Growth Increase in the Herbaceous Plant Centella asiatica by the Plant Growth-Promoting Rhizobacteria Priestia megaterium HyangYak-01
Abstract
:1. Introduction
2. Results
2.1. PGPR Activity Results
2.2. Field Test
2.3. Soil Physicochemical Property Analysis
2.4. Whole-Genome Analysis
2.5. Rhizosphere Microbiome Analysis
3. Discussion
4. Materials and Methods
4.1. Rhizobacteria Isolation
4.2. Plant Growth-Promoting Activity Test
4.2.1. Indole-3-Acetic Acid
4.2.2. Phosphate Solubilization
4.2.3. Urease Activity
4.2.4. Denitrification
4.3. Treatment and Plant Sample Collection of Centella asiatica
4.4. Soil Physicochemical Property Analysis
4.5. DNA Extraction, Library Preparation, and Sequencing
4.5.1. Method for 16S rRNA Amplicon Sequencing
4.5.2. Whole-Genome Sequencing
4.5.3. Bioinformatic Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khatoon, Z.; Huang, S.; Rafique, M.; Fakhar, A.; Kamran, M.A.; Santoyo, G. Unlocking the Potential of Plant Growth-Promoting Rhizobacteria on Soil Health and the Sustainability of Agricultural Systems. J. Environ. Manag. 2020, 273, 111118. [Google Scholar] [CrossRef]
- Mohanty, P.; Singh, P.K.; Chakraborty, D.; Mishra, S.; Pattnaik, R. Insight into the Role of PGPR in Sustainable Agriculture and Environment. Front. Sustain. Food Syst. 2021, 5, 667150. [Google Scholar] [CrossRef]
- Zhu, H.; Li, C.; Gao, C. Applications of CRISPR–Cas in Agriculture and Plant Biotechnology. Nat. Rev. Mol. Cell Biol. 2020, 21, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Qaim, M. Role of New Plant Breeding Technologies for Food Security and Sustainable Agricultural Development. Appl. Econ. Perspect. Policy 2020, 42, 129–150. [Google Scholar] [CrossRef]
- Zaidi, S.S.; Vanderschuren, H.; Qaim, M.; Mahfouz, M.M.; Kohli, A.; Mansoor, S.; Tester, M. New Plant Breeding Technologies for Food Security. Science 2019, 363, 1390–1391. [Google Scholar] [CrossRef] [Green Version]
- Cordero, I.; Balaguer, L.; Rincón, A.; Pueyo, J.J. Inoculation of Tomato Plants with Selected PGPR Represents a Feasible Alternative to Chemical Fertilization under Salt Stress. J. Plant Nutr. Soil Sci. 2018, 181, 694–703. [Google Scholar] [CrossRef]
- Ibal, J.C.; Jung, B.K.; Park, C.E.; Shin, J.H. Plant Growth-Promoting Rhizobacteria Used in South Korea. Appl. Biol. Chem. 2018, 61, 709–716. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Prakash, A.; Johri, B.N. Bacillus as PGPR in Crop Ecosystem. In Bacteria in Agrobiology: Crop Ecosystems; Springer: Berlin/Heidelberg, Germany, 2011; pp. 37–59. [Google Scholar]
- Govindasamy, V.; Senthilkumar, M.; Magheshwaran, V.; Kumar, U.; Bose, P.; Sharma, V.; Annapurna, K. Bacillus and Paenibacillus spp.: Potential PGPR for sustainable agriculture. In Plant Growth and Health Promoting Bacteria; Springer: Berlin/Heidelberg, Germany, 2010; pp. 333–364. [Google Scholar]
- Lim, J.H.; Kim, S.D. Synergistic plant growth promotion by the indigenous auxins-producing PGPR Bacillus subtilis AH18 and Bacillus licheniforims K11. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 531–538. [Google Scholar] [CrossRef]
- Probanza, A.; Mateos, J.L.; Lucas Garcia, J.A.; Ramos, B.; De Felipe, M.R.; Gutierrez Manero, F.J. Effects of inoculation with PGPR Bacillus and Pisolithus tinctorius on Pinus pinea L. growth, bacterial rhizosphere colonization, and mycorrhizal infection. Microb. Ecol. 2001, 41, 140–148. [Google Scholar] [CrossRef]
- Chandrika, U.G.; Kumara, P.A.P. Gotu Kola (Centella asiatica): Nutritional properties and plausible health benefits. Adv. Food Nutr. Res. 2015, 76, 125–157. [Google Scholar]
- Sabaragamuwa, R.; Perera, C.O.; Fedrizzi, B. Centella asiatica (Gotu kola) as a neuroprotectant and its potential role in healthy ageing. Trends Food Sci. Technol. 2018, 79, 88–97. [Google Scholar] [CrossRef]
- Bylka, W.; Znajdek-Awiżeń, P.; Studzińska-Sroka, E.; Brzezińska, M. Centella asiatica in cosmetology. Adv. Dermatol. Allergol./Postępy Dermatol. Alergol. 2013, 30, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Ratz-Łyko, A.; Arct, J.; Pytkowska, K. Moisturizing and Antiinflammatory Properties of Cosmetic Formulations Containing Centella asiatica Extract. Indian J. Pharm. Sci. 2016, 78, 27. [Google Scholar] [CrossRef] [Green Version]
- Orhan, I.E. Centella asiatica (L.) Urban: From Traditional Medicine to Modern Medicine with Neuroprotective Potential. Evid.Based Complement. Alternat. Med. 2012, 2012, 946259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maquart, F.X.; Bellon, G.; Gillery, P.; Wegrowski, Y.; Borel, J.P. Stimulation of Collagen Synthesis in Fibroblast Cultures by a Triterpene Extracted from Centella asiatica. Connect. Tissue Res. 1990, 24, 107–120. [Google Scholar] [CrossRef]
- Bonte, F.; Dumas, M.; Chaudagne, C.; Meybeck, A. Asiaticoside and Madecassoside Activity on Human Fibroblast Type I and III Collagen Secretion. Ann. Pharm. Fr. 1995, 53, 38–42. [Google Scholar]
- Hashim, P.; Sidek, H.; Helan, M.H.M.; Sabery, A.; Palanisamy, U.D.; Ilham, M. Triterpene Composition and Bioactivities of Centella asiatica. Molecules 2011, 16, 1310–1322. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.-H.; Kuo, C.-H.; Huang, H.-Y.; Chen, C.-Y.; Chen, C.-Y.; Chen, Y.-K.; Wei, T.-Y.; Lin, Y.-C.; Liu, S.-T. A Plant Endophytic Bacterium Priestia megaterium Strain BP-R2 Isolated from the Halophyte Bolboschoenus planiculmis Enhances Plant Growth under Salt and Drought Stresses. Microorganisms 2022, 10, 2047. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Khadka, B.; Kunwar, I.K.; Lohani, M.; Tamang, M.D.; Oren, A. Robust Demarcation of 17 Distinct Bacillus Species Clades, Proposed as Novel Bacillaceae Genera, by Phylogenomics and Comparative Genomic Analyses: Description of Robertmurraya kyonggiensis sp. nov. and Proposal for an Emended Genus Bacillus Limiting It Only to the Members of the Subtilis and Cereus Clades of Species. Int. J. Syst. Evol. Microbiol. 2020, 70, 5753–5798. [Google Scholar]
- Jung, B.K.; Ibal, J.C.; Pham, H.Q.; Kim, M.C.; Park, G.S.; Hong, S.J.; Jo, H.W.; Park, C.E.; Choi, S.D.; Jung, Y.; et al. Quorum Sensing System Affects the Plant Growth Promotion Traits of Serratia fonticola GS2. Front. Microbiol. 2020, 11, 536865. [Google Scholar] [CrossRef]
- Idris, E.E.; Iglesias, D.J.; Talon, M.; Borriss, R. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects the level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant-Microbe Interact. 2007, 20, 619–626. [Google Scholar] [CrossRef] [Green Version]
- Masepohl, B.; Kaiser, B.; Isakovic, N.; Richard, C.L.; Kranz, R.G.; Klipp, W. Urea utilization in the phototrophic bacterium Rhodobacter capsulatus is regulated by the transcriptional activator NtrC. J. Bacteriol. 2001, 183, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, M.A.; Macomber, L.; Hausinger, R.P. Biosynthesis of the urease metallocenter. J. Biol. Chem. 2013, 288, 13178–13185. [Google Scholar] [PubMed] [Green Version]
- Liang, J.L.; Liu, J.; Jia, P.; Yang, T.T.; Zeng, Q.W.; Zhang, S.C.; Liao, B.; Shu, W.S.; Li, J.T. Novel phosphate-solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zangelmi, E.; Stanković, T.; Malatesta, M.; Acquotti, D.; Pallitsch, K.; Peracchi, A. Discovery of a new, recurrent enzyme in bacterial phosphonate degradation: (R)-1-hydroxy-2-aminoethylphosphonate ammonia-lyase. Biochemistry 2021, 60, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Kiss, S.; Simihaian, M. Improving Efficiency of Urea Fertilizers by Inhibition of Soil Urease Activity; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Goswami, D.; Patel, K.; Parmar, S.; Vaghela, H.; Muley, N.; Dhandhukia, P.; Thakker, J.N. Elucidating multifaceted urease producing marine Pseudomonas aeruginosa BG as a cogent PGPR and bio-control agent. Plant Growth Regul. 2015, 75, 253–263. [Google Scholar]
- Devkota, A.; Jha, P.K. Variation in growth of Centella asiatica along different soil composition. Bot. Res. Int. 2009, 2, 55–60. [Google Scholar]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar]
- Oldfield, E.E.; Wood, S.A.; Bradford, M.A. Direct effects of soil organic matter on productivity mirror those observed with organic amendments. Plant Soil 2018, 423, 363–373. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef] [Green Version]
- Orhan, E.; Esitken, A.; Ercisli, S.; Turan, M.; Sahin, F. Effects of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient contents in organically growing raspberry. Sci. Hortic. 2006, 111, 38–43. [Google Scholar]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [PubMed] [Green Version]
- Li, X.; Wang, C.; Cheng, J.; Zhang, J.; da Silva, J.A.T.; Liu, X.; Duan, X.; Li, T.; Sun, H. Transcriptome analysis of carbohydrate metabolism during bulblet formation and development in Lilium davidii var. unicolor. BMC Plant Biol. 2014, 14, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filion, M.; Hamelin, R.C.; Bernier, L.; St-Arnaud, M. Molecular profiling of rhizosphere microbial communities associated with healthy and diseased black spruce (Picea mariana) seedlings grown in a nursery. Appl. Environ. Microbiol. 2004, 70, 3541–3551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannaa, M.; Han, G.; Jeon, H.W.; Kim, J.; Kim, N.; Park, A.R.; Kim, J.C.; Seo, Y.S. Influence of resistance-inducing chemical elicitors against pine wilt disease on the rhizosphere microbiome. Microorganisms 2020, 8, 884. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Li, H.; Yin, P.; Mei, H.; Li, J.; Zhou, P.; Wang, Y.; Ma, Q.; Jeyaraj, A.; Thangaraj, K.; et al. Integrated application of rapeseed cake and green manure enhances soil nutrients and microbial communities in tea garden soil. Sustainability 2021, 13, 2967. [Google Scholar] [CrossRef]
- Wen, T.; Yuan, J.; He, X.; Lin, Y.; Huang, Q.; Shen, Q. Enrichment of beneficial cucumber rhizosphere microbes mediated by organic acid secretion. Hortic. Res. 2020, 7, 154. [Google Scholar]
- Dai, Z.; Ahmed, W.; Yang, J.; Yao, X.; Zhang, J.; Wei, L.; Ji, G. Seed coat treatment by plant-growth-promoting rhizobacteria Lysobacter antibioticus 13–6 enhances maize yield and changes rhizosphere bacterial communities. Biol. Fertil. Soils 2023, 59, 317–331. [Google Scholar] [CrossRef]
- Li, J.; Luo, C.; Zhang, D.; Cai, X.; Jiang, L.; Zhao, X.; Zhang, G. Diversity of the active phenanthrene degraders in PAH-polluted soil is shaped by ryegrass rhizosphere and root exudates. Soil Biol. Biochem. 2019, 128, 100–110. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Simultaneous detection and quantification of indole-3-acetic acid (IAA) and indole-3-butyric acid (IBA) produced by rhizobacteria from l-tryptophan (Trp) using HPTLC. J. Microbiol. Methods 2015, 110, 7–14. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Brink, B. Urease test protocol. Am. Soc. Microbiol. 2010, 1–7. [Google Scholar]
- Kim, M.; Jeong, S.Y.; Yoon, S.J.; Cho, S.J.; Kim, Y.H.; Kim, M.J.; Ryu, E.Y.; Lee, S.J. Aerobic denitrification of Pseudomonas putida AD-21 at different C/N ratios. J. Biosci. Bioeng. 2008, 106, 498–502. [Google Scholar] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar]
- Lahti, L.; Shetty, S. microbiome: R Package for Microbiome Analysis. Bioconductor 2017, 99, 1–26. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
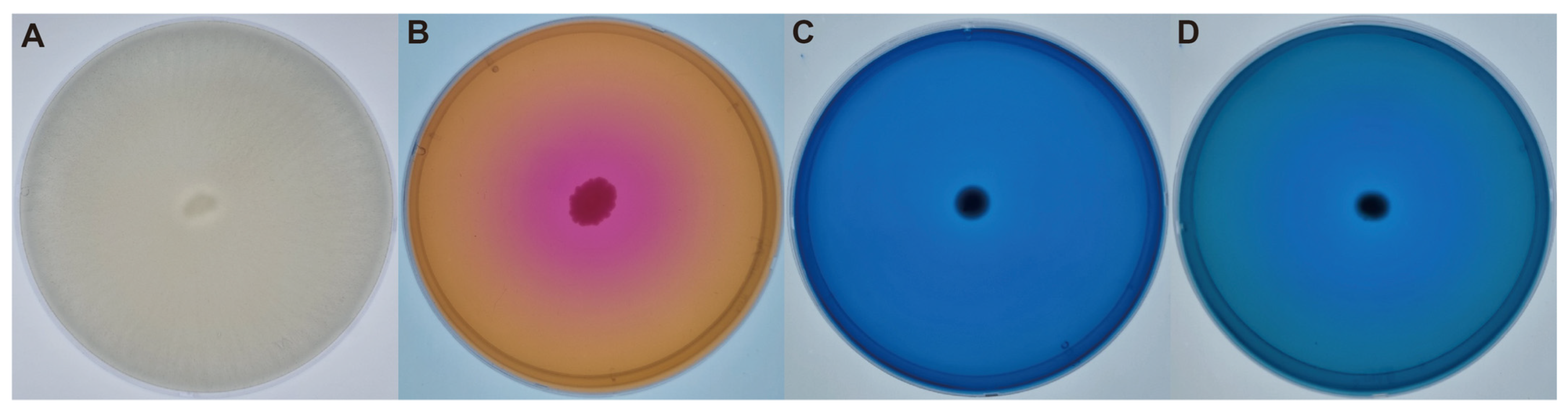
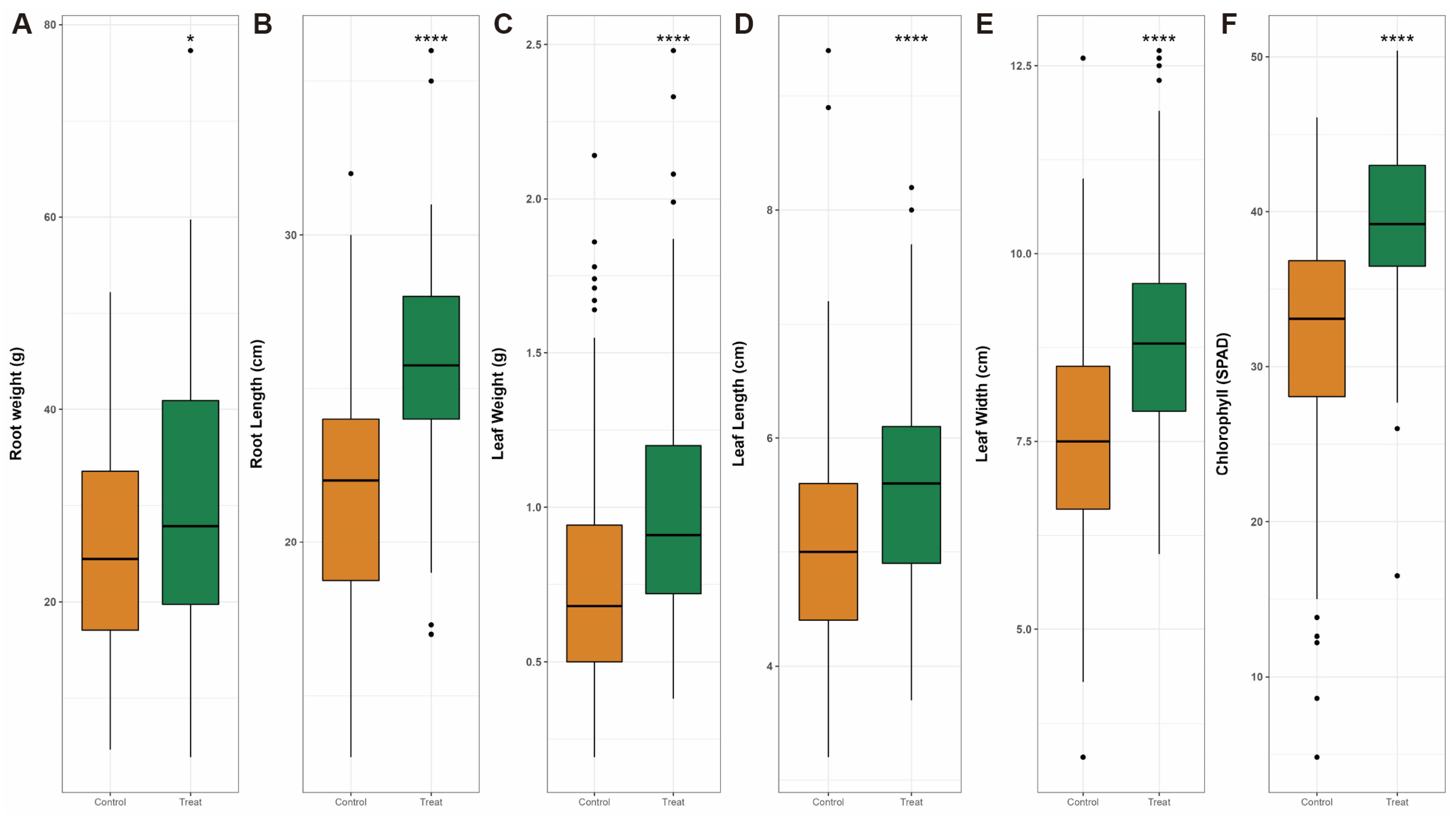
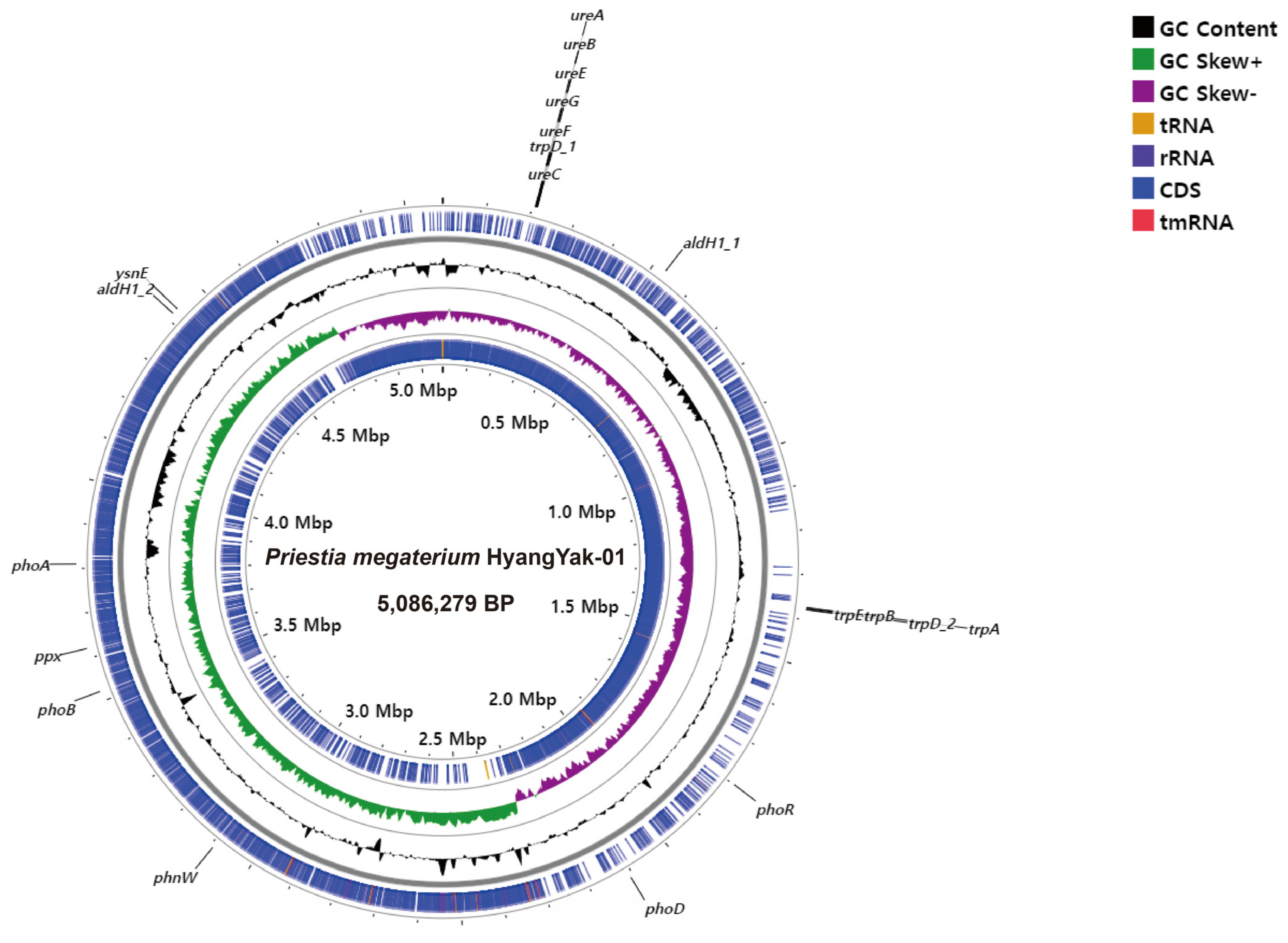

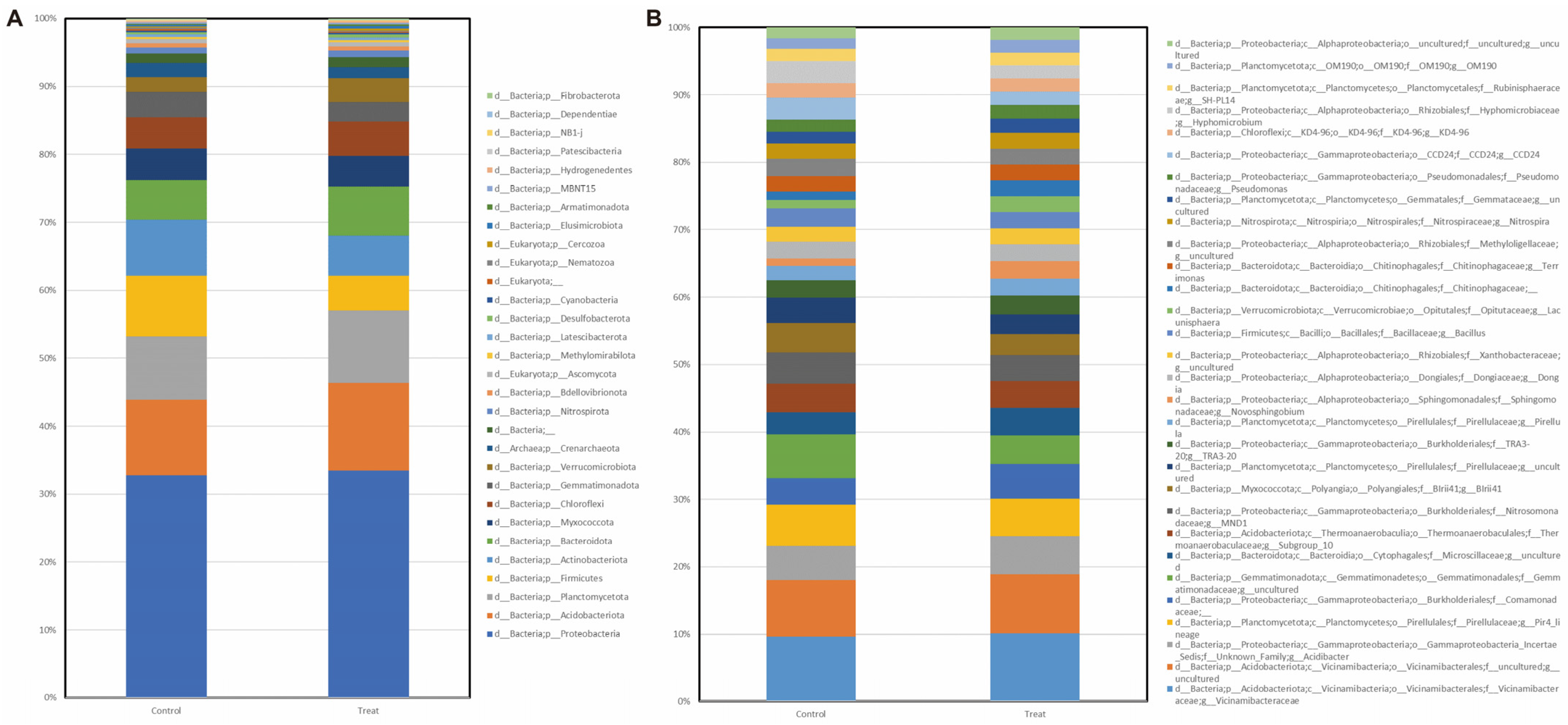
| Group | pH (1:5) | SOM (g kg−1) | Avail. P (mg kg−1) | Extractable Cation (cmolc kg−1) | ||
|---|---|---|---|---|---|---|
| K | Ca | Mg | ||||
| Control | 7.91 ± 0.01 | 19.45 ± 0.06 | 524.42 ± 5.45 | 0.38 ± 0.02 | 11.01 ± 0.01 | 3.89 ± 0.08 |
| Treated | 6.92 ± 0.02 | 16.52 ± 0.02 | 450.2 ± 0.14 | 0.26 ± 0.01 | 11.83 ± 0.03 | 4.16 ± 0.03 |
| Significance | **** | **** | ** | ** | *** | * |
| Features | Values |
|---|---|
| Genomic size (bp) | 5,086,279 |
| GC content (%) | 38.2 |
| Total genes | 5289 |
| CDSs | 5111 |
| Pseudo-genes | 48 |
| Ribosomal RNAs | 44 |
| Transfer RNAs | 126 |
| Other RNAs | 8 |
| Related PGP Traits | Genes |
|---|---|
| IAA-producing | aldH |
| trpE | |
| trpD | |
| trpA | |
| trpB | |
| ysnE | |
| Urease activity | urea |
| ureB | |
| ureC | |
| ureE | |
| ureF | |
| ureG | |
| Phosphate solubilization | phnW |
| phoA | |
| phoB | |
| phoD | |
| phoR | |
| Ppx |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Lim, K.; Ibal, J.C.; Kim, M.-C.; Kim, H.-B.; Baek, C.; Heo, Y.M.; Lee, H.; Kang, S.; Lee, D.-G.; et al. Growth Increase in the Herbaceous Plant Centella asiatica by the Plant Growth-Promoting Rhizobacteria Priestia megaterium HyangYak-01. Plants 2023, 12, 2398. https://doi.org/10.3390/plants12132398
Jo H, Lim K, Ibal JC, Kim M-C, Kim H-B, Baek C, Heo YM, Lee H, Kang S, Lee D-G, et al. Growth Increase in the Herbaceous Plant Centella asiatica by the Plant Growth-Promoting Rhizobacteria Priestia megaterium HyangYak-01. Plants. 2023; 12(13):2398. https://doi.org/10.3390/plants12132398
Chicago/Turabian StyleJo, HyungWoo, Kyeongmo Lim, Jerald Conrad Ibal, Min-Chul Kim, Hye-Been Kim, Chaeyun Baek, Young Mok Heo, Haeun Lee, Seunghyun Kang, Dong-Geol Lee, and et al. 2023. "Growth Increase in the Herbaceous Plant Centella asiatica by the Plant Growth-Promoting Rhizobacteria Priestia megaterium HyangYak-01" Plants 12, no. 13: 2398. https://doi.org/10.3390/plants12132398






