.A.; de Camargo, J.P.C.; Diamante, M.S.; Basílio, L.S.P.; Tecchio, M.A.; Lima, G.P.P. Bioactive compounds and antioxidant capacity of grape pomace flours. LWT 2020, 135, 110053. [Google Scholar] [CrossRef]
- Pap, N.; Fidelis, M.; Azevedo, L.; do Carmo, M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B.; et al. Berry polyphenols and human health: Evidence of antioxidant, anti-inflammatory, microbiota modulation, and cell-protecting effects. Curr. Opin. Food Sci. 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Junior, T.K.; de Moura, C.; do Carmo, M.A.V.; Azevedo, L.; Esmerino, L.A.; Tardivo, R.C.; Kilpeläinen, P.; Granato, D. Chemical Composition, Antioxidant, Antimicrobial and Cytotoxic/Cytoprotective Activity of Non-Polar Extracts of Grape (Vitis labrusca cv. Bordeaux) and Blackberry (Rubus fruticosus) Seeds. Molecules 2021, 26, 4057. [Google Scholar] [CrossRef]
- Teixeira, A.; Eiras-Dias, J.; Castellarin, S.D.; Geros, H. Berry phenolics of grapevine under challenging environments. Int. J. Mol. Sci. 2013, 14, 18711–18739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W. Safety assessment of Vitis vinifera (Grape)-derived ingredients as used in cosmetics. Int. J. Toxicol. 2014, 33, 48–83. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Yun, S.; Chu, D.; He, X.; Zhang, W.; Feng, C. Protective effects of grape seed proanthocyanidins against iron overload-induced renal oxidative damage in rats. J. Trace Elem. Med. Biol. 2020, 57, 126407. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Shen, W.; Wang, Y.; Cao, Y.; Nuerbulati, N.; Chen, W.; Lu, G.; Xiao, W.; Qi, R. Grape Seed Polyphenols Ameliorated Dextran Sulfate Sodium-Induced Colitis via Suppression of Inflammation and Apoptosis. Pharmacology 2020, 105, 9–18. [Google Scholar] [CrossRef]
- Wang, L.; Huang, W.; Zhan, J. Grape Seed Proanthocyanidins Induce Autophagy and Modulate Survivin in HepG2 Cells and Inhibit Xenograft Tumor Growth in Vivo. Nutrients 2019, 11, 2983. [Google Scholar] [CrossRef] [Green Version]
- Arafat, E.A.; Shabaan, D.A. The possible neuroprotective role of grape seed extract on the histopathological changes of the cerebellar cortex of rats prenatally exposed to Valproic Acid: Animal model of autism. Acta Histochem. 2019, 121, 841–851. [Google Scholar] [CrossRef]
- Chen, F.; Wang, H.; Zhao, J.; Yan, J.; Meng, H.; Zhan, H.; Chen, L.; Yuan, L. Grape seed proanthocyanidin inhibits monocrotaline-induced pulmonary arterial hypertension via attenuating inflammation: In vivo and in vitro studies. J. Nutr. Biochem. 2019, 67, 72–77. [Google Scholar] [CrossRef]
- Hakimuddin, F.; Tiwari, K.; Paliyath, G.; Meckling, K. Grape and wine polyphenols down-regulate the expression of signal transduction genes and inhibit the growth of estrogen receptor–negative MDA-MB231 tumors in nu/nu mouse xenografts. Nutr. Res. 2008, 28, 702–713. [Google Scholar] [CrossRef]
- Singh, T.; Sharma, S.D.; Katiyar, S.K. Grape proanthocyanidins induce apoptosis by loss of mitochondrial membrane potential of human non-small cell lung cancer cells in vitro and in vivo. PLoS ONE 2011, 6, e27444. [Google Scholar] [CrossRef]
- Schlachterman, A.; Valle, F.; Wall, K.M.; Azios, N.G.; Castillo, L.; Morell, L.; Washington, A.V.; Cubano, L.A.; Dharmawardhane, S.F. Combined Resveratrol, Quercetin, and Catechin Treatment Reduces Breast Tumor Growth in a Nude Mouse Model. Transl. Oncol. 2008, 1, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singletary, K.W.; Stansbury, M.J.; Giusti, M.; Van Breemen, R.B.; Wallig, M.; Rimando, A. Inhibition of rat mammary tumorigenesis by concord grape juice constituents. J. Agric. Food Chem. 2003, 51, 7280–7286. [Google Scholar] [CrossRef] [PubMed]
- Moraes, D.P.; Lozano-S’anchez, J.; Machado, M.L.; Vizzotto, M.; Lazzaretti, M.; Leyva-Jimenes, F.J.J. Characterization of a new blackberry cultivar BRS Xingu: Chemical composition, phenolic compounds, and antioxidant capacity in vitro and in vivo. Food Chem. 2020, 322, 126783. [Google Scholar] [CrossRef] [PubMed]
- Tomas, M.; Rocchetti, G.; Ghisoni, S.; Giuberti, G.; Capanoglu, E.; Lucini, L. Effect of different soluble dietary fibres on the phenolic profile of blackberry puree subjected to in vitro gastrointestinal digestion and large intestine fermentation. Food Res. Int. 2019, 130, 108954. [Google Scholar] [CrossRef]
- Da Fonseca Machado, A.P.; Rezende, C.A.; Rodrigues, R.A.; Barbero, G.F.; De Tarso Vieira e Rosa, P.; Martínez, J. Encapsulation of anthocyanin-rich extract from blackberry residues by spray-drying, freeze-drying and supercritical antisolvent. Powder Technol. 2018, 340, 553–562. [Google Scholar] [CrossRef]
- Yamashita, C.; Chung, M.M.S.; Dos Santos, C.; Mayer, C.R.M.; Moraes, I.C.F.; Branco, I.G. Microencapsulation of an anthocyanin-rich blackberry (Rubus spp.) by-product extract by freeze-drying. LWT Food Sci. Technol. 2017, 84, 256–262. [Google Scholar]
- Zia-ul-haq, M.; Riaz, M.; De Feo, V.; Jaafar, H.; Moga, M. Rubus fruticosus L.: Constituents, biological activities and health related uses. Molecules 2014, 19, 10998–11029. [Google Scholar] [CrossRef] [Green Version]
- Sautebin, L.; Rossi, A.; Serraino, I.; Dugo, P.; Di Paola, R.; Mondello, L.; Genovese, T.; Britti, D.; Peli, A.; Dugo, G.; et al. Effect of Anthocyanins Contained in a Blackberry Extract on the Circulatory Failure and Multiple Organ Dysfunction Caused by Endotoxin in the Rat. Planta Medica 2004, 70, 745–752. [Google Scholar] [CrossRef]
- Serraino, I.; Dugo, L.; Dugo, P.; Mondello, L.; Mazzon, E.; Dugo, G.; Caputi, A.P.; Cuzzocrea, S. Protective effects of cyanidin-3-O-glucoside from blackberry extract against peroxynitrite-induced endothelial dysfunction and vascular failure. Life Sci. 2003, 73, 1097–1114. [Google Scholar] [CrossRef]
- Kaume, L.; Howard, L.R.; Devareddy, L. The Blackberry Fruit: A Review on Its Composition and Chemistry, Metabolism and Bioavailability, and Health Benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. [Google Scholar] [CrossRef]
- Deolindo, C.T.P.; Monteiro, P.I.; Santos, J.S.; Cruz, A.G.; da Silva, M.C.; Granato, D. Phenolic-rich Petit Suisse cheese manufactured with organic Bordeaux grape juice, skin, and seed extract: Technological, sensory, and functional properties. LWT 2019, 115, 108493. [Google Scholar] [CrossRef]
- Karnopp, A.R.; Oliveira, K.G.; de Andrade, E.F.; Postingher, B.M.; Granato, D. Optimization of an organic yogurt based on sensorial, nutritional, and functional perspectives. Food Chem. 2017, 233, 401–411. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; van Camp, J.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Halin, M.A.; Kanan, K.A.; Nahar, T.; Rahman, M.J.; Ahmed, K.S.; Hossain, H.; Mozumder, N.H.M.R.; Ahmed, M. Metabolic profiling of phenolics of the extracts from the various parts of blackberry plant (Syzygium cumini L.) and their antioxidant activities. LWT Food Sci. Technol. 2022, 167, 113813. [Google Scholar] [CrossRef]
- Zhang, L.; Santos, J.S.; Cruz, T.M.; Marques, M.B.; do Carmo, M.A.V.; Azevedo, L.; Wang, Y.; Granato, D. Multivariate effects of Chinese keemun black tea grades (Camellia sinensis var. sinensis) on the phenolic composition, antioxidant, antihemolytic and cytotoxic/cytoprotection activities. Food Res. Int. 2019, 125, 108516. [Google Scholar] [PubMed]
- Cruz, T.M.; Santos, J.S.; do Carmo, M.A.V.; Hellström, J.; Pihlava, J.M.; Azevedo, L.; Granato, D.; Marques, M.B. Extraction optimization of bioactive compounds from ora-pro-nobis (Pereskia aculeata Miller) leaves and their in vitro antioxidant and antihemolytic activities. Food Chem. 2021, 361, 130078. [Google Scholar] [CrossRef] [PubMed]
- Atrooz, O.; Harb, M.; Al-Qato, M. The Antioxidant and Antihaemolytic Activities and the Polyphenolic Contents of Some Plants Seeds Extracts. Mu’tah Lil-Buhuth Wad-Dirasat Nat. Appl. Sci. Ser. 2007, 22, 11–23. [Google Scholar]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and anticancer properties of berries. Crit. Rev. Food Sci. Nutr. 2018, 58, 2491–2507. [Google Scholar] [CrossRef]
- do Carmo, M.A.V.; Pressete, C.G.; Marques, M.J.; Granato, D.; Azevedo, L. Polyphenols as potential antiproliferative agents: Scientific trends. Curr. Opin. Food Sci. 2018, 24, 26–35. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Granato, D.; Grevink, R.; Zielinski, A.A.F.; Nunes, D.S.; Van Ruth, S.M. Analytical strategy coupled with response surface methodology to maximize the extraction of antioxidants from ternary mixtures of green, yellow, and red teas (Camellia sinensis var. sinensis). J. Agric. Food Chem. 2014, 62, 10283–10296. [Google Scholar] [CrossRef] [PubMed]
- Margraf, T.; Karnopp, A.R.; Rosso, N.D.; Granato, D. Comparison between Folin-Ciocalteu and Prussian Blue assays to estimate the total phenolic content of juices and teas using 96-well microplates. J. Food Sci. 2015, 80, C2397–C2403. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, J.; Gonçalves, J.L.; Alves, V.L.; Câmara, J.S. Chemical Fingerprint of Free Polyphenols and Antioxidant Activity in Dietary Fruits and Vegetables Using a Non-Targeted Approach Based on QuEChERS Ultrasound-Assisted Extraction Combined with UHPLC-PDA. Antioxidants 2020, 9, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maestro Durán, R.; Borja Padilla, R.; Martín Martín, A.; Fiestas de Ursinos, J.A.; Alba Mendoza, J. Biodegradación de los compuestos fenólicos presentes en el alpechín. Grasas Y Aceites 1991, 42, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Seraglio, S.K.T.; Valese, A.C.; Daguer, H.; Bergamo, G.; Azevedo, M.S.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Development and validation of a LC-ESI-MS/MS method for the determination of phenolic compounds in honeydew honeys with the diluted-and-shoot approach. Food Res. Int. 2016, 87, 60–67. [Google Scholar] [CrossRef]
- Santos, J.S.; Alvarenga Brizola, V.R.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef]
- Margraf, T.; Santos, É.N.T.; de Andrade, E.F.; van Ruth, S.M.; Granato, D. Effects of geographical origin, variety and farming system on the chemical markers and in vitro antioxidant capacity of Brazilian purple grape juices. Food Res. Int. 2016, 82, 145–155. [Google Scholar] [CrossRef]
- Xiong, S.L.; Zheng, Y.; Dai, X.; Wang, Q.; Cao, J.; Xiao, J. Antioxidant and immunoregulatory activity of different polysaccharide fractions from tuber of Ophiopogon japonicus. Carbohydr. Polym. 2011, 86, 1273–1280. [Google Scholar] [CrossRef]
- Do Carmo, M.A.V.; Fidelis, M.; Pressete, C.G.; Marques, M.J.; Castro-Gamero, A.M.; Myoda, T.; Granato, D.; Azevedo, L. Hydroalcoholic Myrciaria dubia (camu-camu) seed extracts prevent chromosome damage and act as antioxidant and cytotoxic agents. Food Res. Int. 2019, 125, 108551. [Google Scholar] [CrossRef]
- Othmène, Y.B.; Salem, I.B.; Hamdi, H.; Annabi, E.; Abid-Essefi, S. Tebuconazole induced cytotoxic and genotoxic effects in HCT116 cells through ROS generation. Pestic. Biochem. Physiol. 2021, 174, 104797. [Google Scholar] [CrossRef]
- Granato, D.; Reshamwala, D.; Korpinen, R.; Azevedo, L.; Vieira do Carmo, M.A.; Cruz, T.M.; Marques, M.B.; Wen, M.; Zhang, L.; Marjomäki, V.; et al. From the forest to the plate—Hemicelluloses, galactoglucomannan, glucuronoxylan, and phenolic-rich extracts from unconventional sources as functional food ingredients. Food Chem. 2022, 381, 132284. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, A.A.; Piroski, C.S.; Daniel, T.G.; Cruz, T.M.; Escher, G.B.; Vieira do Carmo, M.A.; Azevedo, L.; Marques, M.B.; Granato, D.; Rosso, N.D. Red Chicory (Cichorium intybus) Extract Rich in Anthocyanins: Chemical Stability, Antioxidant Activity, and Antiproliferative Activity In Vitro. J. Food Sci. 2019, 84, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Morabito, R.; Remigante, A.; Di Pietro, M.L.; Giannetto, A.; La Spada, G.; Marino, A. SO4 = uptake and catalase role in preconditioning after H2O2-induced oxidative stress in human erythrocytes. Pflügers Arch. Eur. J. Physiol. 2017, 469, 235–250. [Google Scholar] [CrossRef] [PubMed]


| Tests | Independent Variables (Original and Coded Values) | Total Phenolic Content (mg GAE/100 g) | Flavonoids (mg QE/100 g) | Condensed Tannins (mg CTE/100 g) | Ortho-Diphenolics (mg [ACE]/100 g) | DPPH (mg AAE/100 g) | Fe2+ Chelating Capacity (mg EDTAE/100 g) | FRAP (mg AAE/100 g) | |
|---|---|---|---|---|---|---|---|---|---|
| Time (min) | 1:X (g/mL) | ||||||||
| 1 | 40 (−1) | 1:40 (−1) | 2916 ± 7 cde | 62.9 ± 1.7 f | 3242 ± 95 e | 554 ± 20 e | 5870 ± 98 def | 66.1 ± 1.8 c | 9032 ± 328 b |
| 2 | 40 (−1) | 1:60 (1) | 3609 ± 24 a | 78.8 ± 2.8 b | 3928 ± 315 de | 744 ± 24 bc | 7069 ± 544 a | 55.5 ± 4.9 d | 10,023 ± 98 a |
| 3 | 60 (1) | 1:40 (−1) | 2914 ± 61 de | 55.9 ± 2.1 g | 3885 ± 353 de | 697 ± 40 cd | 5676 ± 250 f | 45.4 ± 4.1 e | 7518 ± 241 de |
| 4 | 60 (1) | 1:60 (1) | 2747 ± 117 f | 74,1 ± 1.6 c | 4982 ± 438 bc | 700 ± 78 cd | 6173 ± 36 cde | 85.3 ± 5.3 a | 7294 ± 234 e |
| 5 | 35.9 (−1.41) | 1:50 (0) | 3623 ± 105 a | 72.7 ± 3.0 cde | 5561 ± 1092 b | 963 ± 64 a | 6650 ± 141 ab | 46.7 ± 2.6 e | 9596 ± 308 a |
| 6 | 64.1 (1.41) | 1:50 (0) | 3036 ± 45 bc | 69.4 ± 2.6 de | 5030 ± 538 bc | 801 ± 54 b | 5832 ± 79 def | 65.1 ± 6.5 c | 8360 ± 190 c |
| 7 | 50 (0) | 1:35.9 (−1.41) | 2825 ± 12 ef | 68.5 ± 3.3 e | 3993 ± 449 de | 892 ± 11 a | 5517 ± 80 f | 36.1 ± 2.4 f | 7807 ± 166 d |
| 8 | 50 (0) | 1:64.1 (1.41) | 3010 ± 51 bcd | 73.6 ± 3.9 cd | 5695 ± 267 ab | 753 ± 43 bc | 6679 ± 511 ab | 74.2 ± 3.7 b | 7565 ± 579 de |
| 9C | 50 (0) | 1:50 (0) | 3087 ± 72 b | 70.1 ± 2.7 cde | 6384 ± 368 a | 787 ± 80 b | 6388 ± 91 bc | 61.4 ± 1.3 cd | 8661 ± 342 bc |
| 10C | 50 (0) | 1:50 (0) | 3063 ± 100 b | 69.2 ± 2.3 de | 4547 ± 411 cd | 636 ± 16 d | 5786 ± 293 ef | 64.2 ± 7.2 c | 8305 ± 202 c |
| 11C | 50 (0) | 1:50 (0) | 2884 ± 89 e | 84.9 ± 3.0 a | 4022 ± 128 de | 648 ± 12 d | 6237 ± 153 bcd | 61.7 ± 2.9 cd | 8660 ± 249 bc |
| p-Value (homocedasticity) | 0.503 | 0.999 | 0.942 | 0.914 | 0.709 | 0.954 | 0.97 | ||
| p-Value (one-way ANOVA) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Tests | Independent Variables (Original and Coded Values) | Total Phenolic Content (mg GAE/100 g) | Flavonoids (mg QE/100 g) | Condensed Tannins (mg CTE/100 g) | Ortho-Diphenolics (mg ACE/100 g) | DPPH (mg AAE/100 g) | Fe2+ Chelating Capacity (mg EDTAE/100 g) | FRAP (mg AAE/100 g) | |
|---|---|---|---|---|---|---|---|---|---|
| Time (min) | 1:X (g/mL) | ||||||||
| 1 | 40 (−1) | 1:40 (−1) | 923 ± 11 h | 46.2 ± 1.6 e | 579 ± 44 f | 253 ± 32 e | 1769 ± 87 f | 144 ± 8 f | 3060 ± 53 f |
| 2 | 40 (−1) | 1:60 (1) | 1167 ± 6 g | 58.1 ± 1.7 cd | 637 ± 50 ef | 258 ± 3 e | 2246 ± 197 cde | 227 ± 1 a | 3823 ± 143 d |
| 3 | 60 (1) | 1:40 (−1) | 1067 ± 25 cd | 55.9 ± 1.8 d | 594 ± 42 f | 350 ± 35 cd | 2152 ± 147 de | 143 ± 4 f | 3437 ± 92 e |
| 4 | 60 (1) | 1:60 (1) | 1243 ± 13 a | 65.7 ± 1.0 a | 1001 ± 112 ab | 367 ± 13 bcd | 1229 ± 190 g | 215 ± 9 b | 4258 ± 148 a |
| 5 | 35.9 (−1.41) | 1:50 (0) | 1143 ± 9 de | 60.9 ± 0.6 b | 856 ± 19 cd | 426 ± 66 a | 2470 ± 173 bc | 186 ± 7 c | 3893 ± 78 cd |
| 6 | 64.1 (1.41) | 1:50 (0) | 1202 ± 8 b | 63.8 ± 1.6 a | 840 ± 40 cd | 409 ± 31 ab | 2366 ± 194 cd | 185 ± 5 c | 3958 ± 93 bcd |
| 7 | 50 (0) | 1:35.9 (−1.41) | 1073 ± 26 g | 59.4 ± 1.6 bc | 750 ± 45 de | 420 ± 42 ab | 2098 ± 93 e | 120 ± 4 g | 3551 ± 92 e |
| 8 | 50 (0) | 1:64.1 (1.41) | 1170 ± 21 cd | 60.3 ± 0.4 bc | 1114 ± 141 a | 326 ± 18 d | 2760 ± 127 a | 235 ± 5 a | 4295 ± 317 a |
| 9C | 50 (0) | 1:50 (0) | 1105 ± 20 f | 58.4 ± 1.4 c | 872 ± 112 bcd | 382 ± 49 abcd | 2264 ± 78 cde | 175 ± 2 d | 3951 ± 80 bcd |
| 10C | 50 (0) | 1:50 (0) | 1187 ± 30 bc | 59.7 ± 1.1 bc | 872 ± 20 bcd | 372 ± 12 abcd | 2706 ± 94 ab | 166 ± 5 e | 4153 ± 166 ab |
| 11C | 50 (0) | 1:50 (0) | 1129 ± 10 ef | 61.1 ± 0.9 b | 926 ± 96 bc | 391 ± 15 abc | 2634 ± 79 ab | 183 ± 3 cd | 4088 ± 209 abc |
| p-Value (homocedasticity) | 0.949 | 0.976 | 0.889 | 0.699 | 0.948 | 0.79 | 0.879 | ||
| p-Value (one-way ANOVA) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Model Components | Regression Coefficients | Standard Error | t-Value | p-Value | −95% Confidence | +95% Confidence |
|---|---|---|---|---|---|---|
| Total phenolic content | ||||||
| Mean | 3012 | 29.6 | 102 | <0.001 | 2951 | 3073 |
| (1) Time (min)(L) | −212 | 18.1 | −11.7 | <0.001 | −249 | −175 |
| Time (min)(Q) | 140 | 21.6 | 6.5 | <0.001 | 95.7 | 185 |
| (2) Ratio (x/s)(L) | 99 | 18.1 | 5.4 | <0.001 | 61.3 | 136 |
| Ratio (x/s)(Q) | −67 | 21.6 | −3.1 | 0.005 | −112 | −22.5 |
| 1 L to 2 L | −215 | 25.6 | −8.4 | <0.001 | −268 | −162 |
| R2 | 0.905 | |||||
| Adjusted R2 | 0.888 | |||||
| p-value (normality of residues) | 0.763 | |||||
| Free radical scavenging activity (DPPH) | ||||||
| Mean. | 6171 | 52.1 | 118 | <0.001 | 6064 | 6279 |
| (1) Time (min)(L) | −281 | 61.2 | −4.6 | <0.001 | −408 | −155 |
| (2) Ratio (x/s)(L) | 418 | 61.2 | 6.8 | <0.001 | 292 | 544 |
| R2 | 0.706 | |||||
| Adjusted R2 | 0.680 | |||||
| p-value (normality of residues) | 0.945 | |||||
| Ferric-reducing antioxidant power (FRAP) | ||||||
| Mean | 8542 | 99.6 | 85.8 | <0.001 | 8336 | 8747 |
| (1) Time (min)(L) | −751 | 61.1 | −12.3 | <0.001 | −877 | −624 |
| Time (min)(Q) | 254 | 72.9 | 3.5 | 0.002 | 103 | 404 |
| Ra (io/s)(Q) | −396 | 72.9 | −5.4 | <0.001 | −546 | −246 |
| 1 L to 2 L | −304 | 86.2 | −3.5 | 0.002 | −482 | −126 |
| R2 | 0.795 | |||||
| Adjusted R2 | 0.765 | |||||
| p-value (normality of residues) | 0.401 | |||||
| Model Components | Regression Coefficient | Standard Error | t-Value | p-Value | −95% Confidence | +95% Confidence |
|---|---|---|---|---|---|---|
| Total phenolic content | ||||||
| Mean | 1145 | 6.6 | 173 | <0.001 | 1131 | 1159 |
| (1) Time (min)(L) | 38.0 | 5.6 | 6.8 | <0.001 | 26.5 | 49.6 |
| (2) Ratio (x/s)(L) | 69.7 | 5.6 | 12.5 | <0.001 | 58.2 | 81.2 |
| Ratio (x/s)(Q) | −22.6 | 6.4 | −3.5 | 0.002 | −35.8 | −9.5 |
| 1 L to 2 L | −17.3 | 7.9 | −2.2 | 0.038 | −33.6 | −1.0 |
| R2 | 0.706 | |||||
| Adjusted R2 | 0.664 | |||||
| p-value (normality of residues) | 0.648 | |||||
| Fe2+ chelating capacity | ||||||
| Mean | 177 | 1.6 | 110 | <0.001 | 173 | 180 |
| Time (min)(Q) | 5.4 | 1.5 | 3.5 | 0.002 | 2.2 | 8.6 |
| (2) Ratio (x/s)(L) | 39.7 | 1.4 | 29.3 | <0.001 | 36.9 | 42.5 |
| R2 | 0.965 | |||||
| Adjusted R2 | 0.963 | |||||
| p-value (normality of residues) | 0.077 | |||||
| Ferric-reducing antioxidant power (FRAP) | ||||||
| Mean | 4065 | 51.6 | 78.8 | <0.001 | 3959 | 4171 |
| (1) Time (min)(L) | 113 | 31.6 | 3.6 | 0.002 | 47.9 | 178 |
| Time (min)(Q) | −140 | 37.7 | −3.7 | 0.001 | −218 | −62.4 |
| (2) Ratio (x/s)(L) | 330 | 31.6 | 10.4 | <0.001 | 265 | 395 |
| Ratio (x/s)(Q) | −142 | 37.7 | −3.8 | <0.001 | −220 | −63.8 |
| R2 | 0.719 | |||||
| Adjusted R2 | 0.679 | |||||
| p-value (normality of residues) | 0.232 | |||||
| Parameters Evaluated | Predicted Mean Values | −95% Prediction | +95% Prediction | Experimental Mean Values | Relative Error (%) | |
|---|---|---|---|---|---|---|
| Grape | TPC (mg GAE/100 g) | 3847 | 3625 | 4068 | 3623 ± 40 | 5.8 |
| FRAP (mg AAE/100 g) | 10,084 | 9339 | 10,829 | 9395 ± 14 | 6.8 | |
| DPPH (mg AAE/100 g) | 6914 | 6167 | 7661 | 7102 ± 164 | 2.7 | |
| Blackberry | TPC (mg GAE/100 g) | 1249 | 1184 | 1314 | 1295 ± 12 | 3.7 |
| FRAP (mg AAE/100 g) | 4180 | 3811 | 4549 | 3804 ± 45 | 9.0 | |
| Fe2+ (mg EDTA/100 g) | 241 | 225 | 256 | 231 ± 6 | 4.1 |
| Total Phenolic Content (mg GAE/100 g) | Flavonoids (mg QE/100 g) | Condensed Tannins (mg CTE/100 g) | Ortho-Diphenols (mg ACE/100 g) | DPPH (mg AAE/100 g) | Fe2+ Chelating Capacity (mg EDTAE/100 g) | FRAP (mg AAE/100 g) | OH Radical Capture (mg AAE/100 g) | Lipidic Peroxidation Inhibition—IC50 (mg/L) | |
|---|---|---|---|---|---|---|---|---|---|
| Grape | 2778 ± 218 | 61 ± 0.05 | 2609 ± 257 | 729 ± 81 | 4838 ± 654 | 59 ± 2 | 4972 ± 164 | 258,165 ± 17,568 | 124.1 |
| Blackberry | 981 ± 185 | 38.1 ± 0.06 | 409 ± 154 | 233 ± 37 | 1377 ± 117 | 210 ± 8 | 1856 ± 164 | 164,279 ± 5856 | 30.6 |
| Grape (mg/100 g) | Blackberry (mg/100 g) | |
|---|---|---|
| Phenolic acids | ||
| Ferulic acid | 1.3 ± 0.2 | 0.8 ± 0.1 |
| 2,5- Dihydroxybenzoic acid | ND | 33.5 ± 1.6 |
| 3,4-Dihydroxybenzoic acid | 5.7 ± 0.1 | 34.5 ± 0.9 |
| Salicylic acid | ND | 0.2 ± 0.0 |
| p-Coumaric acid | 1.2 ± 0.0 | ND |
| Caffeic acid | ND | 0.1 ± 0.0 |
| Synaptic acid | 0.6 ± 0.0 | 0.9 ± 0.1 |
| Sinapaldehyde | ND | 1.0 ± 0.0 |
| Flavonoids | ||
| (−)-Epicatechin | 833.1 ± 2.7 | 81.5 ± 3.4 |
| (+)-Catechin | 573.8 ± 11.2 | 88.8 ± 3.4 |
| Epigallocatechin-3-gallate | 785.0 ± 1.6 | 8.4 ± 0.1 |
| Quercetin | 231.7 ± 1.2 | 15.2 ± 0.2 |
| Quercetin-3-rutinoside | 72.6 ± 1.0 | 13.1 ± 0.6 |
| Quercetin-3-glucoside | 2.5 ± 0.1 | 35.3 ± 1.6 |
| Hesperidin | 9.1 ± 0.7 | 12.7 ± 0.3 |
| Kaempferol | ND | 1.8 ± 0.1 |
| Kaempferol-3-rutinoside | 1.5 ± 0.1 | ND |
| Taxifolin | 0.4 ± 0.1 | ND |
| Pinocembrine | ND | 1.0 ± 0.1 |
| Galangine | ND | 0.1 ± 0.0 |
| Apigenin | ND | 0.2 ± 0.0 |
| Others | ||
| Coniferaldehyde | ND | 0.5 ± 0.1 |
| Total identified (mg/100 g) | 2519 | 329 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junior, T.K.; de Moura, C.; Cruz, T.M.; Marques, M.B.; Carmo, M.A.V.d.; Deolindo, C.T.P.; Daguer, H.; Azevedo, L.; Granato, D. Optimization of the Green Chemistry-like Extraction of Phenolic Compounds from Grape (Vitis labrusca L.) and Blackberry (Rubus fruticosus L.) Seeds with Concomitant Biological and Antioxidant Activity Assessments. Plants 2023, 12, 2618. https://doi.org/10.3390/plants12142618
Junior TK, de Moura C, Cruz TM, Marques MB, Carmo MAVd, Deolindo CTP, Daguer H, Azevedo L, Granato D. Optimization of the Green Chemistry-like Extraction of Phenolic Compounds from Grape (Vitis labrusca L.) and Blackberry (Rubus fruticosus L.) Seeds with Concomitant Biological and Antioxidant Activity Assessments. Plants. 2023; 12(14):2618. https://doi.org/10.3390/plants12142618
Chicago/Turabian StyleJunior, Tufy Kabbas, Cristiane de Moura, Thiago Mendanha Cruz, Mariza Boscacci Marques, Mariana Araújo Vieira do Carmo, Carolina Turnes Pasini Deolindo, Heitor Daguer, Luciana Azevedo, and Daniel Granato. 2023. "Optimization of the Green Chemistry-like Extraction of Phenolic Compounds from Grape (Vitis labrusca L.) and Blackberry (Rubus fruticosus L.) Seeds with Concomitant Biological and Antioxidant Activity Assessments" Plants 12, no. 14: 2618. https://doi.org/10.3390/plants12142618





