Integrated Characterization of Cassava (Manihot esculenta) Pectin Methylesterase (MePME) Genes to Filter Candidate Gene Responses to Multiple Abiotic Stresses
Abstract
:1. Introduction
2. Results
2.1. The Manihot Esculenta Genome Contains 89 PME Genes
2.2. Chromosome Distribution and Collinear Analysis of MePMEs
2.3. Phylogenetic Analysis of MePMEs
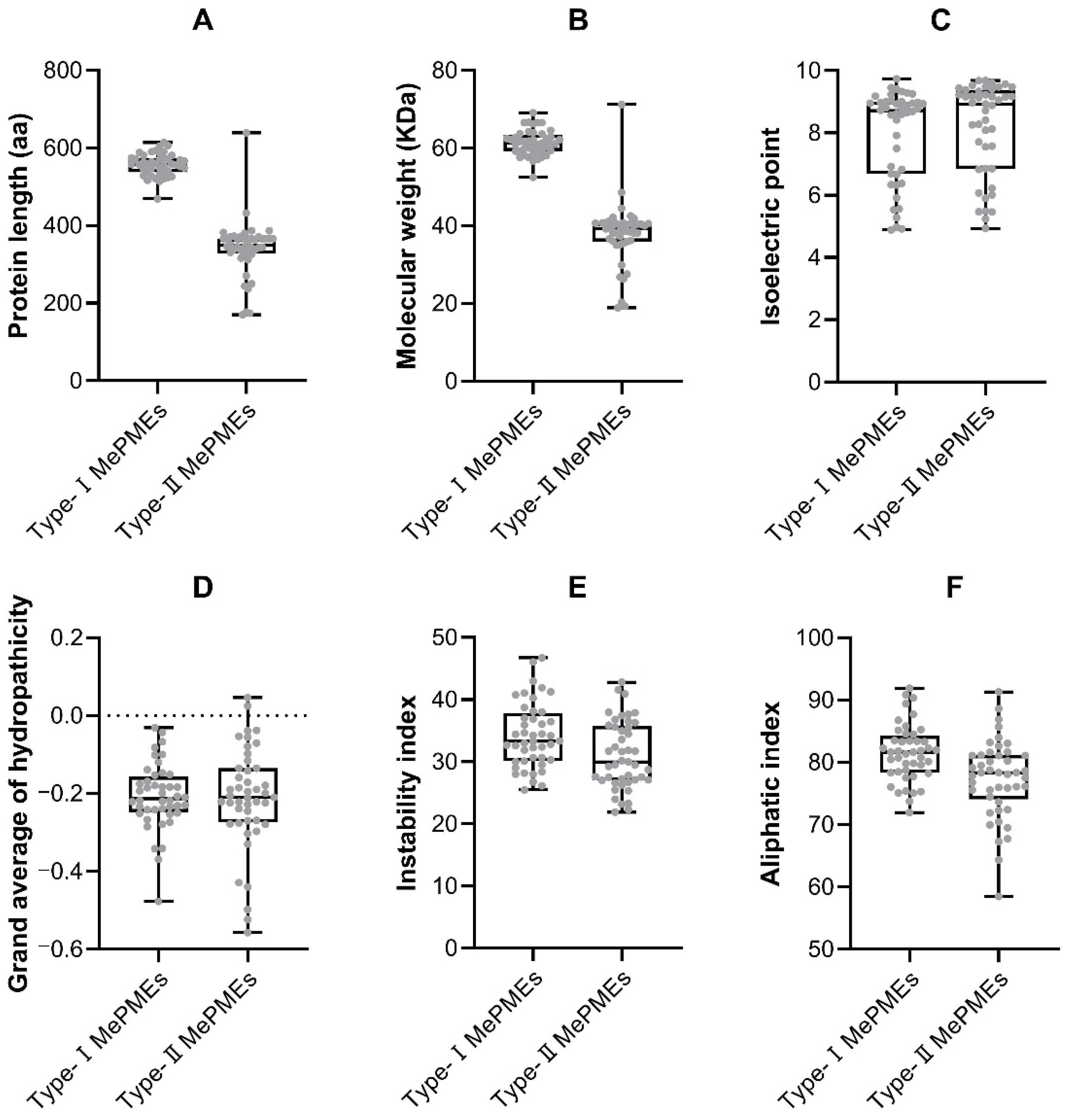
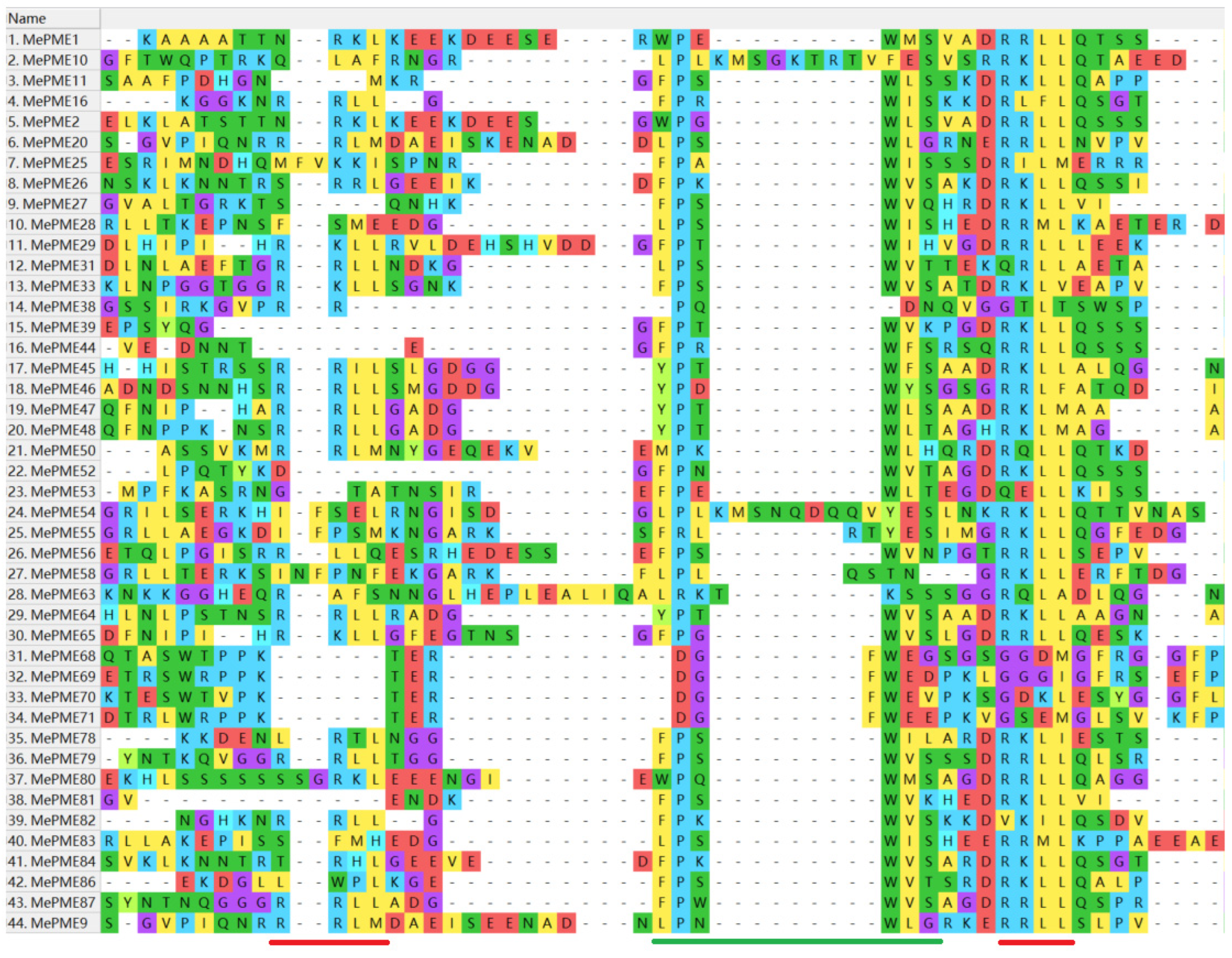
2.4. Gene Structure and Motif Analysis of MePME Genes in Cassava
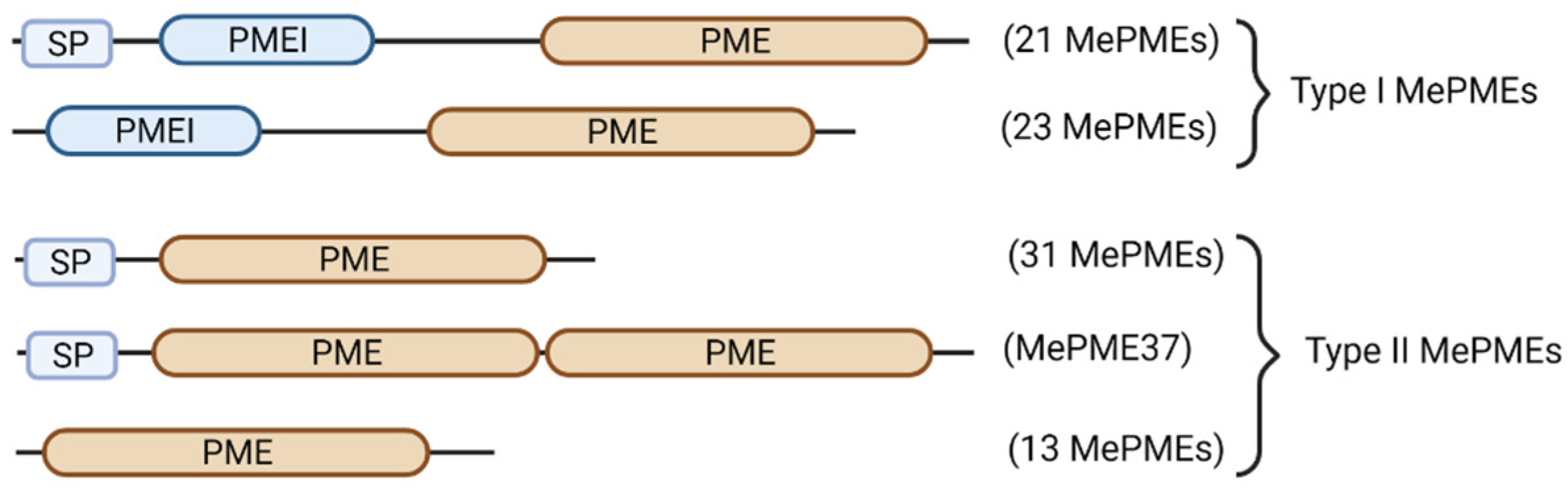
2.5. Domain Analysis of MePMEs
2.6. Cis-Acting Regulatory Elements Analysis of MePMEs
2.7. Expression Analysis of MePMEs in Cassava under Different Stresses
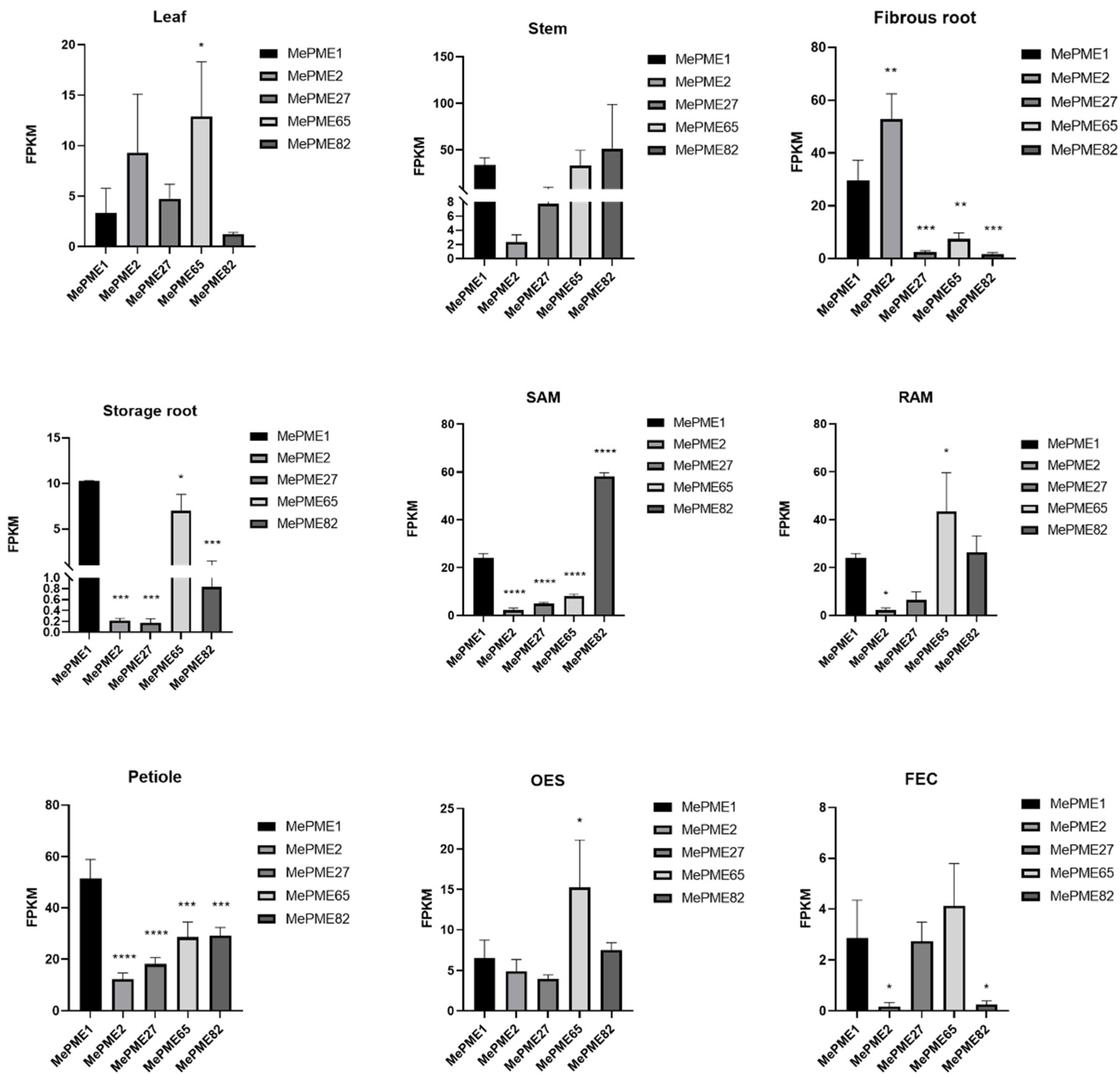
2.8. Expression of Shared MePMEs in Different Tissues of Cassava
3. Discussion
4. Materials and Methods
4.1. Identification of Pectin Methylesterase Gene Family Members in Cassava
4.2. Characterization of MePMEs
4.3. Chromosomal Location, Duplication, Gene Structure, Conserved Motifs and Cis-Acting Regulatory Element Analysis of MePMEs
4.4. Multiple Sequence Alignment and Phylogenetic Analysis of MePMEs with PMEs in Arabidopsis
4.5. Multifunctional MePME Gene Screening and Analysis of Their Expression Patterns
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bosch, M.; Cheung, A.Y.; Hepler, P.K. Pectin methylesterase, a regulator of pollen tube growth. Plant Physiol. 2005, 138, 1334–1346. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.C.; Ren, A.Y.; Tang, X.F.; Qi, G.; Xu, Z.C.; Chai, G.H.; Hu, R.B.; Zhou, G.K.; Kong, Y.Z. MYB52 Negatively Regulates Pectin Demethylesterification in Seed Coat Mucilage. Plant Physiol. 2018, 176, 2737–2749. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Li, R.; Wang, S.; Ding, Z.; Zhou, Q.; Liu, J.; Wang, Y.; Yao, Y.; Hu, X.; Guo, J. Overexpression of MePMEI1 in Arabidopsis enhances Pb tolerance. Front. Plant Sci. 2022, 13, 996981. [Google Scholar] [CrossRef]
- Bilska-Kos, A.; Solecka, D.; Dziewulska, A.; Ochodzki, P.; Jonczyk, M.; Bilski, H.; Sowinski, P. Low temperature caused modifications in the arrangement of cell wall pectins due to changes of osmotic potential of cells of maize leaves (Zea mays L.). Protoplasma 2017, 254, 713–724. [Google Scholar] [CrossRef] [Green Version]
- Draye, M.; Van Cutsern, P. Pectin methylesterases induce an abrupt increase of acidic pectin during strawberry fruit ripening. J. Plant Physiol. 2008, 165, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Blumer, J.M.; Clay, R.P.; Bergmann, C.W.; Albersheim, P.; Darvill, A. Characterization of changes in pectin methylesterase expression and pectin esterification during tomato fruit ripening. Can. J. Bot.-Rev. Can. Bot. 2000, 78, 607–618. [Google Scholar] [CrossRef]
- Yan, R.; Han, C.; Fu, M.R.; Jiao, W.X.; Wang, W.H. Inhibitory Effects of CaCl2 and Pectin Methylesterase on Fruit Softening of Raspberry during Cold Storage. Horticulturae 2022, 8, 1. [Google Scholar] [CrossRef]
- Wu, Q.; Tao, Y.; Zhang, X.L.; Dong, X.Y.; Xia, J.X.; Shen, R.F.; Zhu, X.F. Pectin Methylesterases Enhance Root Cell Wall Phosphorus Remobilization in Rice. Rice Sci. 2022, 29, 179–188. [Google Scholar] [CrossRef]
- Xu, Z.J.; Yang, M.; Li, Z.Y.; Xiao, J.; Yang, X.Q.; Wang, H.; Wang, X.F. Tissue-specific pectin methylesterification and pectin methylesterase activities play a role in lettuce seed germination. Sci. Hortic. 2022, 301, 111134. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zeng, Z.H.; Yan, J.Y.; Fan, W.; Bian, H.W.; Zhu, M.Y.; Yang, J.L.; Zheng, S.J. Association of specific pectin methylesterases with Al-induced root elongation inhibition in rice. Physiol. Plant. 2013, 148, 502–511. [Google Scholar] [CrossRef]
- Lionetti, V. PECTOPLATE: The simultaneous phenotyping of pectin methylesterases, pectinases, and oligogalacturonides in plants during biotic stresses. Front. Plant Sci. 2015, 6, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raiola, A.; Camardella, L.; Giovane, A.; Mattei, B.; De Lorenzo, G.; Cervone, F.; Bellincampi, D. Two Arabidopsis thaliana genes encode functional pectin methylesterase inhibitors. FEBS Lett. 2004, 557, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Camardella, L.; Carratore, V.; Ciardiello, M.A.; Servillo, L.; Balestrieri, C.; Giovane, A. Kiwi protein inhibitor of pectin methylesterase—Amino-acid sequence and structural importance of two disulfide bridges. Eur. J. Biochem. 2000, 267, 4561–4565. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.N.; Chen, H.L.; Zhang, Y.C.; Feng, S.Y.; Wang, S.L.; Shang, Q.; Wu, M.; Li, Z.Q.; Zhang, L.; Guo, H.; et al. Integrative genomics analysis of the ever-shrinking pectin methylesterase (PME) gene family in foxtail millet (Setaria italica). Funct. Plant Biol. 2022, 49, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.H.; Tian, Z.; Li, X.X.; Li, S.P.; Li, Z.Y.; Wang, J.L.; Hu, Z.Y.; Chen, H.Q.; Guo, C.; Xie, M.M.; et al. Systematic analysis of the pectin methylesterase gene family in Nicotiana tabacum and reveal their multiple roles in plant development and abiotic stresses. Front. Plant Sci. 2022, 13, 998841. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Nguyen, H.P.; Lee, C. Genome-wide identification and expression analysis of rice pectin methylesterases: Implication of functional roles of pectin modification in rice physiology. J. Plant Physiol. 2015, 183, 23–29. [Google Scholar] [CrossRef]
- Li, Y.Q.; He, H.Y.; He, L.F. Genome-Wide Analysis of the Pectin Methylesterase Gene Family in Potato. Potato Res. 2021, 64, 1–19. [Google Scholar] [CrossRef]
- Louvet, R.; Cavel, E.; Gutierrez, L.; Guenin, S.; Roger, D.; Gillet, F.; Guerineau, F.; Pelloux, J. Comprehensive expression profiling of the pectin methylesterase gene family during silique development in Arabidopsis thaliana. Planta 2006, 224, 782–791. [Google Scholar] [CrossRef]
- Pinzon-Latorre, D.; Deyholos, M.K. Characterization and transcript profiling of the pectin methylesterase (PME) and pectin methylesterase inhibitor (PMEI) gene families in flax (Linum usitatissimum). BMC Genom. 2013, 14, 742. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.X.; Wu, L.M.; Wang, C.; Wang, Y.; He, L.G.; Wang, Z.J.; Ma, X.F.; Bai, F.X.; Feng, G.Z.; Liu, J.H.; et al. Characterization of pectin methylesterase gene family and its possible role in juice sac granulation in navel orange (Citrus sinensis Osbeck). BMC Genom. 2022, 23, 1–18. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Nguyen, H.P.; Eom, S.H.; Lee, C. Integrative analysis of pectin methylesterase (PME) and PME inhibitors in tomato (Solanum lycopersicum): Identification, tissue-specific expression, and biochemical characterization. Plant Physiol. Biochem. 2018, 132, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.K.; Huang, Z.N.; Song, X.M.; Liu, T.K.; Liu, H.L.; Hou, X.L.; Li, Y. Comprehensive analysis of the polygalacturonase and pectin methylesterase genes in Brassica rapa shed light on their different evolutionary patterns. Sci. Rep. 2016, 6, 25107. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zeng, W.; Wang, X.; Pan, L.; Niu, L.; Lu, Z.; Cui, G.; Wang, Z. Characterization and Transcript Profiling of PME and PMEI Gene Families during Peach Fruit Maturation %J Journal of the American Society for Horticultural Science. J. Am. Soc. Hortic. Sci. 2017, 142, 246–259. [Google Scholar] [CrossRef] [Green Version]
- Li, W.J.; Shang, H.H.; Ge, Q.; Zou, C.S.; Cai, J.; Wang, D.J.; Fan, S.M.; Zhang, Z.; Deng, X.Y.; Tan, Y.N.; et al. Genome-wide identification, phylogeny, and expression analysis of pectin methylesterases reveal their major role in cotton fiber development. BMC Genom. 2016, 17, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, T.; Liu, R.; Wang, W.; An, L.; Chen, T.; Liu, G.; Zhao, Z. Brassinosteroids regulate pectin methylesterase activity and AtPME41 expression in Arabidopsis under chilling stress. Cryobiology 2011, 63, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Wu, H.C.; Wang, Y.D.; Liu, C.H.; Lin, C.C.; Luo, D.L.; Jinn, T.L. PECTIN METHYLESTERASE34 Contributes to Heat Tolerance through Its Role in Promoting Stomatal Movement. Plant Physiol. 2017, 174, 748–763. [Google Scholar] [CrossRef]
- Hocq, L.; Habrylo, O.; Voxeur, A.; Pau-Roblot, C.; Safran, J.; Sénéchal, F.; Fournet, F.; Bassard, S.; Battu, V.; Demailly, H.J.b. Arabidopsis AtPME2 has a pH-dependent processivity and control cell wall mechanical properties. BioRxiv 2021, 3, 433777. [Google Scholar] [CrossRef]
- Tsakali, A.; Asitzoglou, I.-C.; Basdeki, V.; Podia, V.; Adamakis, I.-D.S.; Giannoutsou, E.; Haralampidis, K. The Role of PME2 and PME3 in Arabidopsis Stomatal Development and Morphology &dagger. Biol. Life Sci. Forum 2022, 11, 36. [Google Scholar] [CrossRef]
- Hongo, S.; Sato, K.; Yokoyama, R.; Nishitani, K. Demethylesterification of the Primary Wall by PECTIN METHYLESTERASE35 Provides Mechanical Support to the Arabidopsis Stem. Plant Cell 2012, 24, 2624–2634. [Google Scholar] [CrossRef] [Green Version]
- Levesque-Tremblay, G.; Müller, K.; Mansfield, S.D.; Haughn, G.W. HIGHLY METHYL ESTERIFIED SEEDS Is a Pectin Methyl Esterase Involved in Embryo Development. Plant Physiol. 2015, 167, 725–737. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; He, H.; Fang, L.; Zhang, A. Pectin methylesterase31 positively regulates salt stress tolerance in Arabidopsis. Biochem. Biophys. Res. Commun. 2018, 496, 497–501. [Google Scholar] [CrossRef]
- Yang, J.L.; Li, Y.Y.; Zhang, Y.J.; Zhang, S.S.; Wu, Y.R.; Wu, P.; Zheng, S.J. Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol. 2008, 146, 602–611. [Google Scholar] [CrossRef]
- El-Moneim, D.A.; Contreras, R.; Silva-Navas, J.; Gallego, F.J.; Figueiras, A.M.; Benito, C. Pectin methylesterase gene and aluminum tolerance in Secale cereale. Environ. Exp. Bot. 2014, 107, 125–133. [Google Scholar] [CrossRef]
- Huang, D.J.; Mao, Y.X.; Guo, G.Y.; Ni, D.J.; Chen, L. Genome-wide identification of PME gene family and expression of candidate genes associated with aluminum tolerance in tea plant (Camellia sinensis). BMC Plant Biol. 2022, 22, 1–13. [Google Scholar] [CrossRef]
- Segonne, S.M.; Bruneau, M.; Celton, J.M.; Le Gall, S.; Francin-Allami, M.; Juchaux, M.; Laurens, F.; Orsel, M.; Renou, J.P. Multiscale investigation of mealiness in apple: An atypical role for a pectin methylesterase during fruit maturation. BMC Plant Biol. 2014, 14, 375. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wu, Z.; Zhu, Z.; Xu, L.; Wu, B.; Li, J. Botrytis cinerea mediated cell wall degradation accelerates spike stalk browning in Munage grape. J. Food Biochem. 2022, 46, e14271. [Google Scholar] [CrossRef] [PubMed]
- Romero, I.; Rosales, R.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Short-Term Gaseous Treatments Improve Rachis Browning in Red and White Table Grapes Stored at Low Temperature: Molecular Mechanisms Underlying Its Beneficial Effect. Int. J. Mol. Sci. 2022, 23, 13304. [Google Scholar] [CrossRef]
- Sonnewald, U.; Fernie, A.R.; Gruissem, W.; Schläpfer, P.; Anjanappa, R.B.; Chang, S.H.; Ludewig, F.; Rascher, U.; Muller, O.; van Doorn, A.M.J.T.P.J. The Cassava Source–Sink project: Opportunities and challenges for crop improvement by metabolic engineering. Plant J. 2020, 103, 1655–1665. [Google Scholar] [CrossRef]
- Obata, T.; Klemens, P.A.W.; Rosado-Souza, L.; Schlereth, A.; Gisel, A.; Stavolone, L.; Zierer, W.; Morales, N.; Mueller, L.A.; Zeeman, S.C.; et al. Metabolic profiles of six African cultivars of cassava (Manihot esculenta Crantz) highlight bottlenecks of root yield. Plant J. 2020, 102, 1202–1219. [Google Scholar] [CrossRef] [Green Version]
- Rosado-Souza, L.; David, L.C.; Drapal, M.; Fraser, P.D.; Hofmann, J.; Klemens, P.A.W.; Ludewig, F.; Neuhaus, H.E.; Obata, T.; Perez-Fons, L.; et al. Cassava Metabolomics and Starch Quality. Curr. Protoc. Plant Biol. 2019, 4, e20102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.M.; Yuan, S.; Zhou, Y.J.; Wang, S.J.; Zhou, Q.; Ding, Z.P.; Wang, Y.J.; Yao, Y.; Liu, J.; Guo, J.C. Comparative Transcriptome Profiling of Cassava Tuberous Roots in Response to Postharvest Physiological Deterioration. Int. J. Mol. Sci. 2023, 24, 246. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yuan, S.; He, Y.; Fan, J.; Zhou, Y.; Qiu, T.; Lin, X.; Yao, Y.; Liu, J.; Fu, S.; et al. Genome-Wide Identification and Expression Profiling Analysis of the Galactinol Synthase Gene Family in Cassava (Manihot esculenta Crantz). Agronomy 2018, 8, 250. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Li, R.; Zhou, Y.; Tang, F.; Wang, Y.; Lu, X.; Wang, S.; Yao, Y.; Liu, J.; Hu, X.; et al. Overexpression of Cassava MeAnn2 Enhances the Salt and IAA Tolerance of Transgenic Arabidopsis. Plants 2021, 10, 941. [Google Scholar] [CrossRef]
- Chen, X.; Lai, H.; Li, R.; Yao, Y.; Liu, J.; Yuan, S.; Fu, S.; Hu, X.; Guo, J. Character changes and Transcriptomic analysis of a cassava sexual Tetraploid. BMC Plant Biol. 2021, 21, 188. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, R.; Zhou, Y.; Wang, S.; Zhou, Q.; Ding, Z.; Yao, Y.; Liu, J.; Wang, Y.; Hu, X.; et al. Genome-Wide Identification of Cassava Glyoxalase I Genes and the Potential Function of MeGLYⅠ-13 in Iron Toxicity Tolerance. Int. J. Mol. Sci 2022, 23, 5212. [Google Scholar]
- Li, R.; Ji, Y.; Fan, J.; Yang, C.; Yao, Y.; Zhou, Y.; Duan, R.; Liu, J.; Fu, S.; Hu, X.; et al. Isolation and characterization of a C-repeat binding factor (CBF)-like gene in cassava (Manihot esculenta Crantz). Acta Physiol. Plant. 2014, 36, 3089–3093. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.E.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Yan, Y.; Zhao, S.; Ding, Z.; Tie, W.; Hu, W. Comparative Transcriptomic Analysis of Storage Roots in Cassava During Postharvest Physiological Deterioration. Plant Mol. Biol. Report. 2021, 39, 607–616. [Google Scholar] [CrossRef]
- Hu, W.; Kong, H.; Guo, Y.L.; Zhang, Y.L.; Ding, Z.H.; Tie, W.W.; Yan, Y.; Huang, Q.X.; Peng, M.; Shi, H.T.; et al. Comparative Physiological and Transcriptomic Analyses Reveal the Actions of Melatonin in the Delay of Postharvest Physiological Deterioration of Cassava. Front. Plant Sci. 2016, 7, 736. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.; Chen, Z.; Xia, J.; Zhang, K.; Chen, X.; Zhou, Y.; Bo, W.; Song, S.; Deng, D.; Guo, X.; et al. Chilling acclimation provides immunity to stress by altering regulatory networks and inducing genes with protective functions in Cassava. BMC Plant Biol. 2014, 14, 207. [Google Scholar] [CrossRef] [Green Version]
- Wolf, S.; Rausch, T.; Greiner, S. The N-terminal pro region mediates retention of unprocessed type-I PME in the Golgi apparatus. Plant J. 2009, 58, 361–375. [Google Scholar] [CrossRef]
- Peaucelle, A.; Louvet, R.; Johansen, J.N.; Salsac, F.; Morin, H.; Fournet, F.; Belcram, K.; Gillet, F.; Hofte, H.; Laufs, P.; et al. The transcription factor BELLRINGER modulates phyllotaxis by regulating the expression of a pectin methylesterase in Arabidopsis. Development 2011, 138, 4733–4741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Wang, Y.P.; Wang, X.Y.; Pei, S.Q.; Kong, Y.Z.; Hu, R.B.; Zhou, G.K. Transcription Factors BLH2 and BLH4 Regulate Demethylesterification of Homogalacturonan in Seed Mucilage. Plant Physiol. 2020, 183, 96–111. [Google Scholar] [CrossRef] [Green Version]
- Phan, H.A.; Iacuone, S.; Li, S.F.; Parish, R.W. The MYB80 Transcription Factor Is Required for Pollen Development and the Regulation of Tapetal Programmed Cell Death in Arabidopsis thaliana. Plant Cell 2011, 23, 2209–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, J.F.; Mo, X.L.; Wen, C.J.; Gao, Z.; Chen, X.; Xue, C. FvMYB79 Positively Regulates Strawberry Fruit Softening via Transcriptional Activation of FvPME38. Int. J. Mol. Sci. 2022, 23, 101. [Google Scholar] [CrossRef] [PubMed]
- Municio-Diaz, C.; Muller, E.; Drevensek, S.; Fruleux, A.; Lorenzetti, E.; Boudaoud, A.; Minc, N. Mechanobiology of the cell wall—Insights from tip-growing plant and fungal cells. J. Cell Sci. 2023, 135, 259208. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Isayenkov, S.V. The regulation of plant cell wall organisation under salt stress. Front. Plant Sci. 2023, 14, 1118313. [Google Scholar] [CrossRef]
- Shin, Y.; Chane, A.; Jung, M.; Lee, Y. Recent Advances in Understanding the Roles of Pectin as an Active Participant in Plant Signaling Networks. Plants 2021, 10, 1712. [Google Scholar] [CrossRef]
- Ganie, S.A.; Ahammed, G.J. Dynamics of cell wall structure and related genomic resources for drought tolerance in rice. Plant Cell Rep. 2021, 40, 437–459. [Google Scholar] [CrossRef]
- Sanchez, N.; Gutiérrez-López, G.F.; Cáez-Ramírez, G. Correlation among PME activity, viscoelastic, and structural parameters for Carica papaya edible tissue along ripening. J. Food Sci. 2020, 85, 1805–1814. [Google Scholar] [CrossRef]
- Zeng, C.Y.; Ding, Z.H.; Zhou, F.; Zhou, Y.F.; Yang, R.J.; Yang, Z.; Wang, W.Q.; Peng, M. The Discrepant and Similar Responses of Genome-Wide Transcriptional Profiles between Drought and Cold Stresses in Cassava. Int. J. Mol. Sci. 2017, 18, 2668. [Google Scholar] [CrossRef] [Green Version]
- Weber, M.; Deinlein, U.; Fischer, S.; Rogowski, M.; Geimer, S.; Tenhaken, R.; Clemens, S. A mutation in the Arabidopsis thaliana cell wall biosynthesis gene pectin methylesterase 3 as well as its aberrant expression cause hypersensitivity specifically to Zn. Plant J. 2013, 76, 151–164. [Google Scholar] [CrossRef]
- Raiola, A.; Lionetti, V.; Elmaghraby, I.; Immerzeel, P.; Mellerowicz, E.J.; Salvi, G.; Cervone, F.; Bellincampi, D. Pectin Methylesterase Is Induced in Arabidopsis upon Infection and Is Necessary for a Successful Colonization by Necrotrophic Pathogens. Mol. Plant-Microbe Interact. 2011, 24, 432–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixit, S.; Upadhyay, S.K.; Singh, H.; Sidhu, O.P.; Verma, P.C.; Chandrashekar, K. Enhanced Methanol Production in Plants Provides Broad Spectrum Insect Resistance. PLoS ONE 2013, 8, e79664. [Google Scholar] [CrossRef] [PubMed]
- Bredeson, J.V.; Lyons, J.B.; Prochnik, S.E.; Wu, G.A.; Ha, C.M.; Edsinger-Gonzales, E.; Grimwood, J.; Schmutz, J.; Rabbi, I.Y.; Egesi, C.; et al. Sequencing wild and cultivated cassava and related species reveals extensive interspecific hybridization and genetic diversity. Nat. Biotechnol. 2016, 34, 562–570. [Google Scholar] [CrossRef] [Green Version]
- Mohan, R.; Venugopal, S. Computational structural and functional analysis of hypothetical proteins of Staphylococcus aureus. Bioinformation 2012, 8, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [Green Version]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Lamesch, P.; Berardini, T.Z.; Li, D.; Swarbreck, D.; Wilks, C.; Sasidharan, R.; Muller, R.; Dreher, K.; Alexander, D.L.; Garcia-Hernandez, M.; et al. The Arabidopsis Information Resource (TAIR): Improved gene annotation and new tools. Nucleic Acids Res. 2011, 40, D1202–D1210. [Google Scholar] [CrossRef]
- Zhao, P.; Guo, X.; Wang, B.; Zhang, X.; Sun, J.; Ruan, M.; Peng, M. Overexpression of MeH1.2 gene inhibited plant growth and increased branch root differentiation in transgenic cassava. Crop. Sci. 2021, 61, 2639–2650. [Google Scholar] [CrossRef]
- Wilson, M.C.; Mutka, A.M.; Hummel, A.W.; Berry, J.; Chauhan, R.D.; Vijayaraghavan, A.; Taylor, N.J.; Voytas, D.F.; Chitwood, D.H.; Bart, R.S. Gene expression atlas for the food security crop cassava. New Phytol. 2017, 213, 1632–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Li, R.; Zhou, Y.; Fernie, A.R.; Ding, Z.; Zhou, Q.; Che, Y.; Yao, Y.; Liu, J.; Wang, Y.; et al. Integrated Characterization of Cassava (Manihot esculenta) Pectin Methylesterase (MePME) Genes to Filter Candidate Gene Responses to Multiple Abiotic Stresses. Plants 2023, 12, 2529. https://doi.org/10.3390/plants12132529
Wang S, Li R, Zhou Y, Fernie AR, Ding Z, Zhou Q, Che Y, Yao Y, Liu J, Wang Y, et al. Integrated Characterization of Cassava (Manihot esculenta) Pectin Methylesterase (MePME) Genes to Filter Candidate Gene Responses to Multiple Abiotic Stresses. Plants. 2023; 12(13):2529. https://doi.org/10.3390/plants12132529
Chicago/Turabian StyleWang, Shijia, Ruimei Li, Yangjiao Zhou, Alisdair R. Fernie, Zhongping Ding, Qin Zhou, Yannian Che, Yuan Yao, Jiao Liu, Yajie Wang, and et al. 2023. "Integrated Characterization of Cassava (Manihot esculenta) Pectin Methylesterase (MePME) Genes to Filter Candidate Gene Responses to Multiple Abiotic Stresses" Plants 12, no. 13: 2529. https://doi.org/10.3390/plants12132529





