Mutations in Selected ABA-Related Genes Reduce Level of Arabidopsis thaliana Susceptibility to the Beet Cyst Nematode Heterodera schachtii
Abstract
:1. Introduction
2. Results
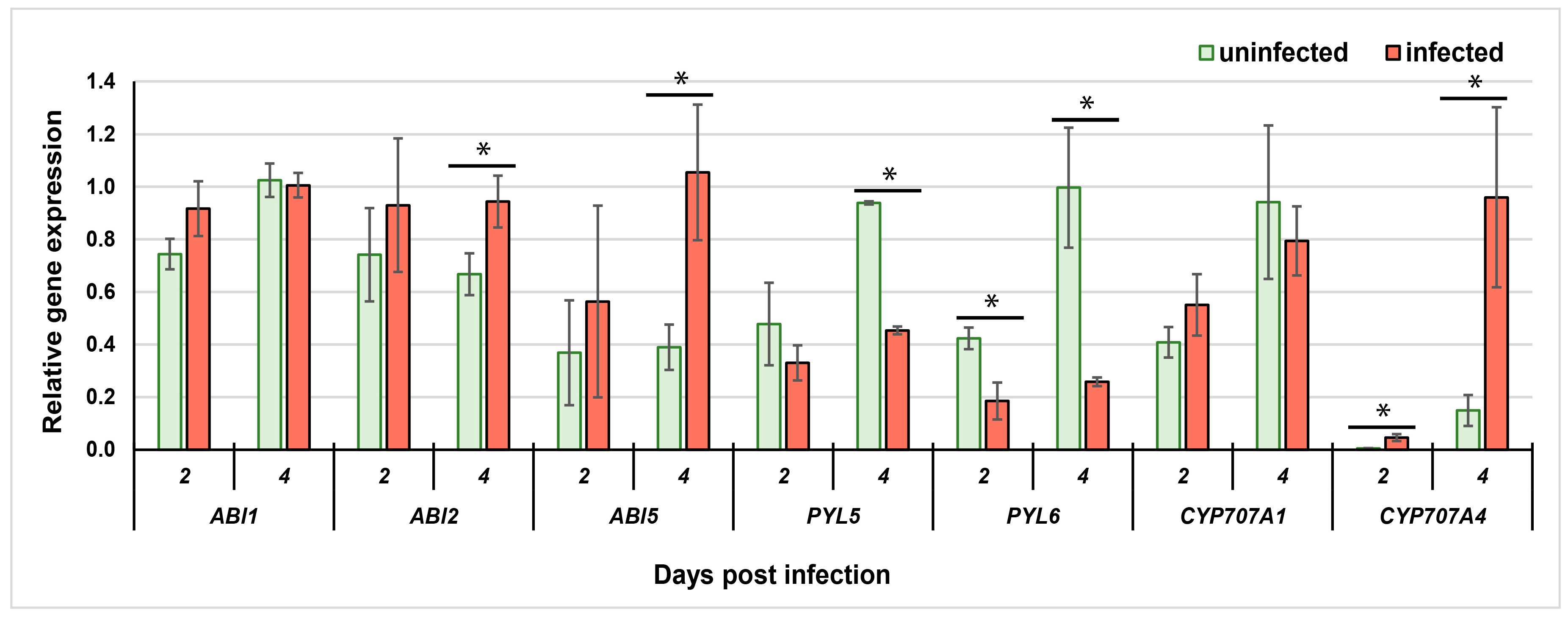
2.1. Analysis of Changes in A. thaliana Gene Expression at Early Stage of Nematode Parasitism
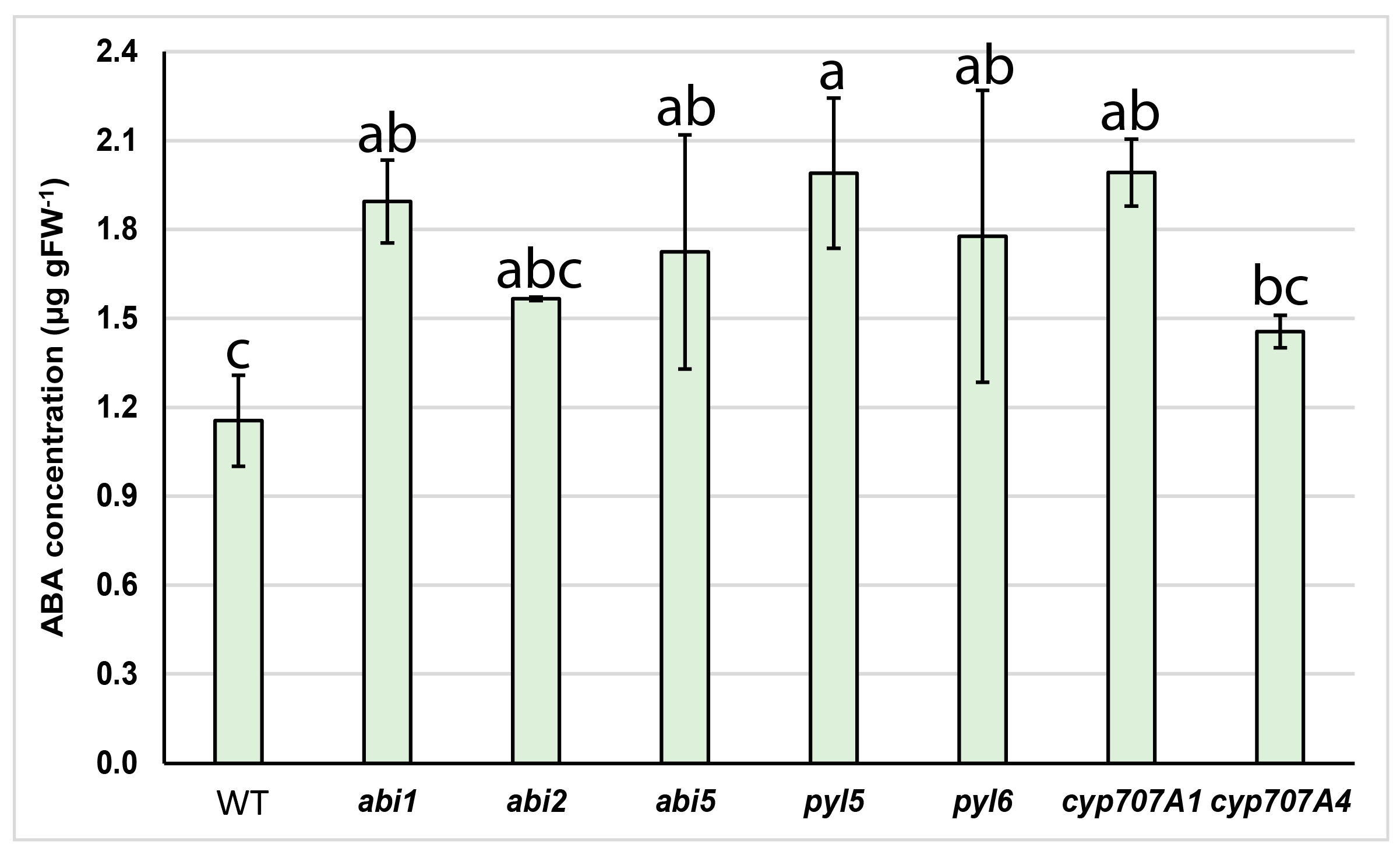
2.2. Endogenous ABA Concentrations in Arabidopsis Mutants
2.3. Susceptibility Level of Arabidopsis Mutants to H. schachtii
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Nematode Culture, Infection Assays and Measurements
4.3. Endogenous ABA Concentration
4.4. RNA Isolation and cDNA Synthesis
4.5. Quantitative Real-Time PCR
4.6. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Decraemer, W.; Hunt, D.J. Structure and classification. In Plant Nematology; Perry, R.N., Moens, M., Eds.; CABI: Wallingford, UK, 2006; pp. 3–32. [Google Scholar]
- Nicol, J.M.; Turner, S.J.; Coyne, D.L.; den Nijs, L.; Hockland, S.; Maafi, Z.T. Current nematode threats to world agriculture. In Genomics and Molecular Genetics of Plant–Nematode Interactions; Jones, J.T., Gheysen, G., Fenoll, C., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 21–44. [Google Scholar]
- Sobczak, M.; Golinowski, W. Cyst nematodes and syncytia. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Jones, J.T., Gheysen, G., Fenoll, C., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 61–82. [Google Scholar]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golinowski, W.; Grundler, F.M.W.; Sobczak, M. Changes in the structure of Arabidopsis thaliana during female development of the plant-parasitic nematode Heterodera schachtii. Protoplasma 1996, 194, 103–116. [Google Scholar] [CrossRef]
- Haegeman, A.; Mantelin, S.; Jones, J.T.; Gheysen, G. Functional roles of effectors of plant-parasitic nematodes. Gene 2012, 492, 19–31. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting Mechanisms of Defense Against Biotrophic and Necrotrophic Pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Ton, J.; Flors, V.; Mauch-Mani, B. The multifaceted role of ABA in disease resistance. Trends Plant Sci. 2009, 14, 310–317. [Google Scholar] [CrossRef]
- Cao, F.Y.; Yoshioka, K.; Desveaux, D. The roles of ABA in plant-pathogen interactions. J. Plant Res. 2011, 124, 489–499. [Google Scholar] [CrossRef]
- Bittner, F.; Oreb, M.; Mendel, R.R. ABA3 Is a Molybdenum Cofactor Sulfurase Required for Activation of Aldehyde Oxidase and Xanthine Dehydrogenase in Arabidopsis thaliana. J. Biol. Chem. 2001, 276, 40381–40384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, L.; Zhu, J.-K. Update on abscisic acid biosynthesis. Plant Physiol. 2003, 133, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Ng, L.M.; Melcher, K.; Teh, B.T.; Xu, H.E. Abscisic acid perception and signaling: Structural mechanisms and applications. Acta Pharmacol. Sin. 2014, 35, 567–584. [Google Scholar] [CrossRef] [Green Version]
- Weng, J.K.; Ye, M.; Li, B.; Noel, J.P. Co-evolution of Hormone Metabolism and Signaling Networks Expands Plant Adaptive Plasticity. Cell 2016, 166, 881–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahar, K.; Kyndt, T.; Nzogela, Y.B.; Gheysen, G. Abscisic acid interacts antagonistically with classical defense pathways in rice-migratory nematode interaction. New Phytol. 2012, 196, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Van Montagu, M.; Gheysen, G. Exogenous application of abscisic acid to potato plants suppresses reproduction of Meloidogyne incognita. In Mededelingen van de Faculteit Landbouwkundige en Toegepaste Biologische Wetenschappen; Universiteit Gent: Gent, Belgium, 1995; Volume 60, pp. 1033–1035. [Google Scholar]
- Sijmons, P.C.; Grundler, F.M.W.; Mende, N.; Burrows, P.R.; Wyss, U. Arabidopsis thaliana as a new model host for plant-parasitic nematodes. Plant J. 1991, 1, 245–254. [Google Scholar] [CrossRef]
- Kammerhofer, N.; Radakovic, Z.; Regis, J.M.A.; Dobrev, P.; Vankova, R.; Grundler, F.M.W.; Siddique, S.; Hofmann, J.; Wieczorek, K. Role of stress-related hormones in plant defence during early infection of the cyst nematode Heterodera schachtii in Arabidopsis. New Phytol. 2015, 207, 778–789. [Google Scholar] [CrossRef] [Green Version]
- Fuller, V.L.; Lilley, C.J.; Urwin, P.E. Nematode resistance. New Phytol. 2008, 180, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Van Schie, C.C.N.; Takken, F.L.W. Susceptibility Genes 101: How to Be a Good Host. Annu. Rev. Phytopathol. 2014, 52, 551–581. [Google Scholar] [CrossRef] [PubMed]
- Gheysen, G.; Mitchum, M.G. Phytoparasitic nematode control of plant hormone pathways. Plant Physiol. 2019, 179, 1212–1226. [Google Scholar] [CrossRef] [Green Version]
- Szakasits, D.; Heinen, P.; Wieczorek, K.; Hofmann, J.; Wagner, F.; Kreil, D.P.; Sykacek, P.; Grundler, F.M.W.; Bohlmann, H. The transcriptome of syncytia induced by the cyst nematode Heterodera schachtii in Arabidopsis roots. Plant J. 2009, 57, 771–784. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, M.; Kushiro, T.; Jikumaru, Y.; Abrams, S.R.; Kamiya, Y.; Seki, M.; Nambara, E. ABA 9′-hydroxylation is catalyzed by CYP707A in Arabidopsis. Phytochemistry 2011, 72, 717–722. [Google Scholar] [CrossRef] [Green Version]
- Saez, A.; Robert, N.; Maktabi, M.H.; Schroeder, J.I.; Serrano, R.; Rodriguez, P.L. Enhancement of Abscisic Acid Sensitivity and Reduction of Water Consumption in Arabidopsis by Combined Inactivation of the Protein Phosphatases Type 2C ABI1 and HAB1. Plant Physiol. 2006, 141, 1389–1399. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Jiang, Y.; Shi, M.; Wu, X.; Wu, G. ABI5 acts downstream of miR159 to delay vegetative phase change in Arabidopsis. New Phytol. 2021, 231, 339–350. [Google Scholar] [CrossRef]
- Antoni, R.; Gonzalez-Guzman, M.; Rodriguez, L.; Peirats-Llobet, M.; Pizzio, G.A.; Fernandez, M.A.; De Winne, N.; De Jaeger, G.; Dietrich, D.; Bennett, M.J.; et al. PYRABACTIN RESISTANCE1-LIKE8 Plays an Important Role for the Regulation of Abscisic Acid Signaling in Root. Plant Physiol. 2013, 161, 931–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, M.; Kuwahara, A.; Seo, M.; Kushiro, T.; Asami, T.; Hirai, N.; Kamiya, Y.; Koshiba, T.; Nambara, E. CYP707A1 and CYP707A2, Which Encode Abscisic Acid 8′-Hydroxylases, Are Indispensable for Proper Control of Seed Dormancy and Germination in Arabidopsis. Plant Physiol. 2006, 141, 97–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiśniewska, A.; Wojszko, K.; Różańska, E.; Lenarczyk, K.; Kuczerski, K.; Sobczak, M. Arabidopsis thaliana Myb59 Gene Is Involved in the Response to Heterodera schachtii Infestation, and Its Overexpression Disturbs Regular Development of Nematode-Induced Syncytia. Int. J. Mol. Sci. 2021, 22, 6450. [Google Scholar] [CrossRef]
- Wiśniewska, A.; Wojszko, K.; Różańska, E.; Lenarczyk, K.; Sobczak, M. Arabidopsis thaliana AtHRS1 gene is involved in the response to Heterodera schachtii infection and its overexpression hampers development of syncytia and involves a jasmonic acid-dependent mechanism. J. Plant Physiol. 2022, 272, 153680. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, C.; Ruijter, J.M.; Lekanne Deprez, R.H.; Moorman, A.F.M. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]

| Gene Name | Gene Acc. No. | Mutant NASC Stock No./Polymorphism | Gene Product | Gene Function | Mutant Phenotype |
|---|---|---|---|---|---|
| ABI1 (ABA-insensitive1) | AT4G26080 | SALK_072009 /abi1-2 | 2C protein phosphatase | ABA signalling | [23] |
| ABI2 (ABA-insensitive2) | AT5G57050 | SAIL_547_C10 | 2C protein phosphatase | nr * | |
| ABI5 (ABA-insensitive5) | AT2G36270 | SALK_013163 /abi5-2 | transcription factor | [24] | |
| PYL5 (PYR1-like5) | AT5G05440 | SM_3_532 | Receptor | nr * | |
| PYL6 (PYR1-like6) | AT2G40330 | SAIL_1179_D01 | Receptor | [25] | |
| CYP707A1 | AT4G19230 | SALK_069127 /CYP707A1-1 | cytochrome P450 monooxygenase | ABA catabolism | [26] |
| CYP707A4 | AT3G19270 | SALK_201208C | cytochrome P450 monooxygenase | nr * |
| Gene Name | Gene Acc. No. | Primers 5′-3′ | Used for |
|---|---|---|---|
| ABI1 | AT4G26080 | TGAATATAGGAAGTCTGAAGCAAGTG CGAAACAGCATCTTCCATCTC | Genotyping |
| ATGTCGAGATCCATTGGCGAT TTGCCATCTCACACGCTTCT | Real-time PCR | ||
| ABI2 | AT5G57050 | TTCCTTCTCCTCTTTTCTCCG TTGATCCGAGATCGATGAATC | Genotyping |
| CGACTCTAGGGCGGTTTTGT AGGTATCTATCGCCAATGGATCT | Real-time PCR | ||
| ABI5 | AT2G36270 | CAATGGAAGTTCGGAATCATG CACTCGTTTTCTTCTTAAAGCG | Genotyping |
| AGTGGATGGTCCAGTGGAGA CAACTCCGCCAATGCATGTT | Real-time PCR | ||
| CYP707A1 | AT4G19230 | CATGAACGTATTGGGTTTTGG TCCTGATATTGAATCCATCGC | Genotyping |
| AGAAGCTGTCGAAGATGTCGAA GGGTTTTGGAGCCACCTCG | Real-time PCR | ||
| CYP707A4 | AT3G19270 | TCGATCATTTACAACATAAG GGAAGACCGTTTTGAGGTAT | Genotyping |
| AAGGTCGTGTGCTAACCCAA AGCCTTTTGCTCAGCCTTAAC | Real-time PCR | ||
| PYL5 | AT5G05440 | TGGACACAAGGATCAACCAAC CAGCGAACAAGATCAAAAAGC | Genotyping |
| CCGTGGTGGTGGAGTCTTAC AGAGACTGAAGGTTGCACCG | Real-time PCR | ||
| PYL6 | AT2G40330 | GCCTCGAGACAGTAGAAGATTG CGTATGACTCAACGACACGTG | Genotyping |
| TATCGGAGACGGTCGAGAGG ACCAACGACGCTGAAACTGA | Real-time PCR | ||
| UBP22 | AT5G10790 | CACAAGGGGATGTTGGAATCAG ACTCACATCCTCTCACCACTTC | Real-time PCR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Różańska, E.; Krępski, T.; Wiśniewska, A. Mutations in Selected ABA-Related Genes Reduce Level of Arabidopsis thaliana Susceptibility to the Beet Cyst Nematode Heterodera schachtii. Plants 2023, 12, 2299. https://doi.org/10.3390/plants12122299
Różańska E, Krępski T, Wiśniewska A. Mutations in Selected ABA-Related Genes Reduce Level of Arabidopsis thaliana Susceptibility to the Beet Cyst Nematode Heterodera schachtii. Plants. 2023; 12(12):2299. https://doi.org/10.3390/plants12122299
Chicago/Turabian StyleRóżańska, Elżbieta, Tomasz Krępski, and Anita Wiśniewska. 2023. "Mutations in Selected ABA-Related Genes Reduce Level of Arabidopsis thaliana Susceptibility to the Beet Cyst Nematode Heterodera schachtii" Plants 12, no. 12: 2299. https://doi.org/10.3390/plants12122299






