Effect of Microwave and Ultrasound-Assisted Extraction on the Phytochemical and In Vitro Biological Properties of Willow (Salix alba) Bark Aqueous and Ethanolic Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Content
2.2. Antioxidant Activity
2.3. Antibacterial and Antifungal Activities
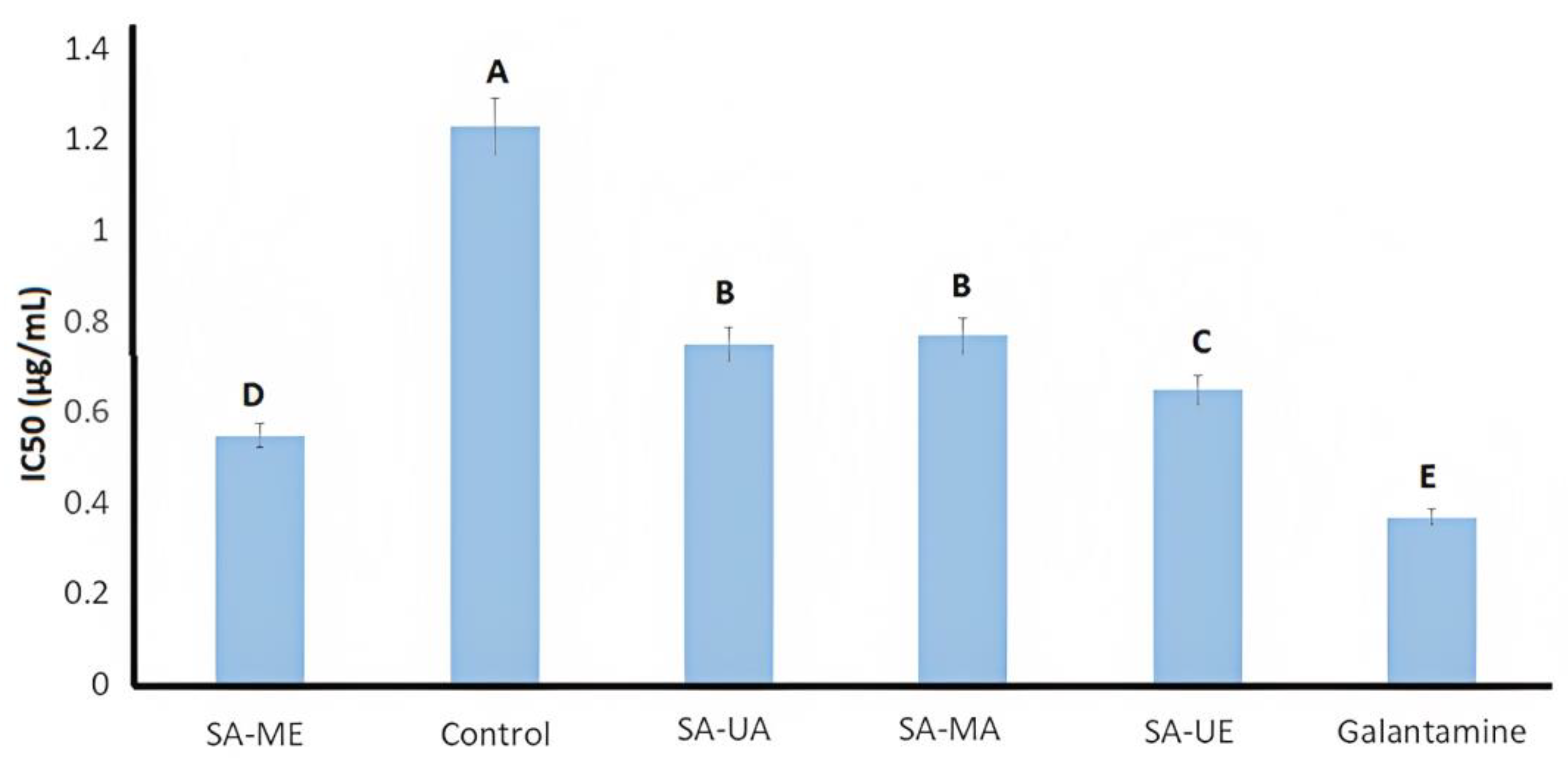
2.4. AChE Inhibition Activity of Willow (Salix alba) Bark Extracts
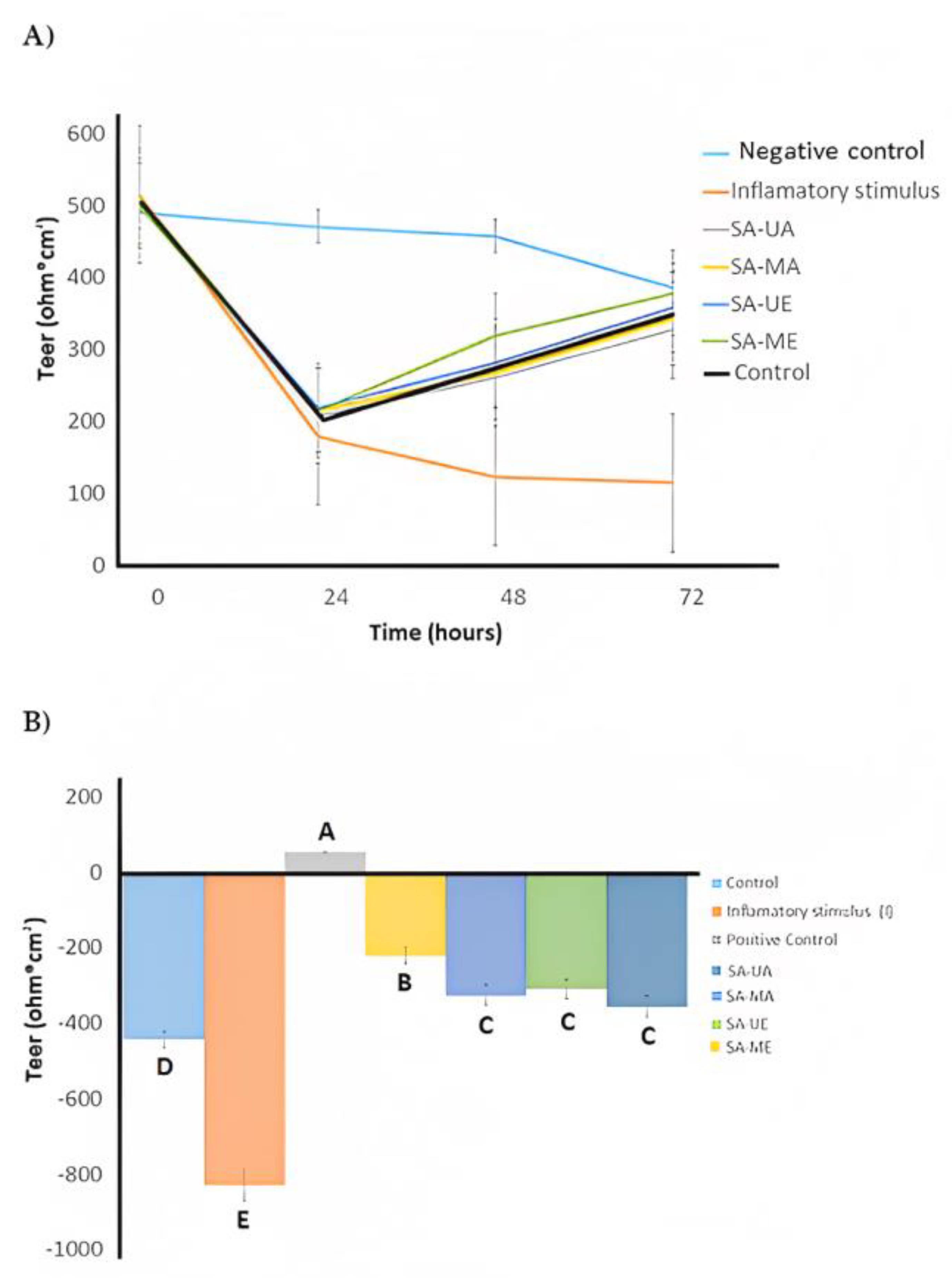
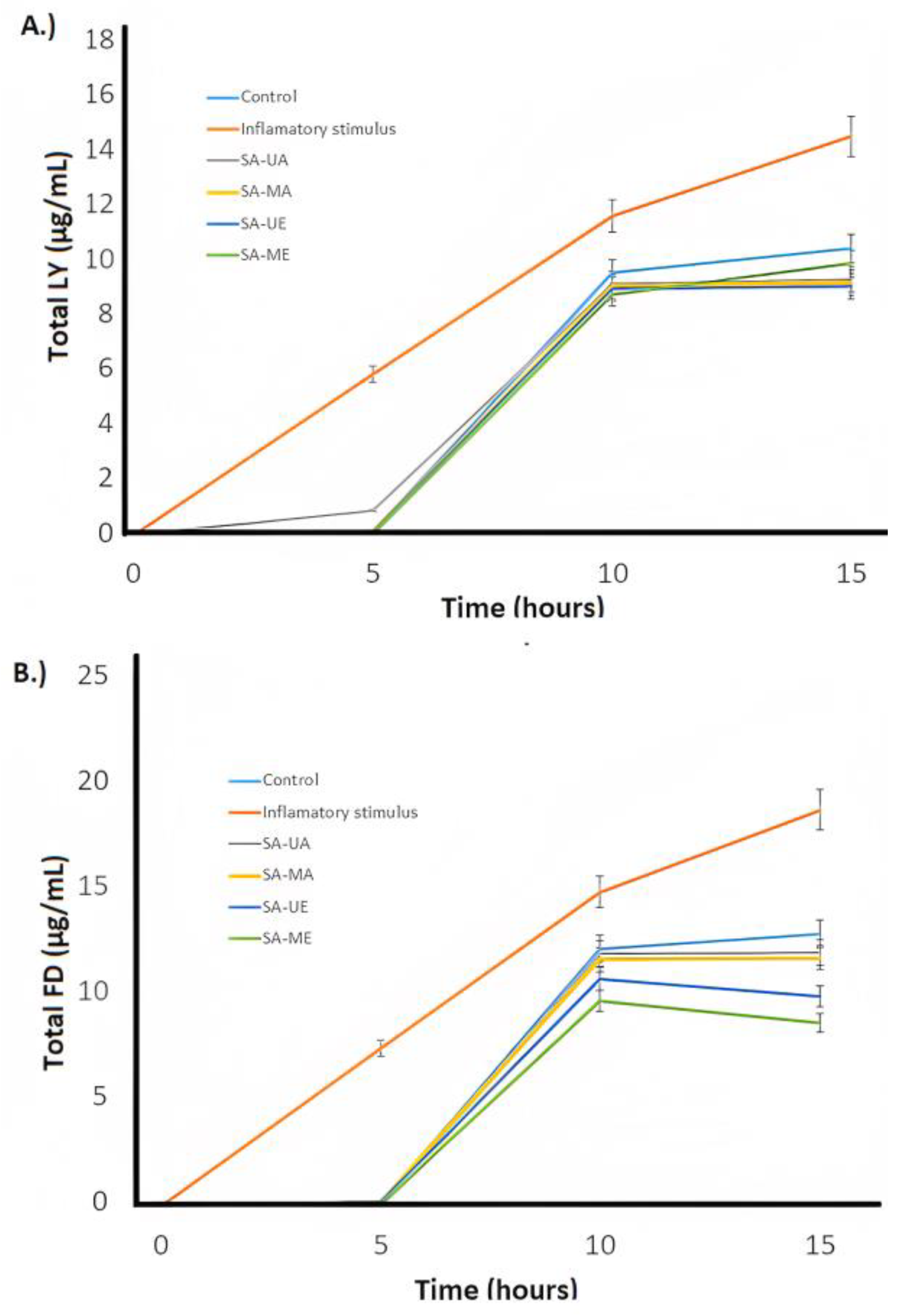
2.5. Anti-Inflammatory Activities of Willow (Salix alba) Bark Extracts
3. Materials and Methods
3.1. Materials
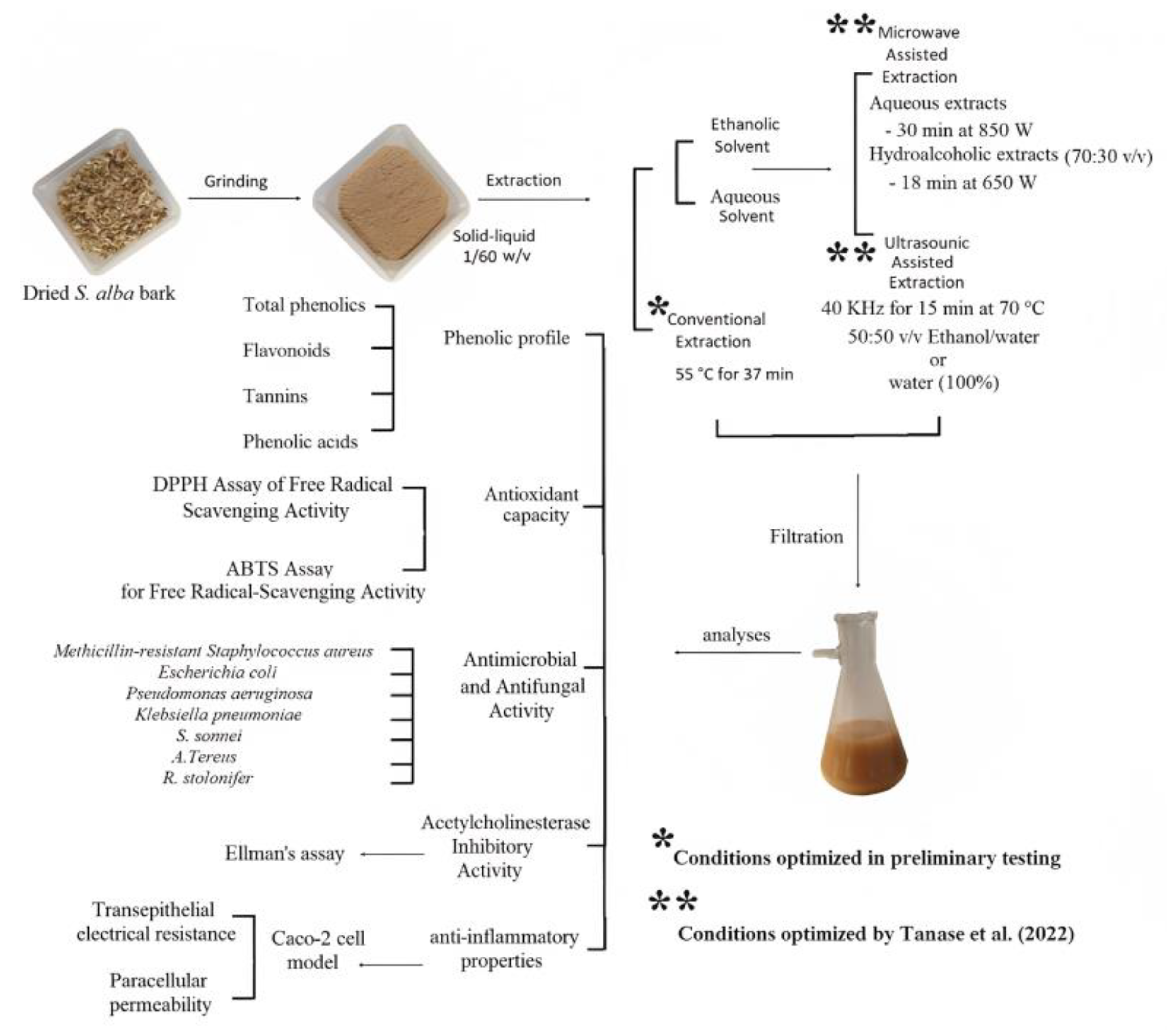
3.2. Phytochemical Extraction
3.3. Phytochemical Characterization
3.3.1. Total Phenolic Content (TPC)
3.3.2. Phenolic Acids
3.3.3. Total Flavonoids Content (TFC)
3.3.4. Tannin Content
3.4. In Vitro Antioxidant Activity—Free Radical Scavenging Assays
3.4.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH•) Assay
3.4.2. ABTS•+ (2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) Assay
3.5. Antimicrobial and Antifungal Activities
3.6. Acetylcholinesterase (AChE) Inhibitory Activity
3.7. Anti-Inflammatory Activity
3.7.1. Caco-2 Cells Maintenance
3.7.2. Caco-2 Cells Treatments
3.7.3. Apparent Permeability Coefficient (Papp)
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chavan, S.S.; Damale, M.G.; Devanand, B.; Sangshetti, J.N. Antibacterial and antifungical drugs from natural source: A review of clinical development. Nat. Prod. Clin. Trials 2018, 1, 114. [Google Scholar]
- Limongelli, F.; Cruppi, P.; Clodoveo, M.L.; Corbo, F.; Muraglia, M. Overview of the Polyphenols in Salicornia: From Recovery to Health-Promoting Effect. Molecules 2022, 27, 7954. [Google Scholar] [CrossRef]
- Baker, P.; Charlton, A.; Johnston, C.; Leahy, J.J.; Lindegaard, K.; Pisano, I.; Skinner, C. A review of willow (Salix spp.) as an intedrated biorefinery feedstock. Ind. Crops Prod. 2022, 189, 115823. [Google Scholar] [CrossRef]
- Gligoric, E.; Igic, R.; Suvajdzi, C.L.; Grujic-Letic, N. Species of the Genus Salix L.; Biochemical Screening and Molecular Docking Approach to Potential Acetylcholonesterase Inhibitors. Appl. Sci. 2019, 9, 1842. [Google Scholar] [CrossRef] [Green Version]
- Nordin, N.A.; Lawai, V.; Ngaini, Z.; Abd Halim, A.N.; Hwang, S.S.; Linton, R.E.; Neilsen, P.M. In vitro cytotoxicity evaluation of thiourea derivates bearing Salix sp. Constituent against HK-1 cell lines. Nat. Prod. Res. 2020, 34, 1505–1514. [Google Scholar] [CrossRef]
- Wu, Y.; Dobermann, D.; Beale, M.H.; Ward, J.L. Acutifoliside, a novel benzoic acid glycoside from Salix acutifolia. Nat. Prod. Res. 2016, 30, 1731–1739. [Google Scholar] [CrossRef]
- European Medicinal Agency (EMA). Assessment Report on Salix (Various Species Including S. purpurea, S. daphnoides Vill., S. fragilis L.); Cortex; EMA: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Tawfeek, N.; Mahmound, M.F.; Hamdan, D.I.; Sobeh, M.; Farrag, N.; Wink, M.; El-Shazly, A.M. Phytochemistry, pharmacology and medicinal uses of plants of the genus Salix: An updated review. Front. Pharmacol. 2021, 12, 593856. [Google Scholar] [CrossRef]
- Ramos, P.; Moreirinha, C.; Silva, S.; Costa, E.; Veiga, M.; Coscueta, E.; Santos, S.; Almeida, A.; Pintado, M.; Freire, C.; et al. The Health-Promoting Potential of Salix spp. Bark Polar Extracts: Key Insights on Phenolic Composition and In vitro Bioactivity and Biocompatibility. Antioxidants 2019, 8, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gligorić, E.; Igić, R.; Čonić, B.S.; Kladar, N.; Teofilović, B.; Grujić, N. Chemical profiling and biological activities of “green” extracts of willow species (Salix L., Salicaceae): Experimental and chemometric approaches. Sustain. Chem. Pharm. 2023, 32, 100981. [Google Scholar] [CrossRef]
- Piątczak, E.; Dybowska, M.; Płuciennik, E.; Kośla, K.; Kolniak-Ostek, J.; Kalinowska-Lis, U. Identification and accumulation of phenolic compounds in the leaves and bark of Salix alba (L.) and their biological potential. Biomolecules 2020, 10, 1391. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural products for human health: Phenolic compounds potential health benefits and toxicity. In Utilisation of Bioactive Compounds from Agricultural and Food Waste; Bhuyan, D.J., Basu, A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 27–59. [Google Scholar]
- Förster, N.; Antoniadou, K.; Zander, M.; Baur, S.; Mittermeier-Kleßinger, V.K.; Dawid, C.; Mewis, I. Chemoprofiling as breeding tool for pharmaceutical use of Salix. Front. Plant Sci. 2021, 12, 579820. [Google Scholar] [CrossRef] [PubMed]
- Warmiński, K.; Stolarski, M.J.; Gil, Ł.; Krzyżaniak, M. Willow bark and wood as a source of bioactive compounds and bioenergy feedstock. Ind. Crops Prod. 2021, 171, 113976. [Google Scholar] [CrossRef]
- Blicharski, T.; Oniszczuk, T.; Kasprzak, K.; Kocira, S.; Oniszczuk, A. Microwave-assisted extraction of polyphenols from Sambucus nigra flowers. Przem. Chem. 2017, 96, 926–929. [Google Scholar]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Silva, A.M.; Pinto, D.; Moreira, M.M.; Costa, P.C.; Delerue-Matos, C.; Rodrigues, F. Valorization of kiwiberry leaves recovered by ultrasound-assisted extraction for skin application: A response surface methodology approach. Antioxidants 2022, 11, 763. [Google Scholar] [CrossRef]
- Hoskin, R.T.; Plundrich, N.; Vargochik, A.; Lila, M.A. Continuous flow microwave-assisted aqueous extraction of pomace phytoactives for production of protein-polyphenol particles and a protein-enriched ready-to-drink beverage. Future Foods 2022, 5, 100137. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Hussien, N.N.; Marzoog, T.R.; Awad, H.A. Phenolic content, antioxidant, antimicrobial and cytotoxic activities of ethanolic extract of Salix alba. Am. J. Biochem. Biotechnol. 2013, 9, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Gligoric, E.I.; Igic, R.; Suvajdzic, L.D.; Teofilovic, B.D.; Grujic-Letic, N.N. Salix eleagnos Scop. a novel source of antioxidant and anti-inflammatory compounds: Biochemical screening and in silico approaches. S. Afr. J. Bot. 2020, 128, 339–348. [Google Scholar] [CrossRef]
- Oroian, M.; Dranca, F.; Ursachi, F. Comparative evaluation of maceration, microwave and ultrasonic-assisted extraction of phenolic compounds from propolis. J. Food Sci. Technol. 2020, 57, 70–78. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Extraction of phenolic compounds from lime peel waste using ultrasonic-assisted and microwave-assisted extractions. Food Biosci. 2019, 28, 66–73. [Google Scholar] [CrossRef]
- Gharekhani, M.; Ghorbani, M.; Rasoulnejad, N. Microwave-assisted extraction of phenolic and flavonoid compounds from Eucalyptus camaldulensis Dehn leaves as compared with ultrasound-assisted extraction. Lat. Am. Appl. Res. 2012, 42, 305–310. [Google Scholar]
- Ince, A.E.; Sahin, S.; Sumnu, G. Comparison of microwave and ultrasound-assisted extraction techniques for leaching of phenolic compounds from nettle. J. Food Sci. Technol. 2014, 51, 2776–2782. [Google Scholar] [CrossRef] [Green Version]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, S.S.; Julio, C.Ã.; Arma, M.L. Extracción asistida por microondas para la obtención del extracto hidroalcohólico de Aloe vera L.(sá bila). Rev. Cuba. De Plantas Med. 2022, 27, e973. [Google Scholar]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A. Microwave-assisted extraction for Hibiscus sabdariffa bioactive compounds. J. Pharm. Biomed. Anal. 2018, 156, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Blasi, F.; Montesano, D.; Ghisoni, S.; Marcotullio, M.C.; Sabatini, S.; Cossignani, L.; Lucini, L. Impact of conventional/non-conventional extraction methods on the untargeted phenolic profile of Moringa oleifera leaves. Food Res. Int. 2019, 115, 319–327. [Google Scholar] [CrossRef]
- Wang, T.; Xu, H.; Dong, R.; Wu, S.; Guo, Y.; Wang, D. Effectiveness of targeting the NLRP3 inflammasome by using natural polyphenols: A systematic review of implications on health effects. Food Res. Int. 2023, 165, 112567. [Google Scholar] [CrossRef]
- Tanase, C.; Nicolescu, A.; Nisca, A.; Ștefănescu, R.; Babotă, M.; Mare, A.D.; Man, A. Biological Activity of Bark Extracts from Northern Red Oak (Quercus rubra L.): An Antioxidant, Antimicrobial and Enzymatic Inhibitory Evaluation. Plants 2022, 11, 2357. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.M.; El-Hashash, M.M.; Mohamed, H.R.; Abdel-Lateef, E.E.S. Phytochemical investigation and in vitro antioxidant activity of different leaf extracts of Salix mucronata Thunb. J. Appl. Pharm. Sci. 2015, 5, 080–085. [Google Scholar] [CrossRef] [Green Version]
- Bhuyan, D.; Basu, A. Phenolic Compounds: Potential Health Benefits and Toxicity. In Utilisation of Bioactive Compounds from Agricultural and Food Waste; Laylor and Francis: Boca Raton, FL, USA, 2017; pp. 27–34. [Google Scholar]
- Kaye, K.S.; Fraimow, H.S.; Abrutyn, E. Pathogens resistant to antimicrobial agents: Epidemiology, molecular mechanisms and clinical management. Infect. Dis. Clin. N. Am. 2014, 18, 467–511. [Google Scholar] [CrossRef]
- Lima, M.C.; Paiva de Sousa, C.; Fernandez-Prada, C.; Harel, J.; Dubreuil, J.D.; de Souza, E.L. A review of the current evidence of fruit phenolic compounds as potential antimicrobials against pathogenic bacteria. Microb. Pathog. 2019, 130, 259–270. [Google Scholar] [CrossRef]
- Jang, Y.S.; Lee, D.E.; Hong, J.H.; Kim, K.A.; Kim, B.; Cho, Y.R.; Ra, M.J.; Jung, S.M.; Yu, J.N.; An, S.; et al. Phytochemical Investigation of Marker Compounds from Indigenous Korean Salix Species and Their Antimicrobial Effects. Plants 2023, 12, 104. [Google Scholar] [CrossRef]
- Javed, B.; Nawaz, K.; Munazir, M. Phytochemical Analysis and Antibacterial Activity of Tannins Extracted from Salix alba L. Against Different Gram-Positive and Gram-Negative Bacterial Strains. Iranian Journal of Science and Technology. Trans. A Sci. 2020, 44, 1303–1314. [Google Scholar]
- Otshudi, A.L.; Apers, S.; Pieters, L.; Claeys, M.; Pannecouque, C. Biologically active bisbenzylisoquinoline alkaloids from the root bark of Epinetrum villosum. J. Ethnopharmacol. 2005, 102, 89–94. [Google Scholar] [CrossRef]
- Senthamilselvi, M.M.; Kesavan, D.; Sulochana, N. An anti-inflammatory and anti-microbial flavone glycoside from flowers of Cleome viscosa. Org. Med. Chem. Lett. 2012, 2, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppo, E.; Marchese, A. Antibacterial activity of polyphenols. Curr. Pharm. Biotechnol. 2014, 15, 380–390. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, M.; He, L.; Fu, P.; Wang, L.; Zhou, J. Optimization of microwave-assisted enzymatic extraction of polyphenols from waste peanut shells and evaluation of its antioxidant and antibacterial activities in vitro. Food Bioprod. Process. 2013, 91, 158–168. [Google Scholar] [CrossRef]
- Şahin, S.; Samli, R.; Tan, A.S.B.; Barba, F.J.; Chemat, F.; Cravotto, G.; Lorenzo, J.M. Solvent-free microwave-assisted extraction of polyphenols from olive tree leaves: Antioxidant and antimicrobial properties. Molecules 2017, 22, 1056. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Bai, J.; Bu, X.; Tang, Y.; Han, W.; Li, D.; Wang, L.; Yang, Y.; Xu, Y. Microwave-assisted aqueous two-phase extraction of phenolic compounds from Ribes nigrum L. and its antibacterial effect on foodborne pathogens. Food Control 2021, 119, 107449. [Google Scholar] [CrossRef]
- Patel, S.; Raghuwanshi, R.; Masood, M.; Acharya, A.; Kumar-Jain, S. Medicinal plants with acetylcholinesterase inhibitory activity. Rev. Neurosci. 2017, 29, 491–529. [Google Scholar] [CrossRef] [PubMed]
- Kračmarová, A.; Drtinová, L.; Pohanka, M. Possibility of acetylcholinesterase overexpression in Alzheimer disease patients after therapy with acetylcholinesterase inhibitors. Acta Med. 2015, 58, 37–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivatsan, M. An analysis of acetylcholinesterase sequence for predicting mechanisms of its non-catalytic actions. Bioinformation 2006, 1, 281–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomathi, R.; Manian, S. Analgesic and acetylcholinesterase inhibition potential of polyphenols from Scolopia crenata (Flacourtiaceae): An endemic medicinal plant of India. Ind. Crops Prod. 2015, 73, 134–143. [Google Scholar] [CrossRef]
- Erdogan-Orhan, I.; Altun, M.L.; Sever-Yilmaz, B.; Saltan, G. Anti-acetylcholinesterase and antioxidant assets of the major components (salicin, amentoflavone, and chlorogenic acid) and the extracts of Viburnum opulus and Viburnum lantana and their total phenol and flavonoid contents. J. Med. Food 2011, 14, 434–440. [Google Scholar] [CrossRef]
- Zaiter, A.; Becker, L.; Petit, J.; Zimmer, D.; Karam, M.C.; Baudelaire, É.; Dicko, A. Antioxidant and antiacetylcholinesterase activities of different granulometric classes of Salix alba (L.) bark powders. Powder Technol. 2016, 301, 649–656. [Google Scholar] [CrossRef]
- Le, N.; Herz, C.; Gomes, J.; Förster, N.; Antoniadou, K.; Mittermeier-Kleßinger, V.; Mewis, I.; Dawid, C.; Ulrichs, C.; Lamy, E. Comparative Anti-Inflammatory Effects of Salix Cortex Extracts and Acetylsalicylic Acid in SARS-CoV-2 Peptide and LPS-Activated Human In vitro Systems. Int. J. Mol. Sci. 2021, 22, 6766. [Google Scholar] [CrossRef]
- Shara, M.; Stohs, S.J. Efficacy and safety of white willow bark (Salix alba) extracts. Phytother. Res. 2015, 29, 1112–1116. [Google Scholar] [CrossRef]
- Mastrogiovanni, F.; Mukhopadhya, A.; Lacetera, N.; Ryan, M.T.; Romani, A.; Bernini, R.; Sweeney, T. Anti-inflammatory effects of pomegranate peel extracts on in vitro human intestinal caco-2 cells and ex vivo porcine colonic tissue explants. Nutrients 2019, 11, 548. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Zhang, H.; Liu, R.; Huang, C.L.; Li, H.; Deng, Z.Y.; Tsao, R. Do short chain fatty acids and phenolic metabolites of the gut have synergistic anti-inflammatory effects?–New insights from a TNF-α-induced Caco-2 cell model. Food Res. Int. 2021, 139, 109833. [Google Scholar] [CrossRef]
- Djemaa-Landri, K.; Hamri-Zeghichi, S.; Belkhiri-Beder, W.; Krisa, S.; Cluzet, S.; Richard, T.; Valls, J.; Kadri, N.; Madani, K. Phenolic content, antioxidant and anti-inflammatory activities of some Algerian olive stone extracts obtained by conventional solvent and microwave-assisted extractions under optimized conditions. J. Food Meas. Charact. 2021, 15, 4166–4180. [Google Scholar] [CrossRef]

| Treatment | TPC (mg/g DW) | DPPH• (μg/mL) | ABTS•+ (μg/mL) |
|---|---|---|---|
| SA-C | 136.6 ± 0.8 a | 22 ± 1 a | 28 ± 2 d |
| SA-UA | 147.6 ± 0.2 b | 20 ± 1 a | 25 ± 1 c |
| SA-MA | 147.8 ± 0.2 b | 17 ± 1 b | 22 ± 2 b |
| SA-UE | 158.1 ± 0.9 c | 15 ± 1 c | 20 ± 1 bc |
| SA-ME | 169.8 ± 0.9 e | 12 ± 1 d | 16 ± 2 a |
| Inhibition Zone, mm | SA-C | SA-UA | SA-MA | SA-UE | SA-ME |
|---|---|---|---|---|---|
| Bacteria | |||||
| S. aureus * | 11 ± 1 b | 15 ± 2 a | 15 ± 1 a | 13 ± 1 a | 11 ± 1 b |
| P. aeruginosa | 8 ± 1 b | 10 ± 2 a | 7± 1 b | 9 ± 1 a | 9 ± 2 a |
| E. coli | ND | ND | ND | ND | ND |
| K. pneumoniae | ND | ND | ND | ND | ND |
| S. sonnei | ND | ND | ND | ND | ND |
| Fungi | |||||
| A. terreus | ND | ND | ND | ND | ND |
| R. stolonifer | ND | ND | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleman, R.S.; Marcia, J.; Duque-Soto, C.; Lozano-Sánchez, J.; Montero-Fernández, I.; Ruano, J.A.; Hoskin, R.T.; Moncada, M. Effect of Microwave and Ultrasound-Assisted Extraction on the Phytochemical and In Vitro Biological Properties of Willow (Salix alba) Bark Aqueous and Ethanolic Extracts. Plants 2023, 12, 2533. https://doi.org/10.3390/plants12132533
Aleman RS, Marcia J, Duque-Soto C, Lozano-Sánchez J, Montero-Fernández I, Ruano JA, Hoskin RT, Moncada M. Effect of Microwave and Ultrasound-Assisted Extraction on the Phytochemical and In Vitro Biological Properties of Willow (Salix alba) Bark Aqueous and Ethanolic Extracts. Plants. 2023; 12(13):2533. https://doi.org/10.3390/plants12132533
Chicago/Turabian StyleAleman, Ricardo S., Jhunior Marcia, Carmen Duque-Soto, Jesús Lozano-Sánchez, Ismael Montero-Fernández, Juan A. Ruano, Roberta Targino Hoskin, and Marvin Moncada. 2023. "Effect of Microwave and Ultrasound-Assisted Extraction on the Phytochemical and In Vitro Biological Properties of Willow (Salix alba) Bark Aqueous and Ethanolic Extracts" Plants 12, no. 13: 2533. https://doi.org/10.3390/plants12132533





