Polysaccharides from the Sargassum and Brown Algae Genus: Extraction, Purification, and Their Potential Therapeutic Applications
Abstract
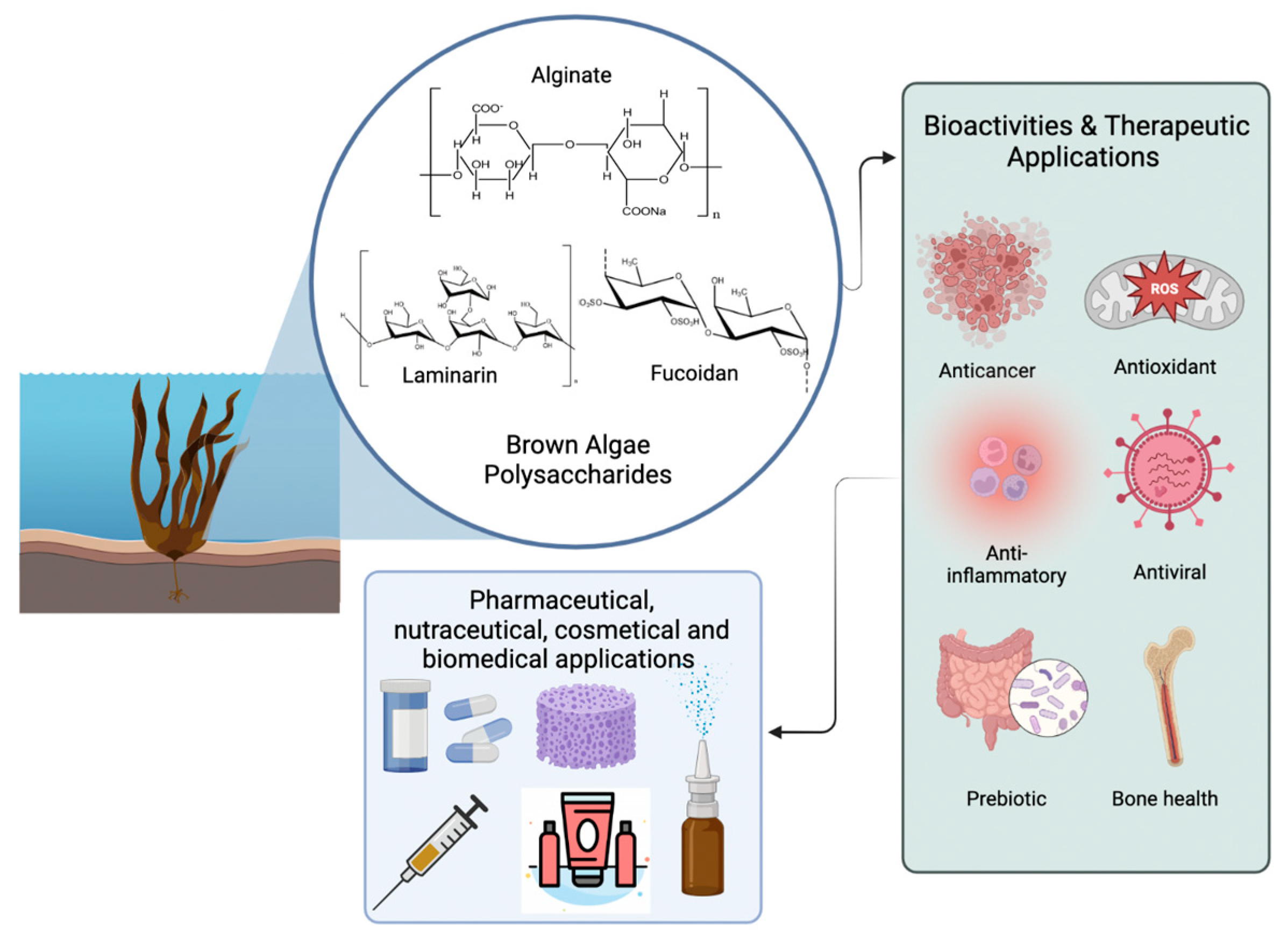
:1. Introduction
2. Advanced Methods for Extraction and Purification of Brown Seaweed Polysaccharides
| Algae | Purification Method | Purification Conditions | Yield | Reference |
|---|---|---|---|---|
| Sargassum ilicifolium | Chromatography | Dissolved in 5 mL distilled water. Loaded to a pre-equilibrated DEAE cellulose 52 columns. The stepwise gradient elution with 0.05 M Tris–HCl buffer (pH 7.0) containing 0.5, 2.0, 3.5, and 5.0 M NaCl. Fractions of 4 mL per tube were collected and monitored at 490 nm by the phenol–sulfuric acid (H2SO4) method. | Fucoidan yield from sonication–microwave extraction: 8 ± 0.9%. Hot water extraction: 6 ± 0.5%. | [44] |
| Sargassum autumnale | Chromatography | Twice the volume of 99.5% ethanol solution was added to the enzyme-assisted hydrolysates precipitate and collected by centrifugation. | Different extraction methods: Distilled water extract: 17.23 ± 0.28. Ultraflo extract: 23.50 ± 0.56. Protamex extract: 23.89 ± 0.42. | [46] |
| Sargassum aquifolium | Isopropyl alcohol purification process | Not reported | 21.74 ± 2135 | [47] |
| Sargassum patens | Dialysis | Pr: (NH4)2SO4 85% Pu: DI (kDa n.s.) | 8.2% | [38] |
| Sargassum polycystum | Ultrafiltration | Not reported | 7.27% | [48] |
| Sargassum siliquosum | Anion-exchange chromatography | Protein and uronic acid were removed. | 5.08 ± 1.17% | [49] |
| Sargassum natans | Gel permeation chromatography | Not reported | Maximum of 21.21% | [13] |
| Sargassum fusiforme | Lyophilization | Not reported | On the sample ASFF: 11.24 ± 0.94a | [50] |
| Sargassum swartzii | High-performance liquid chromatography | A total of 1 mg of lyophilized SP was dissolved in 1 mL distilled water. Filtered through a 0.22 μm syringe tip filter and subjected to HPLC analysis. | Hot water extraction: 3.6% HCl method: 1.2% | [45] |
3. Structural Description of the Main Polysaccharides Found in Brown Macroalgae
3.1. Fucoidans
3.2. Alginate
3.3. Laminarin
4. Exploring the Potential Relationship between Polysaccharides Structures and Their Bioactivities
4.1. Anticancer Activity
| Brown Algae | Polysaccharide | Bioactivity | Bioassay | Bioactivity Results | Reference |
|---|---|---|---|---|---|
| L. japonica | Laminarin | Anticancer | Cell viability detected by WST-8 cell proliferation assay, flow cytometry in 96-well plate in human HCC cell lines, including Bel-7404 and HepG2, when incubated with different concentrations of laminarin. Hepa 1–6 tumor-bearing mice were injected with different concentrations and tumors were measured. | Cell viability; ;aminarin concentrations of 35 mg/mL significantly decreased the Bel-7404 viability, with only 46.20% at 48 h. HepG2 was only 42.85% of that of cells without treatment. Apoptosis rate of Bel-7404 was 2.72 higher with laminarine and 8.18 times higher for HepG2 than without treatment. Tumor growth inhibition was higher at 1200 mg/k.d of laminarin with 67.92%. | [59] |
| Padina pavonioca | Sulfated polysaccharides | Anticancer Antioxidant | DPPH Cell viability: MTT assay cytotoxic activity in HeLa cancer cell lines cultured in DMEM supplemented. | A total of 1 mg/mL increases scavenging activity up to 63%. Low doses (0.05–0.1 mg/mL) exhibit cytotoxic activity in HeLa cancer cell line. | [66] |
| Sargassum ilicifolium | Fucoidan | Antioxidant Osteogenic ability (bone regeneration) | DPPH expression of osteoblast differentiation media in DMEM supplemented with murine mesenchymal stem cells (C3H10T1/2). | IC50 0.96 mg/mL (crude) and 2.51 mg/mL (purified). A total of 1 μg/mL of purified fucoidan provides cell proliferation (130%) on C3H10T1/2 at 48 h. | [38] |
| Silvetia Compressa Ecklonia arborea | Sulfated polysaccharides (fucoidan, laminarin, alginate) and phlorotannins | Antioxidant | DPPH & ORAC | DPPH IC50 (mg/mL) S.Compressa 1.7 E. arborea 3.7 ORAC (mmol Trolox equivalent/g) S.Compressa 0.817 E. arborea 0.801. | [67] |
| Sargassum siliquosum | Fucoidan | Antioxidant Anti-inflammatory | DPPH (absorbance of 50% methanol solution mixed with the sample solution was the blank). Cell viability: RAW264.7 cell line in 24-well plate. TNF-α content level after lipopolysaccharide LPS exposure reflected the anti-inflammatory activity. Quercetin was used as a positive control. | Antioxidant results: EC50 of purified fucoidan 2.58 mg/mL, higher antioxidant ability showed crude extract with an EC50 of 0.34 mg/mL. Cell Viability: Inhibition of TNF-α reached 14.8% with 0.25 µg/mL of fucoidan-treated compared to LPS control. | [36] |
| Sargassum horneri | Alginic acid | Anti-Inflammatory | Cell culture RAW 264.7 mouse macrophages and HaCaT (human keratinocytes) cultured in DMEM, 10% FBS, and 1% antibiotics. In 24-well plates, HaCaT were seeded with SHA and Chinnese fine dust CFD at 125 µg/mL under optimized conditions. MTT assay was used for cell viability. | Cell viability: A concentration of SHA 25–100 µg/mL decreased viability of HaCaT in a range of 20%. | [37] |
| Sargassum horneri | Fucoidan | Anti-inflammatory | Cell culture and viability assay RAW 264.7 macrophages were cultured in DMEM, 10% FBS, and 1% antibiotics. MTT test in 24-well plate. Negative control had untreated macrophages, positive control was only treated with PBS. | Fucoidan in concentrations of 12.5–50 μg/mL inhibited the production in LPS-activated RAW 264.7 macrophages with IC50 = 40 µg/mL. | [68] |
| Sargassum fulvellum | Sulfated polysaccharides | Anti-inflammatory | Anti-inflammatory activity in RAW 264.7 macrophages cultured in DMEM medium. Stress induced by E. coli lipopolysaccharides. In vivo tests applied in zebrafish embryos to measure their survival rate after 3 days. Levels of ROS, heartbeat, and cell death after stress induced by E. coli lipopolysaccharides. | Viability of RAW 264.7 cells increased by 94.6%, while the production of nitric oxide (NO) in RAW 264.7 decreased by 40.7%. Zebrafish survival, cell death, and ROS/NO production decreased in a dose-dependent manner. | [29] |
| Turbinaria decurrens | Fucoidan | Anti-inflammatory | Swiss albino mice were subjected to formalin-induced paw edema. The mice were treated with the extracted fucoidan, which was administered orally, to evaluate the anti-inflammatory effect. | The mice treated with fucoidan showed reduction in the perception of the wound by the mice. The licking time was reduced by more than 50%. In addition, the anti-inflammatory effect of the fucoidan in paw edema showed a reduction of 52%. | [69] |
| Sargassum fusiforme | Sulfated polysaccharides | Anti-inflammatory | Sulfated polysaccharides extracted by an enzymatic method were tested in RAW 264.7 cells stressed with lipopolysaccharide (LPS). To study the anti-inflammatory effects of the polysaccharides, the level of expression of NO and inflammatory cytokines, such as TNF-α, IL-1β, IL-6, and PGE2, were determined. | The sulfated polysaccharides showed an anti-inflammatory activity with a dose-dependent behavior. The addition of the polysaccharides successfully reduced the expression of TNF-α, IL-1β, IL-6, and PGE2. Additionally, the production of NO was reduced, while the cellular viability increased. | [70] |
| L. japonica | Polysaccharides | Antiviral | The polysaccharides isolated by ethanol precipitation were tested in HEK293 cells infected with the respiratory syncytial virus (RSV) to study the antiviral activity of the polysaccharides. The expression of IRF3 and IFN-α were analyzed. | The polysaccharide extracts demonstrated significant antiviral activity against RSV by increasing IFN-α expression via regulation of the IRF3 signaling pathway in HEK293 cells. | [71] |
| Sargassum polycystum | Fucoidan fraction-2 (Fu-F2) | Antibacterial | The MIC and MBC were determined for bacterial strains Streptococcus mutans, Staphylococcus aureus, Pseudomonas aeruginosa, and E. coli. A 48-well microtiter plate was used. The antibacterial activity was determined by disk diffusion assay. Test bacteria were grown on Luria-Bertani agar medium and a crude fucoidan loaded disk was placed with the standard antibiotic disk (tetracycline). | The highest antibacterial activity (21 ± 1.0 mm) was obtained at 50 µg/mL against Pseudomonas aeruginosa, and the lowest activity (16 ± 0.53 mm) was against Staphylococcus aureus. | [72] |
| Fucus vesiculosus | Fucoidan | Anti-angiogenesis | Use of crude extracted fucoidan over endothelial cells and chicken embryos. | A concentration of fucoidan at 0.5 mg/mL was able to prevent the formation of tubular structures in epithelial cells. Chicken embryos presented a reduction in blood vessel formation, as well as in the tumoral mass. | [73] |
| Fucus distichus subsp. evanescens | Fucoidan | Anti-angiogenesis | Measurement of the gene expression of the angiopoietins 1 and 2, vascular endothelial growth factors, and stromal-derived factors in mono- and co-cultured systems of human outgrowth endothelial and human mesenchymal stem cells. Cells were treated for seven days with extracts obtained enzymatically from Fucus distichus subsp. evanescence. The anti-angiogenic activity of co-cultured cells was analyzed by measuring the length and area of the tube-like structures created by the endothelial and mesenchymal cells. | In monoculture: The fucoidan extract downregulates the expression of vascular endothelial growth factor and stromal-derived factor-1 in mesenchymal stem cells; however, the angiopoietins-1 and angiopoietins-2 in the outgrowth endothelial cells’ levels were not affected by the fucoidan extract. The fucoidan extract with the higher sulfate content was able to disturb the formation of the tube-like structures; length and area were both reduced. | [74] |
| Ascophyllum nodosum | Sodium alginate | Prebiotic | Alg-MAE (microwave-assisted extraction) L. delbruecki ssp. bulgaricus and L. Casei growth media. Inulin (positive prebiotic control), glucose (the negative control in a 96-well-plate. | Alg- MAE improved the growth rate of L. delbruecki ssp. bulgaricus by 75% (at 0.10% (w/v) inclusion), 150% (at 0.50% (w/v)), 40% (at 0.10% (w/v)), and 34% (at 0.30% (w/v)) for L. casei when compared to the unsupplemented media. | [24] |
| Sargassum glaucescens | Fucoidan | Hair growth-promoting (alopecia treatment) | Cell proliferation: Human follicle dermal papilla cells (HFDPC) in DMEM with 1% FBS for 24 h, then treated with different molecular weight fucoidans (HHP-1-MW, SCW-1, and SCW-5; 1 mg/mL). HFDPC treated by glucose (1 mg/mL) as control; cell viability was measured with CCK-8 assay. Hair follicle culture assay: 5-week-old male C57BL/6 mice. Cultured in William’s medium with or without the supplementation of SCW-5 (1 mg/mL) or minoxidil (1 µM). | Cell proliferation was higher than glucose or PBS treated control. SCW-1 was the most effective with cell viability (200%) of HFDPC. The hair follicles treated by SCW-5 after 69 days of treatment had better hair growth than the minoxidil and control groups (p < 0.05). | [75] |
| Sargassum angustifolium | Fucoidan | Wound healing | The wound healing effect of crude fucoidan extracts on adipose-derived mesenchymal stem cells (ADMSCs) was determined by MTT and scratch assays. | The crude extracts of fucoidans were demonstrated by the MTT assay to improve growth up to 1.5 times. With the scratching technique, an increase in cell migration of 76 and 142% was observed after 48 and 72 h of incubation, respectively, in ADMSC cells. | [76] |
4.2. Antioxidant Activity
4.3. Anti-Inflammatory Activity
4.4. Antiviral Activity
4.5. Non-Conventional Activities
5. Challenges in Research and Marketing of Products Based on Brown Algae Polysaccharides
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milledge, J.J.; Harvey, P.J. Golden Tides: Problem or Golden Opportunity? The Valorisation of Sargassum from Beach Inundations. J. Mar. Sci. Eng. 2016, 4, 60. [Google Scholar] [CrossRef] [Green Version]
- Louime, C.; Fortune, J.; Gervais, G.; Rio, R.; Juan, S.; Rico, P. Sargassum Invasion of Coastal Environments: A Growing Concern. Am. J. Environ. Sci. 2017, 13, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Amador-Castro, F.; García-Cayuela, T.; Alper, H.S.; Rodriguez-Martinez, V.; Carrillo-Nieves, D. Valorization of pelagic sargassum biomass into sustainable applications: Current trends and challenges. J. Environ. Manag. 2021, 283, 112013. [Google Scholar] [CrossRef]
- Dai, C.; Wang, S. The Structure and Function of the Sargassum fusiforme Microbiome under Different Conditions. J. Mar. Sci. Eng. 2022, 10, 1401. [Google Scholar] [CrossRef]
- Oliyaei, N.; Moosavi-Nasab, M.; Mazloomi, S.M. Therapeutic activity of fucoidan and carrageenan as marine algal polysaccharides against viruses. 3 Biotech 2022, 12, 154. [Google Scholar] [CrossRef]
- Jönsson, M.; Allahgholi, L.; Sardari, R.R.R.; Hreggviosson, G.O.; Karlsson, E.N. Extraction and Modification of Macroalgal Polysaccharides for Current and Next-Generation Applications. Molecules 2020, 25, 930. [Google Scholar] [CrossRef] [Green Version]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef]
- Ciko, A.M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds from Marine Macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef] [Green Version]
- Vlaisavljević, S.; Rašeta, M.; Berežni, S.; Passamonti, S.; Tramer, F. Four selected commercial seaweeds: Biologically active compounds, antioxidant and cytotoxic properties. Int. J. Food Sci. Nutr. 2021, 72, 757–766. [Google Scholar] [CrossRef]
- Los Ficocoloides en la Industria. Available online: https://repositorio.usil.edu.pe/items/a25f3eba-1057-4142-a378-b1e8bf5b9ef3 (accessed on 9 December 2022).
- Marliana Baba, B.; Aida Wan Mustapha, W.; Seng Joe, L. Malaysian journal of analytical sciences effect of extraction methods on the yield, fucose content and purity of fucoidan from Sargassum sp. obtained from pulau langkawi, malaysia (Kesan Kaedah Pengekstrakan Fukoidan Terhadap Hasil, Kandungan Fukosa dan Ketulenan Fukoidan daripada Sargassum sp. dari Pulau Langkawi, Malaysia). Malays. J. Anal. Sci. 2018, 22, 87–94. [Google Scholar] [CrossRef]
- Bălașa, A.F.; Chircov, C.; Grumezescu, A.M. Marine Biocompounds for Neuroprotection—A Review. Mar. Drugs 2020, 18, 290. [Google Scholar] [CrossRef]
- Liu, J.; Wu, S.Y.; Chen, L.; Li, Q.J.; Shen, Y.Z.; Jin, L.; Zhang, X.; Chen, P.C.; Wu, M.J.; Choi, J.; et al. Different extraction methods bring about distinct physicochemical properties and antioxidant activities of Sargassum fusiforme fucoidans. Int. J. Biol. Macromol. 2020, 155, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Kalasariya, H.S.; Yadav, V.K.; Yadav, K.K.; Tirth, V.; Algahtani, A.; Islam, S.; Gupta, N.; Jeon, B.H. Seaweed-Based Molecules and Their Potential Biological Activities: An Eco-Sustainable Cosmetics. Molecules 2021, 26, 5313. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Jiang, H.; Mao, X.; Ci, F.; Huang, Y.; Jiang, H.; Mao, X.; Ci, F. Laminarin and Laminarin Oligosaccharides Originating from Brown Algae: Preparation, Biological Activities, and Potential Applications. JOUC 2021, 20, 641–653. [Google Scholar] [CrossRef]
- Zargarzadeh, M.; Amaral, A.J.R.; Custódio, C.A.; Mano, J.F. Biomedical applications of laminarin. Carbohydr. Polym. 2020, 232, 115774. [Google Scholar] [CrossRef]
- Ismail, M.M.; Mostafa, M.H.; El Sikaily, A.M. Phytochemical variation, antioxidant and antidiabetic capacity of extracts of common brown seaweeds from the Red Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2022, 26, 445–462. [Google Scholar] [CrossRef]
- Gullón, B.; Gagaoua, M.; Barba, F.J.; Gullón, P.; Zhang, W.; Lorenzo, J.M. Seaweeds as promising resource of bioactive compounds: Overview of novel extraction strategies and design of tailored meat products. Trends Food Sci. Technol. 2020, 100, 1–18. [Google Scholar] [CrossRef]
- Pradhan, B.; Bhuyan, P.P.; Patra, S.; Nayak, R.; Behera, P.K.; Behera, C.; Behera, A.K.; Ki, J.S.; Jena, M. Beneficial effects of seaweeds and seaweed-derived bioactive compounds: Current evidence and future prospective. Biocatal. Agric. Biotechnol. 2022, 39, 102242. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Kovačević, D.B.; Garofulić, I.E.; Dragović-Uzelac, V. Advanced Technologies for the Extraction of Marine Brown Algal Polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef] [Green Version]
- Otero, P.; Carpena, M.; Garcia-Oliveira, P.; Echave, J.; Soria-Lopez, A.; Garcia-Perez, P.; Fraga-Corral, M.; Cao, H.; Nie, S.; Xiao, J.; et al. Seaweed polysaccharides: Emerging extraction technologies, chemical modifications and bioactive properties. Crit. Rev. Food Sci. Nutr. 2021, 63, 1901–1929. [Google Scholar] [CrossRef]
- Okolie, C.L.; Mason, B.; Mohan, A.; Pitts, N.; Udenigwe, C.C. Extraction technology impacts on the structure-function relationship between sodium alginate extracts and their in vitro prebiotic activity. Food Biosci. 2020, 37, 100672. [Google Scholar] [CrossRef]
- Gomez, L.P.; Alvarez, C.; Zhao, M.; Tiwari, U.; Curtin, J.; Garcia-Vaquero, M.; Tiwari, B.K. Innovative processing strategies and technologies to obtain hydrocolloids from macroalgae for food applications. Carbohydr. Polym. 2020, 248, 116784. [Google Scholar] [CrossRef]
- Karthika Parvathy, K.R.; Ajanth Praveen, M.; Balasubramanian, P.; Mallick, B. Impact of advanced extraction technologies and characterization of freeze-dried brown seaweed polysaccharides. Dry. Technol. 2020, 39, 371–382. [Google Scholar] [CrossRef]
- Ekezie, F.G.C.; Sun, D.W.; Cheng, J.H. Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Zhang, Z. Potential applications of alginate oligosaccharides for biomedicine—A mini review. Carbohydr. Polym. 2021, 271, 118408. [Google Scholar] [CrossRef]
- Gan, W.; Tserkezis, C.; Cai, Q.; Falin, A.; Mateti, S.; Nguyen, M.; Aharonovich, I.; Watanabe, K.; Taniguchi, T.; Huang, F.; et al. Atomically thin boron nitride as an ideal spacer for metal-enhanced fluorescence. ACS Nano 2019, 13, 12184–12191. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M. Enzyme-assisted extraction of Nizamuddinia zanardinii for the recovery of sulfated polysaccharides with anticancer and immune-enhancing activities. J. Appl. Phycol. 2019, 31, 1391–1402. [Google Scholar] [CrossRef]
- Saravana, P.S.; Tilahun, A.; Gerenew, C.; Tri, V.D.; Kim, N.H.; Do Kim, G.; Woo, H.C.; Chun, B.S. Subcritical water extraction of fucoidan from Saccharina japonica: Optimization, characterization and biological studies. J. Appl. Phycol. 2018, 30, 579–590. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Ummat, V.; Tiwari, B.; Rajauria, G. Exploring Ultrasound, Microwave and Ultrasound–Microwave Assisted Extraction Technologies to Increase the Extraction of Bioactive Compounds and Antioxidants from Brown Macroalgae. Mar. Drugs 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. A green approach for alginate extraction from Sargassum muticum brown seaweed using ultrasound-assisted technique. Int. J. Biol. Macromol. 2019, 124, 451–459. [Google Scholar] [CrossRef]
- Youssouf, L.; Lallemand, L.; Giraud, P.; Soulé, F.; Bhaw-Luximon, A.; Meilhac, O.; D’Hellencourt, C.L.; Jhurry, D.; Couprie, J. Ultrasound-assisted extraction and structural characterization by NMR of alginates and carrageenans from seaweeds. Carbohydr. Polym. 2017, 166, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Huang, C.Y.; Chen, C.Y.; Chang, C.C.; Huang, C.Y.; Di Dong, C.; Chang, J.S. Structure and Biological Activity Analysis of Fucoidan Isolated from Sargassum siliquosum. ACS Omega 2020, 5, 32447–32455. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Jayawardena, T.U.; Sanjeewa, K.K.A.; Wang, L.; Jeon, Y.J.; Lee, W.W. Anti-inflammatory potential of alginic acid from Sargassum horneri against urban aerosol-induced inflammatory responses in keratinocytes and macrophages. Ecotoxicol. Environ. Saf. 2018, 160, 24–31. [Google Scholar] [CrossRef]
- Devi , G.V.Y.; Nagendra, A.H.; Shenoy, P.S.; Chatterjee, K.; Venkatesan, J. Isolation and purification of fucoidan from Sargassum ilicifolium: Osteogenic differentiation potential in mesenchymal stem cells for bone tissue engineering. J. Taiwan Inst. Chem. Eng. 2022, 136, 104418. [Google Scholar] [CrossRef]
- Javee, A.; Malairaj, S.; Subramani, N. Biosynthesis, protease optimization and purification of alkaline serine from Shewanella algae and its potential application as silver recovery. Sustain. Chem. Pharm. 2022, 25, 100595. [Google Scholar] [CrossRef]
- Corrêa, P.S.; Morais Júnior, W.G.; Martins, A.A.; Caetano, N.S.; Mata, T.M. Microalgae Biomolecules: Extraction, Separation and Purification Methods. Processes 2020, 9, 10. [Google Scholar] [CrossRef]
- Pansare, S.K.; Patel, S.M. Lyophilization Process Design and Development: A Single-Step Drying Approach. J. Pharm. Sci. 2019, 108, 1423–1433. [Google Scholar] [CrossRef]
- Khan, A.; Ali, J.; Jamil, S.U.U.; Zahra, N.; Tayaba, T.B.; Iqbal, M.J.; Waseem, H. Removal of micropollutants. In Environmental Micropollutants. Advances in Pollution Research; Elsevier: Amsterdam, The Netherlands, 2022; pp. 443–461. [Google Scholar] [CrossRef]
- Andrew, S.M.; Titus, J.A.; Zumstein, L. Dialysis and concentration of protein solutions. Curr. Protoc. Toxicol. 2002, 10, A-3H. [Google Scholar] [CrossRef]
- Echave, J.; Fraga-Corral, M.; Garcia-Perez, P.; Popović-Djordjević, J.; Avdović, E.H.; Radulović, M.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Seaweed Protein Hydrolysates and Bioactive Peptides: Extraction, Purification, and Applications. Mar. Drugs 2021, 19, 500. [Google Scholar] [CrossRef]
- Mohd Fauziee, N.A.; Chang, L.S.; Wan Mustapha, W.A.; Md Nor, A.R.; Lim, S.J. Functional polysaccharides of fucoidan, laminaran and alginate from Malaysian brown seaweeds (Sargassum polycystum, Turbinaria ornata and Padina boryana). Int. J. Biol. Macromol. 2021, 167, 1135–1145. [Google Scholar] [CrossRef]
- Geun Lee, H.; Jayawardena, T.U.; Liyanage, N.M.; Song, K.M.; Choi, Y.S.; Jeon, Y.J.; Kang, M.C. Antioxidant potential of low molecular weight fucoidans from Sargassum autumnale against H2O2-induced oxidative stress in vitro and in zebrafish models based on molecular weight changes. Food Chem. 2022, 384, 132591. [Google Scholar] [CrossRef]
- Permatasari, A.A.A.P.; Rosiana, I.W.; Wiradana, P.A.; Lestari, M.D.; Widiastuti, N.K.; Kurniawan, S.B.; Widhiantara, I.G. Extraction and characterization of sodium alginate from three brown algae collected from Sanur Coastal Waters, Bali as biopolymer agent. Biodiversitas J. Biol. Divers. 2022, 23, 1655–1663. [Google Scholar] [CrossRef]
- Bibi, A.; Rehman, S.; Yaseen, A.; Zhang, J.; Wehrle, E.; Latifah, R.N.; Rahmania, S.; Rohmah, B.L. The effect of extraction time on the quality of brown seaweed Na-Alginate Sargassum polycisteum as the base material for SBK Edible Film. J. Phys. Conf. Ser. 2022, 2190, 012001. [Google Scholar] [CrossRef]
- Vanavil, B.; Selvaraj, K.; Aanandhalakshmi, R.; Usha, S.K.; Arumugam, M. Bioactive and thermostable sulphated polysaccharide from Sargassum swartzii with drug delivery applications. Int. J. Biol. Macromol. 2020, 153, 190–200. [Google Scholar] [CrossRef]
- Mohammed, A.; Rivers, A.; Stuckey, D.C.; Ward, K. Alginate extraction from Sargassum seaweed in the Caribbean region: Optimization using response surface methodology. Carbohydr. Polym. 2020, 245, 116419. [Google Scholar] [CrossRef]
- Chen, S.; Sathuvan, M.; Zhang, X.; Zhang, W.; Tang, S.; Liu, Y.; Cheong, K.L. Characterization of polysaccharides from different species of brown seaweed using saccharide mapping and chromatographic analysis. BMC Chem. 2021, 15, 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Zhang, Y.; Yang, Y.; Wang, P.; Imre, B.; Wong, A.C.Y.; Hsieh, Y.S.Y.; Wang, D. Brown Algae Carbohydrates: Structures, Pharmaceutical Properties, and Research Challenges. Mar. Drugs 2021, 19, 620. [Google Scholar] [CrossRef]
- Ramos-de-la-Peña, A.M.; Contreras-Esquivel, J.C.; Aguilar, O.; González-Valdez, J. Structural and bioactive roles of fucoidan in nanogel delivery systems. A review. Carbohydr. Polym. Technol. Appl. 2022, 4, 100235. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Lee, J.S.; Kim, W.S.; Jeon, Y.J. The potential of brown-algae polysaccharides for the development of anticancer agents: An update on anticancer effects reported for fucoidan and laminaran. Carbohydr. Polym. 2017, 177, 451–459. [Google Scholar] [CrossRef]
- Mišurcová, L.; Škrovánková, S.; Samek, D.; Ambrožová, J.; Machů, L. Health Benefits of Algal Polysaccharides in Human Nutrition. Adv. Food Nutr. Res. 2012, 66, 75–145. [Google Scholar] [CrossRef]
- Cancer. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 29 October 2021).
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Martinelli, C.; Pucci, C.; Ciofani, G. Nanostructured carriers as innovative tools for cancer diagnosis and therapy. APL Bioeng. 2019, 3, 011502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, L.; Li, C.M.; Li, Y.F.; Huang, T.M.; Chao, N.X.; Luo, G.R.; Mo, F.R. Laminarin from Seaweed (Laminaria japonica) Inhibits Hepatocellular Carcinoma Through Upregulating Senescence Marker Protein-30. Cancer Biother. Radiopharm. 2020, 35, 277. [Google Scholar] [CrossRef] [Green Version]
- Etman, S.M.; Elnaggar, Y.S.R.; Abdallah, O.Y. Fucoidan, a natural biopolymer in cancer combating: From edible algae to nanocarrier tailoring. Int. J. Biol. Macromol. 2020, 147, 799–808. [Google Scholar] [CrossRef]
- PArumugam, P.; Arunkumar, K.; Sivakumar, L.; Murugan, M.; Murugan, K. Anticancer effect of fucoidan on cell proliferation, cell cycle progression, genetic damage and apoptotic cell death in HepG2 cancer cells. Toxicol. Rep. 2019, 6, 556. [Google Scholar] [CrossRef]
- Al Monla, R.; Dassouki, Z.; Sari-Chmayssem, N.; Mawlawi, H.; Gali-Muhtasib, H. Fucoidan and Alginate from the Brown Algae Colpomenia sinuosa and Their Combination with Vitamin C Trigger Apoptosis in Colon Cancer. Molecules 2022, 27, 358. [Google Scholar] [CrossRef]
- Reig-Vano, B.; Tylkowski, B.; Montané, X.; Giamberini, M. Alginate-based hydrogels for cancer therapy and research. Int. J. Biol. Macromol. 2020, 170, 424–436. [Google Scholar] [CrossRef]
- Shaikh, M.A.J.; Alharbi, K.S.; Almalki, W.H.; Imam, S.S.; Albratty, M.; Meraya, A.M.; Alzarea, S.I.; Kazmi, I.; Al-Abbasi, F.A.; Afzal, O.; et al. Sodium alginate based drug delivery in management of breast cancer. Carbohydr. Polym. 2022, 292, 119689. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Qi, C.; Yu, W.; Han, X.; Jiarui, Z.; Qing, Z.; Aiguo, J.; Shuliang, S. Advances in Research on the Bioactivity of Alginate Oligosaccharides. Mar. Drugs 2020, 18, 144. [Google Scholar] [CrossRef] [Green Version]
- Arunkumar, K.; Raja, R.; Kumar, V.B.S.; Joseph, A.; Shilpa, T.; Carvalho, I.S. Antioxidant and cytotoxic activities of sulfated polysaccharides from five different edible seaweeds. J. Food Meas. Charact. 2021, 15, 567–576. [Google Scholar] [CrossRef]
- Múzquiz De La Garza, A.R.; Tapia-Salazar, M.; Maldonado-Muñiz, M.; De La Rosa-Millán, J.; Gutiérrez-Uribe, J.A.; Santos-Zea, L.; Barba-Dávila, B.A.; Ricque-Marie, D.; Cruz-Suárez, L.E. Nutraceutical Potential of Five Mexican Brown Seaweeds. Biomed. Res. Int. 2019, 2019, 3795160. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, S.Y.; Kim, H.S.; Ahn, G.; Kim, J.; Jeon, Y.J. Fucoidan isolated from invasive Sargassum horneri inhibit LPS-induced inflammation via blocking NF-κB and MAPK pathways. Algal Res. 2019, 41, 101561. [Google Scholar] [CrossRef]
- Manikandan, R.; Parimalanandhini, D.; Mahalakshmi, K.; Beulaja, M.; Arumugam, M.; Janarthanan, S.; Palanisamy, S.; You, S.G.; Prabhu, N.M. Studies on isolation, characterization of fucoidan from brown algae Turbinaria decurrens and evaluation of it’s in vivo and in vitro anti-inflammatory activities. Int. J. Biol. Macromol. 2020, 160, 1263–1276. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Jayawardena, T.U.; Jeon, Y.J.; Ryu, B.M. Anti-inflammatory and anti-melanogenesis activities of sulfated polysaccharides isolated from Hizikia fusiforme: Short communication. Int. J. Biol. Macromol. 2020, 142, 545–550. [Google Scholar] [CrossRef]
- Cao, Y.G.; Hao, Y.; Li, Z.H.; Liu, S.T.; Wang, L. xin Antiviral activity of polysaccharide extract from Laminaria japonica against respiratory syncytial virus. Biomed. Pharmacother. 2016, 84, 1705–1710. [Google Scholar] [CrossRef]
- Palanisamy, S.; Vinosha, M.; Rajasekar, P.; Anjali, R.; Sathiyaraj, G.; Marudhupandi, T.; Selvam, S.; Prabhu, N.M.; You, S.G. Antibacterial efficacy of a fucoidan fraction (Fu-F2) extracted from Sargassum polycystum. Int. J. Biol. Macromol. 2019, 125, 485–495. [Google Scholar] [CrossRef]
- Oliveira, C.; Granja, S.; Neves, N.M.; Reis, R.L.; Baltazar, F.; Silva, T.H.; Martins, A. Fucoidan from Fucus vesiculosus inhibits new blood vessel formation and breast tumor growth in vivo. Carbohydr. Polym. 2019, 223, 115034. [Google Scholar] [CrossRef] [Green Version]
- Ohmes, J.; Xiao, Y.; Wang, F.; Mikkelsen, M.D.; Nguyen, T.T.; Schmidt, H.; Seekamp, A.; Meyer, A.S.; Fuchs, S. Effect of Enzymatically Extracted Fucoidans on Angiogenesis and Osteogenesis in Primary Cell Culture Systems Mimicking Bone Tissue Environment. Mar. Drugs 2020, 18, 481. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Huang, C.Y.; Yang, C.C.; Lee, T.M.; Chang, J.S. Hair growth-promoting effects of Sargassum glaucescens oligosaccharides extracts. J. Taiwan Inst. Chem. Eng. 2022, 134, 104307. [Google Scholar] [CrossRef]
- Amiri Goushki, M.; Sabahi, H.; Kabiri, M. In Vitro Evaluation of the Wound Healing Properties and Safety Assessment of Fucoidan Extracted from Sargassum angustifolium. Curr. Appl. Sci. Technol. 2023, 23, 10-55003. [Google Scholar] [CrossRef]
- Güven, K.C.; Coban, B.; Özdemir, O. Pharmacology of Marine Macroalgae. Encycl. Mar. Biotechnol. 2020, 1, 585–615. [Google Scholar] [CrossRef]
- Remya, R.R.; Samrot, A.V.; Suresh Kumar, S.; Mohanavel, V.; Karthick, A.; Kumar Chinnaiyan, V.; Umapathy, D.; Muhibbullah, M. Bioactive Potential of Brown Algae. Adsorpt. Sci. Technol. 2022, 2022, 9104835. [Google Scholar] [CrossRef]
- Rajauria, G.; Ravindran, R.; Garcia-Vaquero, M.; Rai, D.K.; Sweeney, T.; O’Doherty, J. Molecular characteristics and antioxidant activity of laminarin extracted from the seaweed species Laminaria hyperborea, using hydrothermal-assisted extraction and a multi-step purification procedure. Food Hydrocoll. 2021, 112, 106332. [Google Scholar] [CrossRef]
- Manggau, M.; Kasim, S.; Fitri, N.; Aulia, N.S.; Agustiani, A.N.; Raihan, M.; Nurdin, W.B. Antioxidant, anti-inflammatory and anticoagulant activities of sulfate polysaccharide isolate from brown alga Sargassum policystum. IOP Conf. Ser. Earth Environ. Sci. 2022, 967, 012029. [Google Scholar] [CrossRef]
- Nogueira, M.T.; Chica, L.R.; Yamashita, C.; Nunes, N.S.S.; Moraes, I.C.F.; Branco, C.C.Z.; Branco, I.G. Optimal conditions for alkaline treatment of alginate extraction from the brown seaweed Sargassum cymosum C. Agardh by response surface methodology. Appl. Food Res. 2022, 2, 100141. [Google Scholar] [CrossRef]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef]
- Park, J.C.; Han, S.H.; Mook-Jung, I. Peripheral inflammatory biomarkers in Alzheimer’s disease: A brief review. BMB Rep. 2020, 53, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Silva, N.J.; Nagashima, M.; Li, J.; Kakuk-Atkins, L.; Ashrafzadeh, M.; Hyde, D.R.; Hitchcock, P.F. Inflammation and matrix metalloproteinase 9 (Mmp-9) regulate photoreceptor regeneration in adult zebrafish. Glia 2020, 68, 1445–1465. [Google Scholar] [CrossRef] [Green Version]
- Rawat, C.; Kukal, S.; Dahiya, U.R.; Kukreti, R. Cyclooxygenase-2 (COX-2) inhibitors: Future therapeutic strategies for epilepsy management. J. Neuroinflamm. 2019, 16, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Lomartire, S.; Gonçalves, A.M.M. Antiviral Activity and Mechanisms of Seaweeds Bioactive Compounds on Enveloped Viruses—A Review. Mar. Drugs 2022, 20, 385. [Google Scholar] [CrossRef]
- Kaparapu, J.; Krishna Prasad, M.; Mohan Narasimha Rao, G. Antiviral potentials of marine algal bioactive compounds for coronavirus drug discovery. Coronavirus Drug Discov. 2022, 2, 225–245. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Ferrandis-Montesinos, M.; Wang, R. Antiviral Properties of Alginate-Based Biomaterials: Promising AntiviralAgents against SARS-CoV-2. ACS Appl. Bio Mater. 2021, 4, 5897–5907. [Google Scholar] [CrossRef]
- Liu, J.; Obaidi, I.; Nagar, S.; Scalabrino, G.; Sheridan, H. The antiviral potential of algal-derived macromolecules. Curr. Res. Biotechnol. 2021, 3, 120–134. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453–454, 1–9. [Google Scholar] [CrossRef]
- Bai, R.G.; Tuvikene, R. Potential Antiviral Properties of Industrially Important Marine Algal Polysaccharides and Their Significance in Fighting a Future Viral Pandemic. Viruses 2021, 13, 1817. [Google Scholar] [CrossRef]
- Sun, Q.L.; Li, Y.; Ni, L.Q.; Li, Y.X.; Cui, Y.S.; Jiang, S.L.; Xie, E.Y.; Du, J.; Deng, F.; Dong, C.X. Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydr. Polym. 2020, 229, 115487. [Google Scholar] [CrossRef]
- Pradhan, B.; Nayak, R.; Patra, S.; Bhuyan, P.P.; Behera, P.K.; Mandal, A.K.; Behera, C.; Ki, J.S.; Adhikary, S.P.; MubarakAli, D.; et al. A state-of-the-art review on fucoidan as an antiviral agent to combat viral infections. Carbohydr. Polym. 2022, 291, 119551. [Google Scholar] [CrossRef]
- Song, S.; Peng, H.; Wang, Q.; Liu, Z.; Dong, X.; Wen, C.; Ai, C.; Zhang, Y.; Wang, Z.; Zhu, B. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020, 11, 7415–7420. [Google Scholar] [CrossRef] [PubMed]
- Kwon, P.S.; Oh, H.; Kwon, S.J.; Jin, W.; Zhang, F.; Fraser, K.; Hong, J.J.; Linhardt, R.J.; Dordick, J.S. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discov. 2020, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, W.; Qiu, H.M.; Cheong, K.L.; Zhong, S. Advances in anti-cancer effects and underlying mechanisms of marine algae polysaccharides. Int. J. Biol. Macromol. 2022, 221, 472–485. [Google Scholar] [CrossRef]
- Li, Z.; Wu, N.; Wang, J.; Yue, Y.; Geng, L.; Zhang, Q. Low molecular weight fucoidan alleviates cerebrovascular damage by promoting angiogenesis in type 2 diabetes mice. Int. J. Biol. Macromol. 2022, 217, 345–355. [Google Scholar] [CrossRef]
- Corino, C.; Di Giancamillo, A.; Modina, S.C.; Rossi, R. Prebiotic Effects of Seaweed Polysaccharides in Pigs. Animals 2021, 11, 1573. [Google Scholar] [CrossRef]
- Zheng, L.X.; Chen, X.Q.; Cheong, K.L. Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. Int. J. Biol. Macromol. 2020, 151, 344–354. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Liu, R.; Pang, Y.; Xiao, T.; Zhang, S.; Liu, Y.; Min, Y. Multifunctional PCL composite nanofibers reinforced with lignin and ZIF-8 for the treatment of bone defects. Int. J. Biol. Macromol. 2022, 218, 1–8. [Google Scholar] [CrossRef]
- Melchor-Martínez, E.M.; Torres Castillo, N.E.; Macias-Garbett, R.; Lucero-Saucedo, S.L.; Parra-Saldívar, R.; Sosa-Hernández, J.E. Modern World Applications for Nano-Bio Materials: Tissue Engineering and COVID-19. Front. Bioeng. Biotechnol. 2021, 9, 597958. [Google Scholar] [CrossRef]
- Iglesias-Mejuto, A.; García-González, C.A. 3D-printed alginate-hydroxyapatite aerogel scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2021, 131, 112525. [Google Scholar] [CrossRef]
- Kuznetsova, T.A.; Andryukov, B.G.; Besednova, N.N.; Zaporozhets, T.S.; Kalinin, A.V. Marine Algae Polysaccharides as Basis for Wound Dressings, Drug Delivery, and Tissue Engineering: A Review. J. Mar. Sci. Eng. 2020, 8, 481. [Google Scholar] [CrossRef]
- Commercial Seaweed Market Size, Share, Trends Report, 2030. Available online: https://www.grandviewresearch.com/industry-analysis/commercial-seaweed-market (accessed on 9 December 2022).
- Global Alginate Market Size|Industry Report, 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/alginate-market (accessed on 19 May 2023).
- Carrageenan Market Size & Share Analysis Report, 2030. Available online: https://www.grandviewresearch.com/industry-analysis/carrageenan-market (accessed on 19 May 2023).
- Beta-Glucan and Fucoidan Market Size, Report to 2030. Available online: https://straitsresearch.com/report/beta-glucan-and-fucoidan-market (accessed on 19 May 2023).
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. Metabolites from Macroalgae and Its Applications in the Cosmetic Industry: A Circular Economy Approach. Resources 2020, 9, 101. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Park, A.Y.; Karpiniec, S.S. Therapies from Fucoidan: New Developments. Mar. Drugs 2019, 17, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haggag, Y.A.; Abd Elrahman, A.A.; Ulber, R.; Zayed, A. Fucoidan in Pharmaceutical Formulations: A Comprehensive Review for Smart Drug Delivery Systems. Mar. Drugs 2023, 21, 112. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.L.; Lee, B.J.; Tran, T.T.D. Current developments in the oral drug delivery of fucoidan. Int. J. Pharm. 2021, 598, 120371. [Google Scholar] [CrossRef]
- Saldarriaga-Hernandez, S.; Hernandez-Vargas, G.; Iqbal, H.M.N.; Barceló, D.; Parra-Saldívar, R. Bioremediation potential of Sargassum sp. biomass to tackle pollution in coastal ecosystems: Circular economy approach. Sci. Total Environ. 2020, 715, 136978. [Google Scholar] [CrossRef] [PubMed]
- Lineamientos Técnicos y de Gestión para la Atención de la Contingencia Ocasionada por Sargazo en el Caribe Mexicano y el Golfo de México. Available online: https://www.gob.mx/semarnat/documentos/lineamientos-tecnicos-y-de-gestion-para-la-atencion-de-la-contingencia-ocasionada-por-sargazo-en-el-caribe-mexicano-y-el-golfo-de-mexico (accessed on 19 May 2023).
- Rosellón-Druker, J.; Calixto-Pérez, E.; Escobar-Briones, E.; González-Cano, J.; Masiá-Nebot, L.; Córdova-Tapia, F. A Review of a Decade of Local Projects, Studies and Initiatives of Atypical Influxes of Pelagic Sargassum on Mexican Caribbean Coasts. Phycology 2022, 2, 254–279. [Google Scholar] [CrossRef]
- Productos|Dianco. Available online: http://diancomexico.com/dianco/productos (accessed on 19 May 2023).


| Algae | Polysaccharide | Pre-Treatment | Extraction Method | Extraction Details | Recovery | References |
|---|---|---|---|---|---|---|
| Sargassum siliquosum | Fucoidan | Washed with (H2O); dried (60 °C) for 48 h; powdered with single-shaft extruder under conditions of 115 °C, 10 kg/h. 360 rpm; sieved with 20 mesh sieves. | MAE & UAE | 1 g powder Solvent: EtOH 10 mL/g 25 °C for 4 h. MAE: 750 W; 10 min; 15 mL/g UAE: 100 W; 10 min; 15 mL/g | MAE 6.94% UAE4.78% | [29] |
| Fucus vesiculosus Fucus serratus Fucus evanescens | Laminarin & Fucoidan | Grind-dried seaweed, washed with EtOH and acetone. | MAE | 1.5 g Solvent: 25 mL sulfuric acid [10 mM] 120 °C for 30 min. Precipitation of laminarin EtOH (40% v/v); precipitation of fucoidan EtOH (70% v/v) | Laminarin 8.68% Fucoidan 5.56% | [30] |
| Ascophyllum nodosum | Fucoidan | 80% EtOH; 20 h; room temp. 80% EtOH; 5 h; 70 °C. | UAE | 0.01 M HCl, 35 min, 40% amplitude; 20 kHz 2% CaCl2, overnight; 4 °C. | 4.56% | [22] |
| Nizamuddinia zanardinii | Fucoidan | Washed, dried (40 °C), milled, and stored in the freezer. (50 g) suspended in 500 mL of 85% EtOH, stirred (24 h, 25 °C), rinsed with acetone, and dried under laminar hood (22 ± 2 °C). | EAE & UAE & EUAE (Alcalase) | EAE (2.5 mL/dry material weight, pH 7, solid-to-solvent ratios 1:30 g/mL) for 24 h at 50 °C. UAE sonicated with distilled water (1:76 g/mL) with (frequency 20 kHz, max power 400 W, Ø = 1.3 cm) at 196 and 70 °C for 59 min. EUAE (2.5 mL/dry material weight, pH 7, temperature 50 °C, solid-to-solvent ratios 1:30 g/mL) for 23 h. Sonication (196 W, 70 °C, 59 min) | EAE 5.58% UAE 3.6% EUAE 7.87% | [31] |
| Saccharina Japonica | Fucoidan | Washed, chopped, freeze-dried (−80 °C. 72 h), ground. Samples that passed through a 710 μm sieving mesh were used. | SCWE | 200 cm3 batch system 0.1% NaOH 80 bars S/L ratio 0.05 g mL−1, 127.01 °C, 300 rpm, 11.98 min | 13.56% | [32] |
| Nizamuddinia zanardinii | Fucoidan | Washed with water, dried at 40 °C for 72 h, sieved (<0.5 mm). | SCWE | 29 min, 150 °C. Raw material to water ratio 21 g (mL) | 25.98% | [31] |
| Ascophyllum nodosum | Fucoidan | Oven-dried (50 °C). Ground for 9 days (1 mm particle size). Maceration 10 min, 0.1 M HCl, room temperature. | UMAE | Sonication: 50 W, 20 kHz, 100% ultrasonic amplitude. Microwave 2450 MHz 1000 W, 5 min. | 3.53% | [33] |
| Nizamuddinia zanardinii | Fucoidan | Cleaned, rinsed on the spot with seawater. Washed with distilled water and oven-dried at 40 °C for 72 h. Powdered. |
|
|
| [31] |
| Sargassum muticum | Alginate | Washed with H2O. | UAE | v/m 20:1 (wt) Solvent: H2O; 25 °C, 30 min, 1.5 A, 50 W, 40 Hz. | 15% | [34] |
| Sargassum binderi | Alginates | Washed with H2O, dried, milled with a blender. Stored under vacuum. EtOH treatment for dried seaweed (overnight) 25 °C. Filtered with 10 µL Millipore nylon mesh. Washed with distilled water. | UAE | 10 g/L Solvent: H2O pH: 11 150 W 30 min 90 °C. 25 kHz | 27% | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Contreras, E.A.; Araújo, R.G.; Rodríguez-Aguayo, A.A.; Guzmán-Román, M.; García-Venegas, J.C.; Nájera-Martínez, E.F.; Sosa-Hernández, J.E.; Iqbal, H.M.N.; Melchor-Martínez, E.M.; Parra-Saldivar, R. Polysaccharides from the Sargassum and Brown Algae Genus: Extraction, Purification, and Their Potential Therapeutic Applications. Plants 2023, 12, 2445. https://doi.org/10.3390/plants12132445
Flores-Contreras EA, Araújo RG, Rodríguez-Aguayo AA, Guzmán-Román M, García-Venegas JC, Nájera-Martínez EF, Sosa-Hernández JE, Iqbal HMN, Melchor-Martínez EM, Parra-Saldivar R. Polysaccharides from the Sargassum and Brown Algae Genus: Extraction, Purification, and Their Potential Therapeutic Applications. Plants. 2023; 12(13):2445. https://doi.org/10.3390/plants12132445
Chicago/Turabian StyleFlores-Contreras, Elda A., Rafael G. Araújo, Arath A. Rodríguez-Aguayo, Muriel Guzmán-Román, Jesús Carlos García-Venegas, Erik Francisco Nájera-Martínez, Juan Eduardo Sosa-Hernández, Hafiz M. N. Iqbal, Elda M. Melchor-Martínez, and Roberto Parra-Saldivar. 2023. "Polysaccharides from the Sargassum and Brown Algae Genus: Extraction, Purification, and Their Potential Therapeutic Applications" Plants 12, no. 13: 2445. https://doi.org/10.3390/plants12132445











