Bread Wheat Landraces Adaptability to Low-Input Agriculture
Abstract
:1. Introduction
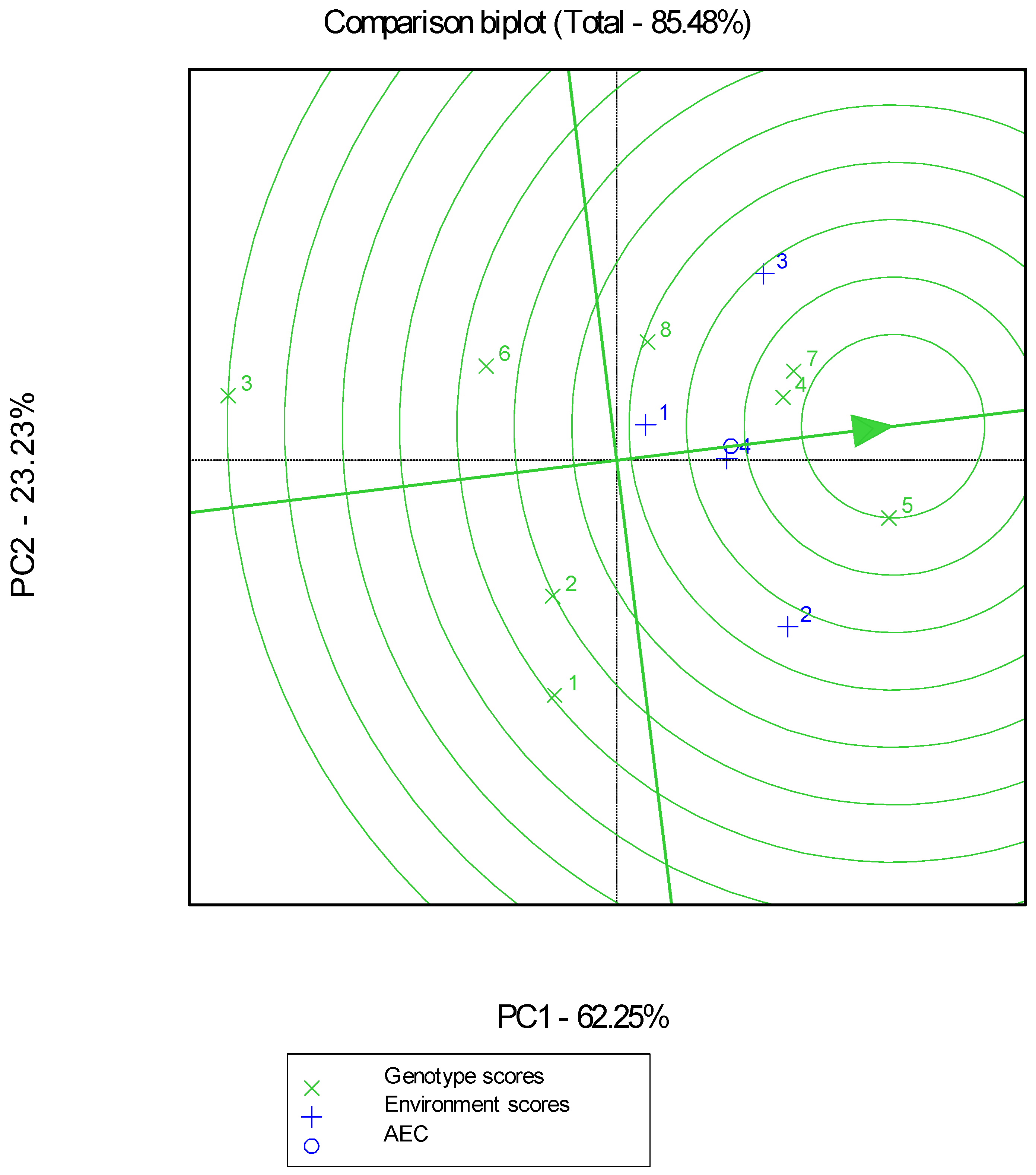
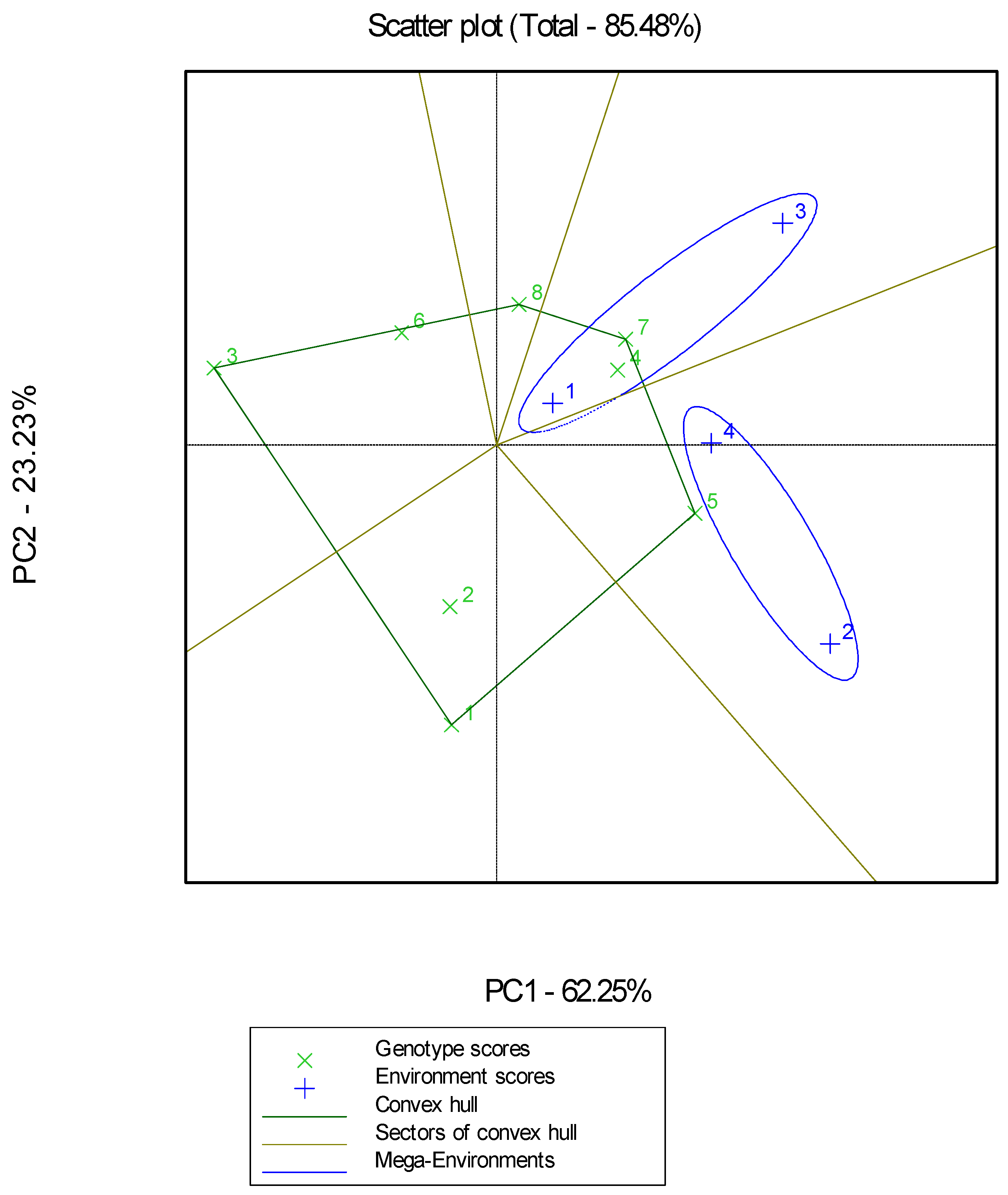
2. Results
- First Season of Experimentation
- Second Season of Experimentation
3. Discussion
4. Materials and Methods
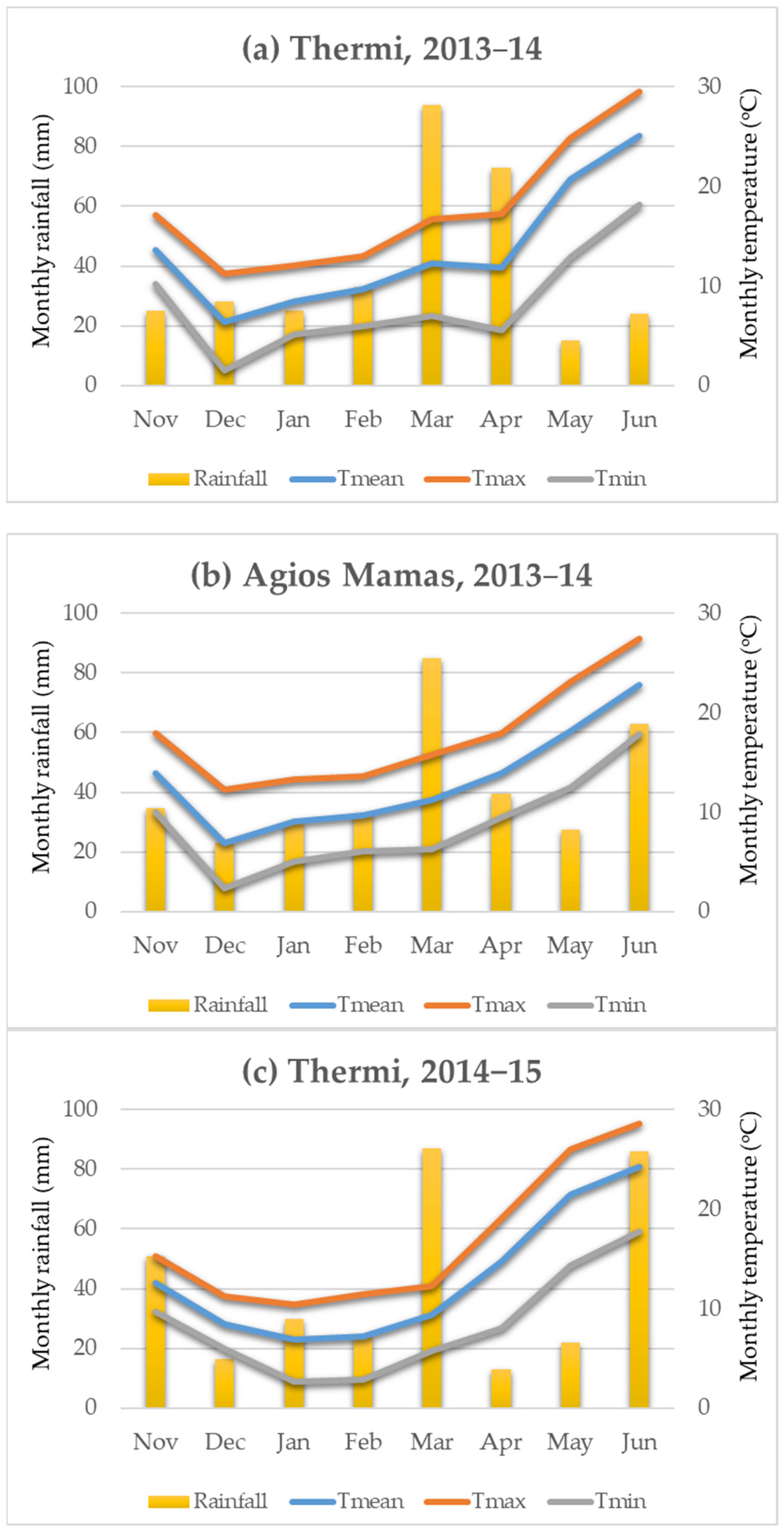
4.1. Genetic Material and Environments
- First Year of Experimentation (Two Environments)
- Second Year of Experimentation (Four Environments)
- L2-AUTH: Low-input environment at the Farm of Aristotle University of Thessaloniki (AUTH);
- L2-IPGRB: Low-input environment at the Farm of IPBGR;
- C2-IPGRB: Conventional field at the Farm of IPBGR;
- O2-IPGRB: Organic field at the Farm of IPBGR, in which a rotation program with vetch and incorporation of green manure every two years is applied.
- It was established randomized complete block design, with four replications on plots of 3.5 m2 in all experimental fields in both cropping seasons (2013–2014, 2014–2015). Moreover, all experiments were established in the middle of November, while the harvest was at the end of June.
4.2. Agronomic and Morphological Ear Characteristics
4.3. Seed Quality Characteristics
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 12 April 2023).
- Peng, J.H.; Sun, D.; Nevo, E. Domestication evolution, genetics and genomics in wheat. Mol. Breed. 2011, 28, 281–301. [Google Scholar] [CrossRef]
- Beres, B.L.; Rahmani, E.; Clarke, J.M.; Grassini, P.; Pozniak, C.J.; Geddes, C.M.; Porker, K.D.; May, W.E.; Ransom, J.K. A Systematic Review of Durum Wheat: Enhancing Production Systems by Exploring Genotype, Environment, and Management (G × E × M) Synergies. Front. Plant Sci. 2020, 11, 568657. [Google Scholar] [CrossRef]
- Xynias, I.N.; Mavromatis, A.G.; Korpetis, E.G.; Pankou, C.I.; Kozub, N.O. Description and Characterization of Hellenic Wheat Germplasm for Agronomical and Seed Quality Parameters Using Phenotypical, Biochemical and Molecular Approaches. Cytol. Genet. 2019, 53, 337–347. [Google Scholar] [CrossRef]
- Martínez-Moreno, F.; Solís, I.; Noguero, D.; Blanco, A.; Özberk, İ.; Nsarellah, N.; Elias, E.; Mylonas, I.; Soriano, J.M. Durum Wheat in the Mediterranean Rim: Historical Evolution and Genetic Resources. Genet. Resour. Crop Evol. 2020, 67, 1415–1436. [Google Scholar] [CrossRef]
- Grundas, S.T. Wheat: The crop. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Trugo, L., Finglas, P.M., Eds.; Academic: San Diego, CA, USA, 2003; pp. 6130–6137. [Google Scholar]
- Leff, B.; Ramankutty, N.; Foley, J.A. Geographic distribution of major crops across the world. Glob. Biogeochem. Cycles 2004, 18, GB1009. [Google Scholar] [CrossRef]
- Hellenic Statistical Authority. Available online: https://www.statistics.gr/en/statistics/-/publication/SPG06/- (accessed on 12 April 2023).
- Newton, A.C.; Akar, T.; Baresel, J.P.; Bebeli, P.J.; Bettencourt, E.; Bladenopoulos, K.V.; Czembor, J.H.; Fasoula, D.A.; Katsiotis, A.; Koutis, K.; et al. Cereal landraces for sustainable agriculture. A review. Agron. Sustain. Dev. 2010, 30, 237–269. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, S.L.; Ceccarelli, S.; Blair, M.W.; Upadhyaya, H.D.; Are, A.K.; Ortiz, R. Landrace Germplasm for Improving Yield and Abiotic Stress Adaptation. Trends Plant Sci. 2016, 21, 31–42. [Google Scholar] [CrossRef]
- Villa, T.C.C.; Maxted, N.; Scholten, M.; Ford-Lloyd, B. Defining and identifying crop landraces. Plant Genet. Res. 2005, 3, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Zeven, A.C. Landraces: A review of definitions and classifications. Euphytica 1998, 104, 127–139. [Google Scholar] [CrossRef]
- Balfourier, F.; Roussel, V.; Strelchenko, P.; Exbrayat-Vinson, F.; Sourdille, P.; Boutet, G.; Koenig, J.; Ravel, C.; Mitrofanova, O.; Beckert, M.; et al. A worldwide bread wheat core collection arrayed in a 384-well plate. Theor. Appl. Genet. 2007, 114, 1265–1275. [Google Scholar] [CrossRef]
- Shlibak, A.A.; Örgeç, M.; Zencirci, N. Wheat Landraces Versus Resistance to Biotic and Abiotic Stresses. In Wheat Landraces; Zencirci, N., Baloch, F.S., Habyarimana, E., Chung, G., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 193–214. [Google Scholar] [CrossRef]
- Brown, A.H.D. Isozymes, plant population genetic structure and genetic conservation. Theoret. Appl. Genet. 1978, 52, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Roupakias, D. Plant Breeding; University Studio Press: Thessaloniki, Greece, 2010; pp. 41–48. (In Greek) [Google Scholar]
- Lopes, M.S.; El-Basyoni, I.; Baenziger, P.S.; Singh, S.; Royo, C.; Ozbek, K.; Aktas, H.; Ozer, E.; Ozdemir, F.; Manickavelu, A.; et al. Exploiting genetic diversity from landraces in wheat breeding for adaptation to climate change. J. Exp. Bot. 2015, 66, 3477–3486. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Kumari, J.; Jacob, S.R.; Prasad, P.; Gangwar, O.P.; Lata, C.; Thakur, R.; Singh, A.K.; Bansal, R.; Kumar, S.; et al. Landraces-potential treasure for sustainable wheat improvement. Genet. Resour. Crop Evol. 2022, 69, 499–523. [Google Scholar] [CrossRef]
- Wood, D.; Lenné, J.M. The conservation of agrobiodiversity on-farm: Questioning the emerging paradigm. Biodivers. Conserv. 1997, 6, 109–129. [Google Scholar] [CrossRef] [Green Version]
- Xynias, I.N.; Mylonas, I.; Korpetis, E.G.; Ninou, E.; Tsaballa, A.; Avdikos, I.D.; Mavromatis, A.G. Durum Wheat Breeding in the Mediterranean Region: Current Status and Future Prospects. Agronomy 2020, 10, 432. [Google Scholar] [CrossRef] [Green Version]
- Wingen, L.U.; West, C.; Leverington-Waite, M.; Collier, S.; Orford, S.; Goram, R.; Yang, C.Y.; King, J.; Allen, A.M.; Burridge, A.; et al. Wheat Landrace Genome Diversity. Genetics 2017, 205, 1657–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koutis, K. Evaluation and Utilization of Wheat Landraces in Conditions of Reduced Inputs. Ph.D. Thesis, Aristotelian University of Thessaloniki, Thessaloniki, Greece, 2011. Available online: http://invenio.lib.auth.gr (accessed on 12 April 2023). (In Greek).
- Lammerts van Bueren, E.T.; Struik, P.C.; Jacobsen, E. Ecological concepts in organic farming and their consequences for an organic crop ideotype. NJAS—Wagen. J. Life Sc. 2002, 50, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Murphy, K.M.; Campbell, K.G.; Lyon, S.R.; Jones, S.S. Evidence of varietal adaptation to organic farming systems. Field Crops Res. 2007, 102, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, M.; Dreccer, F.; Trethowan, R. Drought-adaptive traits derived from wheat wild relatives and landraces. J. Exp. Bot. 2007, 58, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Dotlačil, L.; Hermuth, J.; Stehno, Z.; Dvořáček, V.; Bradová, J.; Leišová, L. How can wheat landraces contribute to present breeding? Czech J. Genet. Plant Breed. 2010, 46, S70–S74. [Google Scholar] [CrossRef] [Green Version]
- Abu-Zaitoun, S.Y.; Chandrasekhar, K.; Assili, S.; Shtaya, M.J.; Jamous, R.M.; Mallah, O.B.; Nashef, K.; Sela, H.; Distelfeld, A.; Alhajaj, N.; et al. Unlocking the Genetic Diversity within A Middle-East Panel of Durum Wheat Landraces for Adaptation to Semi-arid Climate. Agronomy 2018, 8, 233. [Google Scholar] [CrossRef] [Green Version]
- Mylonas, I.; Stavrakoudis, D.; Katsantonis, D.; Korpetis, E. Better farming practices to combat climate change. In Climate Change and Food Security with Emphasis on Wheat; Ozturk, M., Gul, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–29. [Google Scholar]
- Marone, D.; Russo, M.A.; Mores, A.; Ficco, D.B.M.; Laidò, G.; Mastrangelo, A.M.; Borrelli, G.M. Importance of Landraces in Cereal Breeding for Stress Tolerance. Plants 2021, 10, 1267. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, N.W. Genotype (G), environment (E) and GE components of crop yields. Exper. Agric. 1981, 17, 355–362. [Google Scholar] [CrossRef]
- Baker, R.J. Tests for crossover genotype-environmental interactions. Can. J. Plant Sci. 1988, 68, 405–410. [Google Scholar] [CrossRef]
- Yan, W. GGEBiplot-A Windows application for graphical analysis of multi-environment trial data and other types of two-way data. Agron. J. 2001, 93, 1111–1118. [Google Scholar] [CrossRef] [Green Version]
- Yan, W. Singular-value partition in biplot analysis of multienvironment trial data. Agron. J. 2002, 94, 990–996. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S.; Ma, B.; Woods, S.; Cornelius, P.L. GGE biplot vs. AMMI analysis of genotype-by-environment data. Crop Sci. 2007, 47, 643–653. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Available online: https://agriculture.ec.europa.eu/farming/organic-farming/organic-action-plan_en (accessed on 12 April 2023).
- Ben Hassen, T.; El Bilali, H. Impacts of the Russia-Ukraine War on Global Food Security: Towards More Sustainable and Resilient Food Systems? Foods 2022, 11, 2301. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B.; Cacak-Pietrzak, G.; Lenc, L.; Stalenga, J. Rating of Spring Wheat Varieties (Triticum aestivum L.) According to Their Suitability for Organic Agriculture. Agronomy 2020, 10, 1900. [Google Scholar] [CrossRef]
- Rozbicki, J.; Ceglińska, A.; Gozdowski, D.; Jakubczyk, M.; Cacak-Pietrzak, G.; Mądry, W.; Golba, J.; Piechociński, M.; Sobczyński, G.; Studnicki, M.; et al. Influence of the cultivar, environment and management on the grain yield and bread-making quality in winter wheat. J. Cer. Sci. 2015, 61, 126–132. [Google Scholar] [CrossRef]
- Zargar, M.; Polityko, P.; Pakina, E.; Bayat, M.; Vandyshev, V.; Kavhiza, N.; Kiselev, E. Productivity, quality and economics of four spring wheat (Triticum aestivum L.) cultivars as affected by three cultivation technologies. Agron. Res. 2018, 16, 2254–2264. [Google Scholar] [CrossRef]
- Studnicki, M.; Wijata, M.; Sobczyński, G.; Samborski, S.; Rozbicki, J. Assessing grain yield and quality traits stability of spring wheat cultivars at different crop management levels. Cereal Res. Commun. 2018, 46, 180–190. [Google Scholar] [CrossRef]
- Mäder, P.; Hahn, D.; Dubois, D.; Gunst, L.; Alföldi, T.; Bergmann, H.; Oehme, M.; Amadò, R.; Schneider, H.; Graf, U. Wheat quality in organic and conventional farming: Results of 21-year field experiment. J. Sci. Food Agric. 2007, 87, 1826–1835. [Google Scholar] [CrossRef]
- Guzman, C.; Ammar, K.; Govindan, V.; Singh, R. Genetic improvement of wheat grain quality at CIMMYT. Front. Agric. Sci. Eng. 2019, 6, 265–272. [Google Scholar] [CrossRef] [Green Version]
- López-Fernández, M.; Pascual, L.; Faci, I.; Fernández, M.; Ruiz, M.; Benavente, E.; Giraldo, P. Exploring the End-Use Quality Potential of a Collection of Spanish Bread Wheat Landraces. Plants 2021, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Casañas, F.; Simó, J.; Casals, J.; Prohens, J. Toward an evolved concept landrace. Front. Plant Sci. 2017, 8, 145. [Google Scholar] [CrossRef] [Green Version]
- Bladenopoulos, K.V.; Ninou, E.G.; Tsochatzis, E.D.; Mylonas, I.G. Organic Breeding and Cultivation of Barley. Effects on Physical and Chemical Properties. In Barley: Physical Properties, Genetic Factors and Environmental Impacts on Growth; Hasunuma, K., Ed.; Kihara Institute for Biological Research, Yokohama City University: Yokohama, Japan, 2014; pp. 1–20. [Google Scholar]
- Ninou, E.G.; Mylonas, I.G.; Tsivelikas, A.; Ralli, P.; Dordas, C.; Tokatlidis, I.S. Wheat Landraces Are Better Qualified as Potential Gene Pools at Ultra spaced Rather than Densely Grown Conditions. Sci. World J. 2014, 2014, 957472. [Google Scholar] [CrossRef]
- Ninou, E.; Mylonas, I.; Karagianni, I.; Michailidou, S.; Tsivelikas, A.; Sistanis, I.; Avdikos, I.; Korpetis, E.; Papathanasiou, F. Utilization of Intra-Cultivar Variation for Grain Yield and Protein Content within Durum Wheat Cultivars. Agriculture 2022, 12, 661. [Google Scholar] [CrossRef]
- Ninou, E.; Papathanasiou, F.; Vlachostergios, D.N.; Mylonas, I.; Kargiotidou, A.; Pankou, C.; Papadopoulos, I.; Sinapidou, E.; Tokatlidis, I. Intense Breeding within Lentil Landraces for High-Yielding Pure Lines Sustained the Seed Quality Characteristics. Agriculture 2019, 9, 175. [Google Scholar] [CrossRef] [Green Version]
- Koutsika-Sotiriou, M.; Mylonas, I.G.; Ninou, E.; Traka-Mavrona, E. The Cultivation Revival of a Landrace: Pedigree and Analytical Breeding. Euphytica 2010, 176, 15–24. [Google Scholar] [CrossRef]
- Koutis, K.; Mavromatis, A.G.; Baxevanos, D.; Koutsika-Sotiriou, M. Multienvironmental evaluation of wheat landraces by GGE Biplot Analysis for organic breeding. Agric. Sci. 2012, 3, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Smutná, P.; Mylonas, I.; Tokatlidis, I.S. The Use of Stability Statistics to Analyze Genotype × Environments Interaction in Rainfed Wheat under Diverse Agroecosystems. Int. J. Plant Prod. 2021, 15, 261–271. [Google Scholar] [CrossRef]
- Mylonas, I.; Sinapidou, E.; Remoundakis, E.; Sistanis, I.; Pankou, C.; Ninou, E.; Papadopoulos, I.; Papathanasiou, F.; Lithourgidis, A.; Gekas, F.; et al. Improved Plant Yield Efficiency Alleviates the Erratic Optimum Density in Maize. Agron. J. 2020, 112, 1690–1701. [Google Scholar] [CrossRef]
- Sinapidou, E.; Pankou, C.; Gekas, F.; Sistanis, I.; Tzantarmas, C.; Tokamani, M.; Mylonas, I.; Papadopoulos, I.; Kargiotidou, A.; Ninou, E.; et al. Plant Yield Efficiency by Homeostasis as Selection Tool at Ultra-Low Density. A Comparative Study with Common Stability Measures in Maize. Agronomy 2020, 10, 1203. [Google Scholar] [CrossRef]
- Papastylianou, P.; Vlachostergios, D.N.; Dordas, C.; Tigka, E.; Papakaloudis, P.; Kargiotidou, A.; Pratsinakis, E.; Koskosidis, A.; Pankou, C.; Kousta, A.; et al. Genotype × Environment Interaction Analysis of Faba Bean (Vicia faba L.) for Biomass and Seed Yield across Different Environments. Sustainability 2021, 13, 2586. [Google Scholar] [CrossRef]
- Bilsborrow, P.; Cooper, J.; Tétard-Jones, C.; Średnicka-Tober, D.; Barański, M.; Eyre, M.; Schmidt, C.; Shotton, P.; Volakakis, N.; Cakmak, I.; et al. The effect of organic and conventional management on the yield and quality of wheat grown in a long-term field trial. Eur. J. Agron. 2013, 51, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Rempelos, L.; Wang, J.; Sufar, E.K.; Almuayrifi, M.S.B.; Knutt, D.; Leifert, H.; Leifert, A.; Wilkinson, A.; Shotton, P.; Hasanaliyeva, G.; et al. Breeding Bread-Making Wheat Varieties for Organic Farming Systems: The Need to Target Productivity, Robustness, Resource Use Efficiency and Grain Quality Traits. Foods 2023, 12, 1209. [Google Scholar] [CrossRef]
- Mikó, P.; Löschenberger, F.; Hiltbrunner, J.; Aebi, R.; Megyeri, M.; Kovács, G.; Molnár-Láng, M.; Vida, G.; Rakszegi, M. Comparison of bread wheat varieties with different breeding origin under organic and low input management. Euphytica 2014, 199, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Mitura, K.; Cacak-Pietrzak, G.; Feledyn-Szewczyk, B.; Szablewski, T.; Studnicki, M. Yield and Grain Quality of Common Wheat (Triticum aestivum L.) Depending on the Different Farming Systems (Organic vs. Integrated vs. Conventional). Plants 2023, 12, 1022. [Google Scholar] [CrossRef]
- Papadakis, I. Greek types of wheat. In Scientific Bulletin of the “Special Station of Plant Breeding in Thessaloniki”; General Directorate for Settlements of Makedonia: Thessaloniki, Greece, 1929; pp. 6–29, (In Greek and In French). [Google Scholar]
- Payne, R.W. GenStat. WIREs Comput. Stat. 2009, 1, 255–258. [Google Scholar] [CrossRef]
| Source of Variation | df | GY | PH | EL | ELA | NS | TKW | PC | GI | SV | FN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| E | 1 | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| G | 11 | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| G × E | 11 | ** | ** | * | * | ns | ** | ** | * | * | ** |
| Blocks | 3 | ns | * | ns | ns | ns | ns | ns | ns | ns | ns |
| error | 69 | ||||||||||
| CV% | 12.7 | 4.5 | 5.3 | 5.5 | 13.0 | 5.6 | 3.2 | 14.9 | 12.01 | 6.1 |
| 2013–2014 | GY | PH | EL | ELA | NS | TKW | PC | GI | SV | FN |
|---|---|---|---|---|---|---|---|---|---|---|
| Environments | ||||||||||
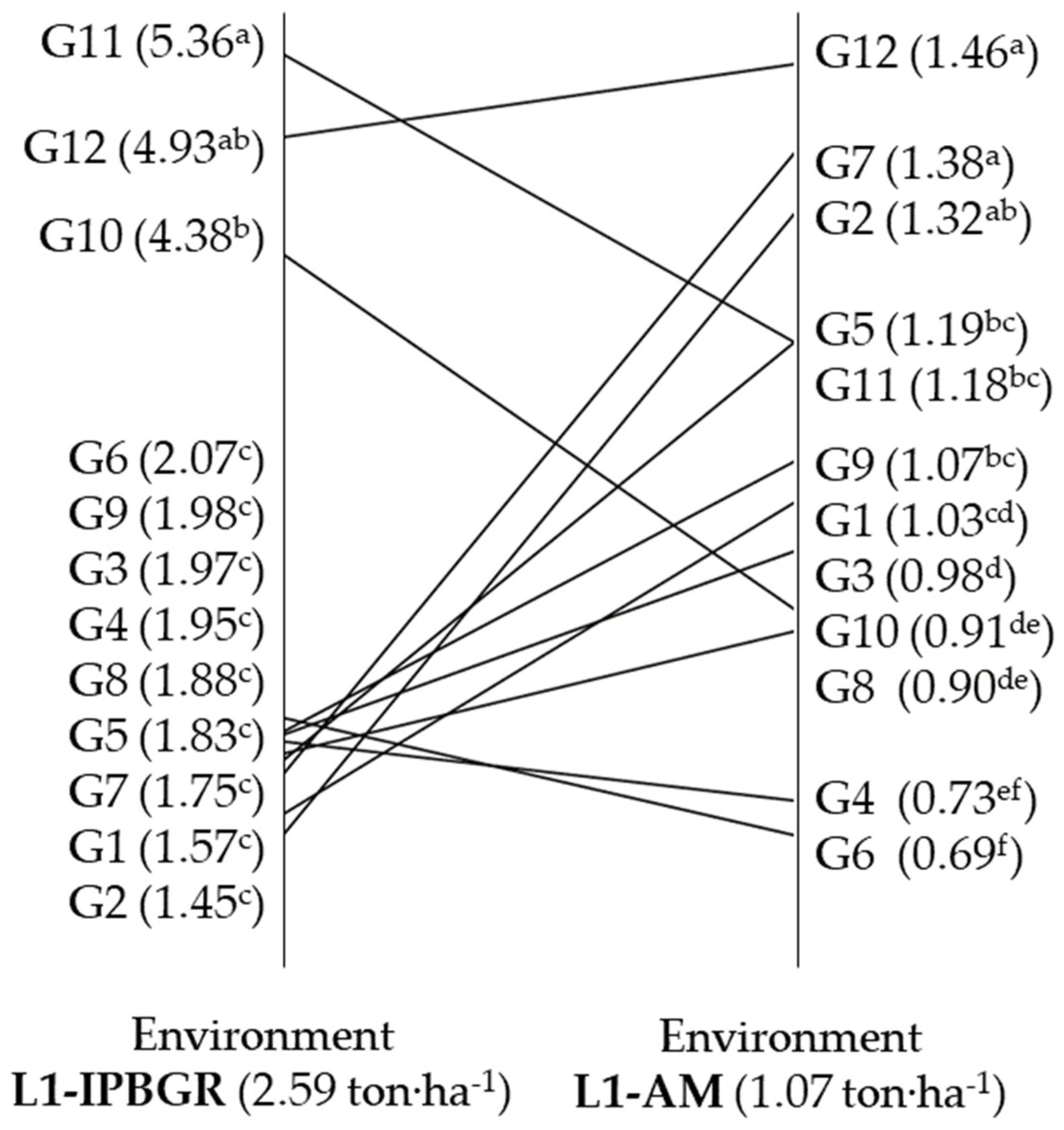
| L1-IPBGR | 2.59 a† | 126.5 a | 12.3 a | 16.8 a | 37.9 b | 36.4 b | 16.01 a | 49.2 a | 35.8 a | 461.2 a |
| L-AM | 1.07 b | 107.3 b | 11.7 b | 16.2 b | 42.2 a | 44.4 a | 14.39 b | 44.8 b | 33.4 b | 383.8 b |
| Genotypes | ||||||||||
| G1 | 1.30 c | 129.8 ab | 11.6 de | 18.0 abc | 35.5 bcd | 38.4 cd | 15.90 a | 44.6 bc | 30.6 def | 445.6 a |
| G2 | 1.39 c | 122.7 bc | 12.9 bc | 18.9 b | 31.1 d | 44.8 a | 15.79 ab | 47.6 bc | 35.3 bcde | 389.4 c |
| G3 | 1.48 c | 129.2 ab | 13.9 ab | 19.3 a | 41.1 bc | 36.1 de | 15.06 bcd | 43.1 bc | 44.5 a | 450.6 a |
| G4 | 1.34 c | 123.8 bc | 14.4 a | 14.5 f | 32.5 cd | 44.5 a | 15.19 abc | 38.1 c | 32.7 cde | 400.5 bc |
| G5 | 1.51 c | 119.4 c | 10.9 ef | 16.3 de | 39.3 bcd | 42.9 ab | 14.82 cd | 41.9 bc | 28.6 ef | 446.4 a |
| G6 | 1.38 c | 134.1 a | 14.1 a | 14.1 f | 35.4 bcd | 41.6 bc | 15.13 abcd | 44.8 bc | 40.1 ab | 431.4 abc |
| G7 | 1.57 c | 129.8 ab | 10.3 f | 16.8 cd | 34.2 cd | 41.7 abc | 15.93 a | 38.7 c | 25.6 f | 437.1 ab |
| G8 | 1.39 c | 128.5 ab | 12.6 cd | 18.0 abc | 43.6 b | 39.3 bcd | 15.32 abc | 46.9 bc | 36.0 bcd | 439.0 ab |
| G9 | 1.53 c | 123.4 bc | 12.0 cde | 17.3 bcd | 35.3 bcd | 42.8 ab | 15.43 abc | 41.7 bc | 35.8 bcd | 465.6 a |
| G10 | 2.65 b | 92.1 d | 10.4 f | 15.1 ef | 56.1 a | 34.3 e | 14.31 d | 53.1 b | 34.0 bcde | 450.4 a |
| G11 | 3.27 a | 74.3 e | 10.9 ef | 15.2 ef | 40.7 bc | 41.7 abc | 14.65 cd | 75.7 a | 34.1 bcde | 264.9 d |
| G12 | 3.20 a | 96.1 d | 9.9 f | 14.6 f | 55.9 a | 37.0 de | 14.86 cd | 47.9 bc | 37.9 abc | 449.1 a |
| GY | PH | EL | ELA | NS | TKW | PC | GI | SV | FN | |
|---|---|---|---|---|---|---|---|---|---|---|
| GY | 1 | −0.946 ** | −0.634 * | −0.499 | 0.721 ** | −0.398 | −0.658 * | 0.745 ** | 0.082 | −0.452 |
| PH | 1 | 0.577 * | 0.456 | −0.611 * | 0.267 | 0.700 * | −0.835 ** | 0.015 | 0.592* | |
| EL | 1 | 0.175 | −0.553 | 0.321 | 0.211 | −0.317 | 0.512 | 0.003 | ||
| ELA | 1 | −0.322 | −0.031 | 0.551 | −0.226 | 0.109 | 0.220 | |||
| NS | 1 | −0.798 ** | −0.710 ** | 0.316 | 0.238 | 0.175 | ||||
| TKW | 1 | 0.423 | −0.147 | −0.360 | −0.343 | |||||
| PC | 1 | −0.497 | −0.298 | 0.196 | ||||||
| GI | 1 | 0.109 | −0.812 ** | |||||||
| SV | 1 | 0.067 | ||||||||
| FN | 1 |
| Source of Variation | df | GY | PH | HD | EL | NS | TKW |
|---|---|---|---|---|---|---|---|
| Environments (E) | 3 | ** | ** | ** | ** | ** | ** |
| Genotypes (G) | 7 | ** | ** | ** | ** | ** | ** |
| G × E | 21 | ** | ns | ** | ** | ns | * |
| Blocks | 3 | * | * | ns | ns | ns | * |
| error | 93 | ||||||
| CV% | 17.1 | 9.8 | 0.6 | 6.3 | 18.3 | 10.6 |
| Second Season | GY | PH | HD | EL | NS | TKW |
|---|---|---|---|---|---|---|
| Environments | ||||||
| L2-AUTH | 1.37 d† | 86.5 bc | 135.5 a | 10.45 a | 40.9 ab | 33.5 c |
| L2-IPGRB | 2.41 b | 83.7 d | 132.0 b | 9.31 b | 37.0 b | 37.3 b |
| C2-IPGRB | 2.70 a | 91.8 ab | 132.2 b | 9.66 b | 38.2 b | 40.6 a |
| O2-IPGRB | 2.05 c | 96.2 a | 132.5 b | 10.29 a | 45.2 a | 42.3 a |
| Genotypes | ||||||
| -Landraces | ||||||
| X1 | 2.01 c | 120.9 a | 136.8 a | 10.9 b | 33.7 de | 36.4 cd |
| X2 | 1.93 c | 115.7 a | 136.6 a | 11.3 ab | 29.3 e | 42.2 ab |
| X3 | 1.48 d | 114.9 a | 135.3 b | 11.8 a | 28.2 e | 41.8 ab |
| X4 | 2.52 a | 87.4 b | 131.5 d | 9.2 d | 55.0 ab | 38.0 bc |
| -Commercial Cultivars | ||||||
| X5 | 2.57 a | 67.5 c | 134.0 c | 8.6 de | 47.4 bc | 32.7 d |
| X6 | 1.97 c | 63.5 c | 136.9 a | 8.4 e | 55.6 a | 32.4 d |
| X7 | 2.47 ab | 64.7 c | 126.3 e | 10.0 c | 33.0 de | 41.0 ab |
| X8 | 2.10 bc | 81.8 b | 126.9 e | 9.2 d | 40.5 cd | 42.9 a |
| Second Season | L2-AUTH | L2-IPGRB | C2-IPGRB | O2-IPGRB | |
|---|---|---|---|---|---|
| df | |||||
| Genotypes | 7 | ** | ** | ** | ** |
| Blocks | 3 | ** | ** | * | ** |
| error | 21 | ||||
| CV% | 12.2 | 14.7 | 9.5 | 14.5 | |
| Genotypes | |||||
| -Landraces | |||||
| X1 | 1.37 bcd† | 2.82 ab | 1.84 d | 2.02 bc | |
| X2 | 1.10 d | 2.72 ab | 2.32 cd | 1.59 c | |
| X3 | 1.22 cd | 1.19 d | 1.97 d | 1.55 c | |
| X4 | 1.83 a | 2.60 abc | 3.10 ab | 2.56 ab | |
| -Commercial Cultivars | |||||
| X5 | 1.25 cd | 3.16 a | 3.09 ab | 2.79 a | |
| X6 | 1.55 abc | 1.79 cd | 2.59 bc | 1.97 bc | |
| X7 | 1.66 ab | 2.76 ab | 3.41 a | 2.04 bc | |
| X8 | 0.99 d | 2.26 bc | 3.25 a | 1.92 bc |
| GY | HD | PH | EL | NS | TKW | |
|---|---|---|---|---|---|---|
| GY | 1 | −0.400 ** | −0.181 * | −0.369 ** | 0.154 | 0.122 |
| HD | 1 | 0.372 ** | 0.269 ** | 0.015 | −0.397 ** | |
| PH | 1 | 0.710 ** | −0.426 ** | 0.313 ** | ||
| EL | 1 | −0.455 ** | 0.225 * | |||
| NS | 1 | −0.330 ** | ||||
| TKW | 1 |
| Soil Characteristics | L1-IPBGR | L1-AM | L2-AUTH | L2-IPBGR | C2-IPBGR | O2-IPBGR |
|---|---|---|---|---|---|---|
| Textural Class | L | C | L | L | L | L |
| Sand (%) | 30 | 30 | 48 | 50 | 48 | 46 |
| Clay (%) | 24 | 44 | 20 | 18 | 20 | 22 |
| Silt (%) | 46 | 26 | 32 | 32 | 32 | 32 |
| pH | 7.74 | 8.04 | 7.85 | 7.89 | 8.00 | 8.11 |
| EC (mS/cm) | 0.596 | 0.920 | 0.834 | 0.503 | 0.483 | 0.508 |
| Organic matter (%) | 2.54 | 2.50 | 1.56 | 2.37 | 1.88 | 2.99 |
| CaCO3 (%) | 1.80 | 5.30 | 5.00 | 3.00 | 3.80 | 7.80 |
| NO3 (mg/L) | 73.32 | 74.32 | 29.46 | 74.21 | 27.30 | 134.16 |
| Nitrogen nitrate | 16.56 | 16.65 | 6.65 | 16.68 | 6.16 | 30.29 |
| P (mg/L) | 4.57 | 4.13 | 31.93 | 31.84 | 30.02 | 67.31 |
| K (mg/L) | 93 | 101 | 377 | 715 | 987 | 1349 |
| Mg2+ exchangeable (mg/L) | 259 | 479 | 416 | 200 | 254 | 309 |
| Ca2+ exchangeable (mg/L) | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| Fe (mg/L) | 4.19 | 4.36 | 7.15 | 3.51 | 3.17 | 3.05 |
| Zn (mg/L) | 0.47 | 0.18 | 1.09 | 0.85 | 1.11 | 1.32 |
| Mn (mg/L) | 7.15 | 5.39 | 5.08 | 7.53 | 8.86 | 10.23 |
| Cu (mg/L) | 2.93 | 1.06 | 1.66 | 1.79 | 2.24 | 2.54 |
| B (mg/L) | 0.46 | 0.48 | 0.14 | 0.16 | 0.22 | 0.45 |
| Descriptor | Conventional | Low-Input | Organic |
|---|---|---|---|
| Planting rate (seeds/m2) | ~400 | ~400 | ~400 |
| Seed Treatment | None | None | None |
| Tillage | Yes | Yes | Yes |
| Starter Fertilizer (source) | (250 Kg·ha−1) (20-10-0) | (200 Kg·ha−1) (20-10-0) | None |
| Spring application of N fertilizer (source) | 67 Kg·N·ha−1(33.5-0-0) | None | None |
| Weed Control | Iodosulfuron-methyl-sodium + mesosulfuron-methyl hiencarbazone + methyl | Iodosulfuron-methyl-sodium + mesosulfuron-methyl hiencarbazone + methyl | None |
| Plant Disease Control | None | None | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korpetis, E.; Ninou, E.; Mylonas, I.; Ouzounidou, G.; Xynias, I.N.; Mavromatis, A.G. Bread Wheat Landraces Adaptability to Low-Input Agriculture. Plants 2023, 12, 2561. https://doi.org/10.3390/plants12132561
Korpetis E, Ninou E, Mylonas I, Ouzounidou G, Xynias IN, Mavromatis AG. Bread Wheat Landraces Adaptability to Low-Input Agriculture. Plants. 2023; 12(13):2561. https://doi.org/10.3390/plants12132561
Chicago/Turabian StyleKorpetis, Evangelos, Elissavet Ninou, Ioannis Mylonas, Georgia Ouzounidou, Ioannis N. Xynias, and Athanasios G. Mavromatis. 2023. "Bread Wheat Landraces Adaptability to Low-Input Agriculture" Plants 12, no. 13: 2561. https://doi.org/10.3390/plants12132561
APA StyleKorpetis, E., Ninou, E., Mylonas, I., Ouzounidou, G., Xynias, I. N., & Mavromatis, A. G. (2023). Bread Wheat Landraces Adaptability to Low-Input Agriculture. Plants, 12(13), 2561. https://doi.org/10.3390/plants12132561









