Variation in the Chemical Composition of Endemic Specimens of Hedychium coronarium J. Koenig from the Amazon and In Silico Investigation of the ADME/Tox Properties of the Major Compounds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition
| RIL | RIC | Constituents | A1 | B1 | C1 | D1 | E1 | F1 |
|---|---|---|---|---|---|---|---|---|
| 924 | 930 | α-thujene | 0 | 0.6 | 0.7 | 0.6 | 0.7 | 0 |
| 932 | 938 | α-pinene | 4.5 | 9.1 | 11.5 | 9.6 | 12.5 | 19.6 |
| 946 | 952 | camphene | 0 | 0.7 | 0.8 | 0.7 | 0.9 | 0 |
| 969 | 975 | sabinene | 0.6 | 1.3 | 1.3 | 2.3 | 2.6 | 0 |
| 974 | 979 | β-pinene | 21.9 | 23 | 28.9 | 25.9 | 30.5 | 7.6 |
| 988 | 990 | myrcene | 0.3 | 1.3 | 1.5 | 1.4 | 2.3 | 10.7 |
| 1002 | 1003 | α-phellandrene | 0 | 0 | 2.3 | 3.4 | 0 | 10.3 |
| 1003 | 1004 | p-mentha-1(7),8-diene | 0 | 0 | 0.4 | 0 | 0 | 0 |
| 1014 | 1017 | α-terpinene | 0 | 0.4 | 0.6 | 0.4 | 0.7 | 1.5 |
| 1020 | 1027 | p-cymene | 0 | 0.8 | 0.5 | 1.3 | 0.5 | 0 |
| 1024 | 1029 | Limonene | 0 | 3 | 3.3 | 3.5 | 4.4 | 0 |
| 1026 | 1032 | 1,8-cineole | 66.1 | 46.2 | 37.4 | 35 | 26 | 33.5 |
| 1036 | 1036 | phenyl acetaldehyde | 0 | 0.1 | 0 | 0.1 | 0.2 | 0 |
| 1044 | 1048 | (E)-β-ocimene | 0 | 0 | 0 | 0 | 0 | 0.2 |
| 1054 | 1060 | γ-terpinene | 0.5 | 0.9 | 1.1 | 1.1 | 1.6 | 3.4 |
| 1086 | 1089 | terpinolene | 0 | 0.3 | 0.4 | 0.4 | 0 | 1.2 |
| 1095 | 1097 | linalool | 1.1 | 0.1 | 0.9 | 0.2 | 0.3 | 0.5 |
| 1098 | 1099 | (E)-sabinene hydrate | 0 | 0 | 0 | 0.7 | 0.1 | 0 |
| 1114 | 1117 | endo-fenchol | 0 | 0 | 0 | 0 | 0 | 0.1 |
| 1128 | 1126 | α-campholenal | 0 | 0 | 0 | 0.1 | 0 | 0 |
| 1132 | 1131 | allocymene | 0 | 0 | 0 | 0 | 0 | 0 |
| 1137 | 1137 | (E)-limonene oxide | 0 | 0.1 | 0 | 0.3 | 0 | 0 |
| 1135 | 1140 | trans-pinocarveol | 0 | 0 | 0 | 0.1 | 0 | 0.2 |
| 1141 | 1142 | Camphor | 0 | 0.1 | 0.9 | 0.2 | 0.3 | 0.1 |
| 1165 | 1166 | borneol | 0.2 | 0.6 | 0.8 | 1.2 | 0.6 | 1.8 |
| 1174 | 1178 | terpinen-4-ol | 1.5 | 1.8 | 2.1 | 1.8 | 1.2 | 2.7 |
| 1179 | 1179 | p-cymen-8-ol | 0 | 0 | 0 | 0.1 | 0 | 0 |
| 1186 | 1190 | α-terpineol | 3.3 | 3.1 | 4.1 | 5.2 | 2.8 | 4.2 |
| 1194 | 1195 | myrtenol | 0 | 0 | 0 | 0 | 0 | 0.2 |
| 1284 | 1289 | bornyl acetate | 0 | 0 | 0 | 0 | 0.1 | 0.2 |
| 1335 | 1340 | δ-elemene | 0 | 0 | 0 | 0.7 | 0.1 | 0 |
| 1346 | 1351 | α-terpinyl acetate | 0 | 0 | 0 | 0.1 | 0 | 0.3 |
| 1417 | 1417 | (E)-caryophyllene | 0 | 0.1 | 0 | 0.3 | 0.3 | 0.5 |
| 1452 | 1455 | α-humulene | 0 | 0 | 0 | 0 | 0 | 0.1 |
| 1505 | 1506 | β-bisabolene | 0 | 0 | 0 | 0.4 | 0.1 | 0 |
| 1582 | 1580 | caryophyllene oxide | 0 | 0 | 0 | 0.1 | 0.1 | 0.3 |
| 1640 | 1644 | epi-α-muurolol | 0 | 0 | 0 | 0.1 | 0.1 | 0 |
| 1759 | 1763 | benzyl benzoate | 0 | 0 | 0.3 | 0 | 0 | 0 |
| Hydrocarbon monoterpenes | 27.8 | 41.4 | 53.3 | 50.6 | 56.7 | 54.5 | ||
| Oxygenated monoterpenes | 72.2 | 52.0 | 46.2 | 45.0 | 31.4 | 43.8 | ||
| Hydrocarbon sesquiterpenes | 0 | 0.1 | 0 | 1.4 | 0.5 | 0.6 | ||
| Oxygenated sesquiterpenes | 0 | 0 | 0 | 0.2 | 0.2 | 0.3 | ||
| Other Class | 0.1 | 0.3 | 0.2 | 0.2 | 0 | |||
| Total | 100 | 93.6 | 99.8 | 97.4 | 89.9 | 99.2 |
| RIL | RIC | Constituents | A2 | B2 | C2 | D2 | E2 | F2 |
|---|---|---|---|---|---|---|---|---|
| 924 | 930 | α-thujene | 0.1 | 0.4 | 0.4 | 0.3 | 0.5 | 0 |
| 932 | 938 | α-pinene | 11.6 | 16.3 | 15.9 | 14.7 | 20.7 | 32.9 |
| 946 | 952 | camphene | 0.1 | 0.3 | 0.3 | 0.2 | 0.2 | 0 |
| 969 | 975 | sabinene | 0.2 | 1.6 | 3.1 | 2.7 | 5.7 | 0 |
| 974 | 979 | β-pinene | 48.9 | 31.1 | 34.8 | 31.6 | 41 | 9.9 |
| 988 | 990 | myrcene | 0.2 | 0.5 | 0.7 | 0.5 | 1.1 | 5.2 |
| 1001 | 1001 | δ-2-carene | 0 | 0 | 0 | 0 | 0 | 0.1 |
| 1002 | 1003 | α-phellandrene | 0 | 0.1 | 0.2 | 0 | 0 | 0 |
| 1014 | 1017 | α-terpinene | 0.2 | 0.5 | 0.3 | 0.4 | 0.2 | 0.4 |
| 1020 | 1027 | p-cymene | 0.1 | 0.3 | 0.2 | 0.2 | 0.1 | 0 |
| 1024 | 1029 | Limonene | 0 | 1.6 | 1.9 | 1.5 | 1.9 | 4.5 |
| 1026 | 1032 | 1,8-cineole | 16.9 | 2.2 | 5.5 | 1.9 | 2.7 | 8.9 |
| 1036 | 1036 | phenyl acetaldehyde | 0 | 0.2 | 0 | 0.3 | 0.6 | 0 |
| 1044 | 1048 | (E)-β-ocimene | 0 | 0 | 0.2 | 0 | 0 | 1.1 |
| 1054 | 1060 | γ-terpinene | 0.5 | 0.9 | 0.7 | 0.7 | 0.4 | 1.2 |
| 1086 | 1089 | terpinolene | 0 | 0.3 | 0.2 | 0.2 | 0.1 | 0.5 |
| 1095 | 1097 | linalool | 1.1 | 0.1 | 0 | 0.1 | 0 | 0 |
| 1098 | 1099 | (E)-sabinene hydrate | 0 | 0.1 | 0 | 0 | 0.1 | 0 |
| 1128 | 1126 | α-campholenal | 0 | 0 | 0 | 0.2 | 0.1 | 0 |
| 1132 | 1131 | allocymene | 0 | 0 | 0 | 0 | 0 | 0.8 |
| 1137 | 1137 | (E)-limonene oxide | 0 | 0.7 | 0 | 0.2 | 0 | 0 |
| 1135 | 1140 | trans-pinocarveol | 0.1 | 0.1 | 0.4 | 0 | 0 | 0.4 |
| 1141 | 1142 | camphor | 1.1 | 0.1 | 0 | 0.1 | 0 | 0 |
| 1140 | 1145 | trans-verbenol | 0 | 0.1 | 0.4 | 0 | 0 | 0 |
| 1160 | 1163 | pinocarvone | 0.3 | 0.1 | 0.4 | 0 | 0 | 0.7 |
| 1165 | 1166 | borneol | 0.3 | 0.6 | 0 | 0.1 | 0.1 | 0 |
| 1174 | 1178 | terpinen-4-ol | 0.7 | 1.5 | 0.9 | 0.1 | 0.4 | 1 |
| 1179 | 1179 | p-cymen-8-ol | 0 | 0.1 | 0 | 0.1 | 0 | 0 |
| 1186 | 1190 | α-terpineol | 2 | 2.7 | 1.6 | 0.7 | 0.4 | 0.5 |
| 1194 | 1195 | myrtenol | 0 | 0 | 0 | 0 | 0 | 0.6 |
| 1204 | 1205 | verbenone | 0 | 0.1 | 0 | 0 | 0 | 0 |
| 1284 | 1289 | bornyl acetate | 0 | 0.1 | 0 | 0 | 0.1 | 0.1 |
| 1285 | 1292 | safrole | 0 | 0 | 0 | 0 | 0 | 0.1 |
| 1298 | 1298 | (E)-pinocarvyl acetate | 0 | 0.2 | 0 | 0 | 0 | 0 |
| 1335 | 1340 | δ-elemene | 0 | 0.1 | 0 | 0 | 0.1 | 0.3 |
| 1346 | 1351 | α-terpinyl acetate | 0.1 | 0 | 0 | 0.2 | 0.1 | 0 |
| 1356 | 1361 | eugenol | 0 | 0 | 0 | 0 | 0 | 0.1 |
| 1389 | 1392 | β-elemene | 0 | 0.6 | 0 | 0 | 0 | 0.2 |
| 1417 | 1417 | (E)-caryophyllene | 3.1 | 15.1 | 13.2 | 20 | 14.1 | 10.5 |
| 1428 | 1432 | (E)-α-ionone | 0 | 0 | 0 | 0 | 0 | 0.1 |
| 1434 | 1437 | γ-elemene | 0 | 0 | 0 | 0 | 0 | 0.1 |
| 1442 | 1448 | guaia-6,9-diene | 0 | 0 | 0 | 0 | 0 | 0.1 |
| 1452 | 1455 | α-humulene | 0.3 | 1 | 0.9 | 1.4 | 0 | 1.5 |
| 1454 | 1457 | (E)-β-farnesene | 0 | 0.3 | 0 | 0 | 0.3 | 0 |
| 1480 | 1486 | germacrene D | 0 | 0 | 0 | 0 | 0 | 0.1 |
| 1487 | 1491 | (E)-β-ionone | 0 | 0 | 0 | 0 | 0 | 0.1 |
| 1505 | 1506 | β-bisabolene | 0 | 0.1 | 0 | 0 | 0.1 | 0 |
| 1513 | 1515 | γ-cadinene | 0 | 0 | 0 | 0 | 0 | 0.1 |
| 1520 | 1522 | 7-epi-α-selinene | 0 | 0 | 0 | 0.2 | 0 | 0 |
| 1522 | 1527 | δ-cadinene | 0 | 0 | 0 | 0 | 0 | 0.1 |
| 1561 | 1563 | (E)-nerolidol | 0 | 0.3 | 0.5 | 0.9 | 0.3 | 0.1 |
| 1577 | 1577 | spathulenol | 0 | 1.2 | 1.1 | 1.3 | 0 | 0 |
| 1582 | 1580 | caryophyllene oxide | 5.7 | 10 | 5.3 | 10.4 | 2.8 | 4.9 |
| 1608 | 1605 | humulene epoxide II | 0 | 0.9 | 0.5 | 1 | 0.2 | 0.5 |
| 1620 | 1624 | dillapiole | 0 | 1 | 0.3 | 1.1 | 0.4 | 0.1 |
| 1627 | 1632 | 1-epi-cubenol | 0 | 1.2 | 0 | 0 | 0 | 0 |
| 1638 | 1640 | epi-α-cadinol | 0 | 1.6 | 1 | 1.1 | 0 | 0 |
| 1639 | 1641 | cariophylla-(12),8(13)-dien-5α-ol | 0 | 0 | 0 | 0 | 0 | 0.3 |
| 1639 | 1642 | cariophylla-(12),8(13)-dien-5β-ol | 0 | 0 | 0 | 0 | 0 | 0.9 |
| 1640 | 1644 | epi-α-muurolol | 0 | 0.6 | 2 | 2.1 | 0.2 | 0 |
| 1644 | 1645 | α-muurolol | 0 | 1.4 | 0 | 0.2 | 0 | 0 |
| 1652 | 1654 | α-cadinol | 0 | 0 | 0.5 | 0 | 0 | 0.1 |
| 1759 | 1763 | benzyl benzoate | 0.3 | 0 | 0.2 | 0 | 0 | 0 |
| Hydrocarbon monoterpenes | 61.9 | 53.9 | 58.9 | 53.0 | 71.9 | 55.8 | ||
| Oxygenated monoterpenes | 22.6 | 8.8 | 9.2 | 3.7 | 4.0 | 12.4 | ||
| Hydrocarbon sesquiterpenes | 3.4 | 17.2 | 14.1 | 21.6 | 14.6 | 13.0 | ||
| Oxygenated sesquiterpenes | 5.7 | 17.2 | 10.9 | 17.0 | 3.5 | 6.8 | ||
| Other Class | 0.3 | 1.2 | 0.5 | 1.4 | 1.0 | 0.3 | ||
| Total | 93.9 | 98.3 | 93.6 | 96.7 | 95.0 | 88.3 |
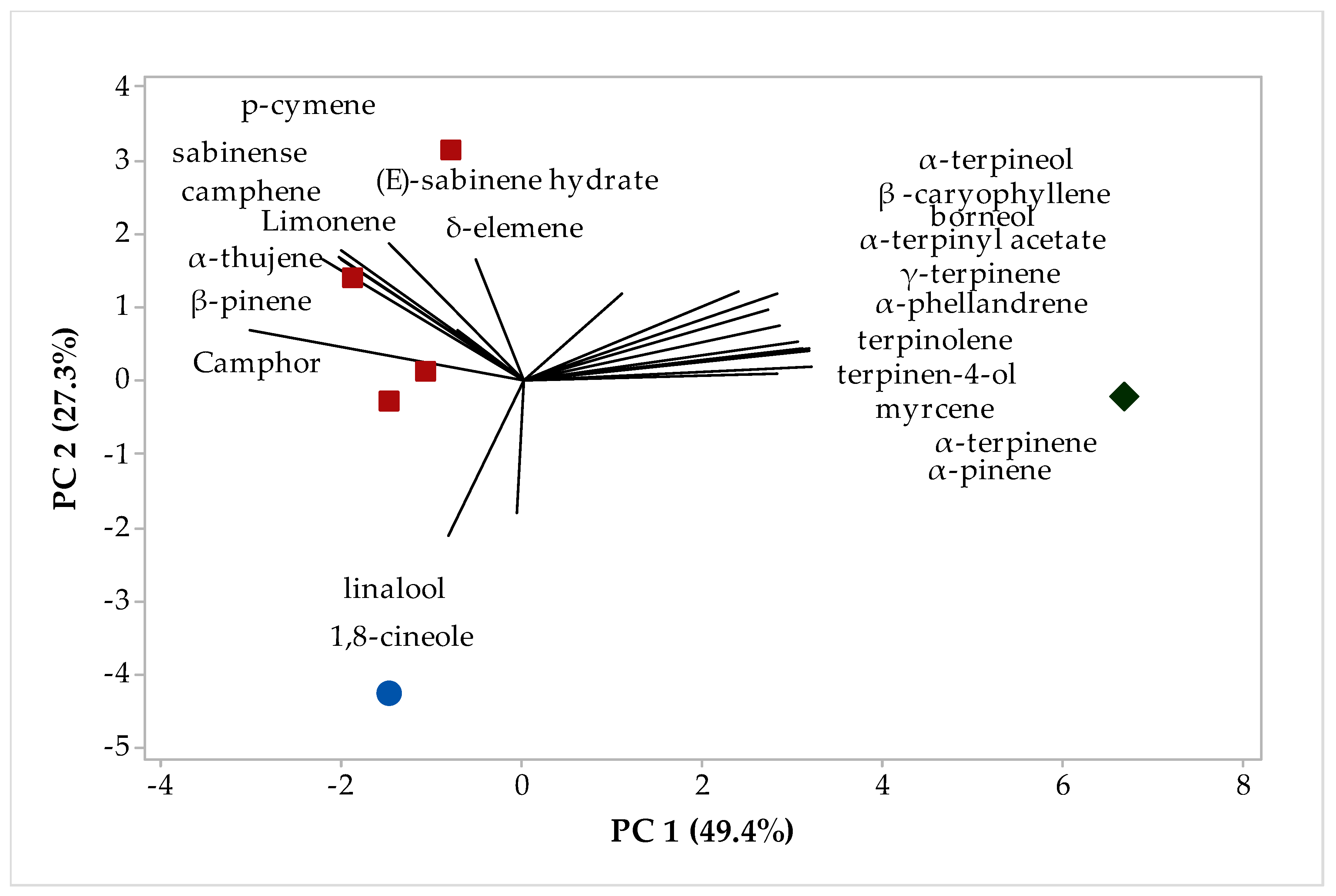
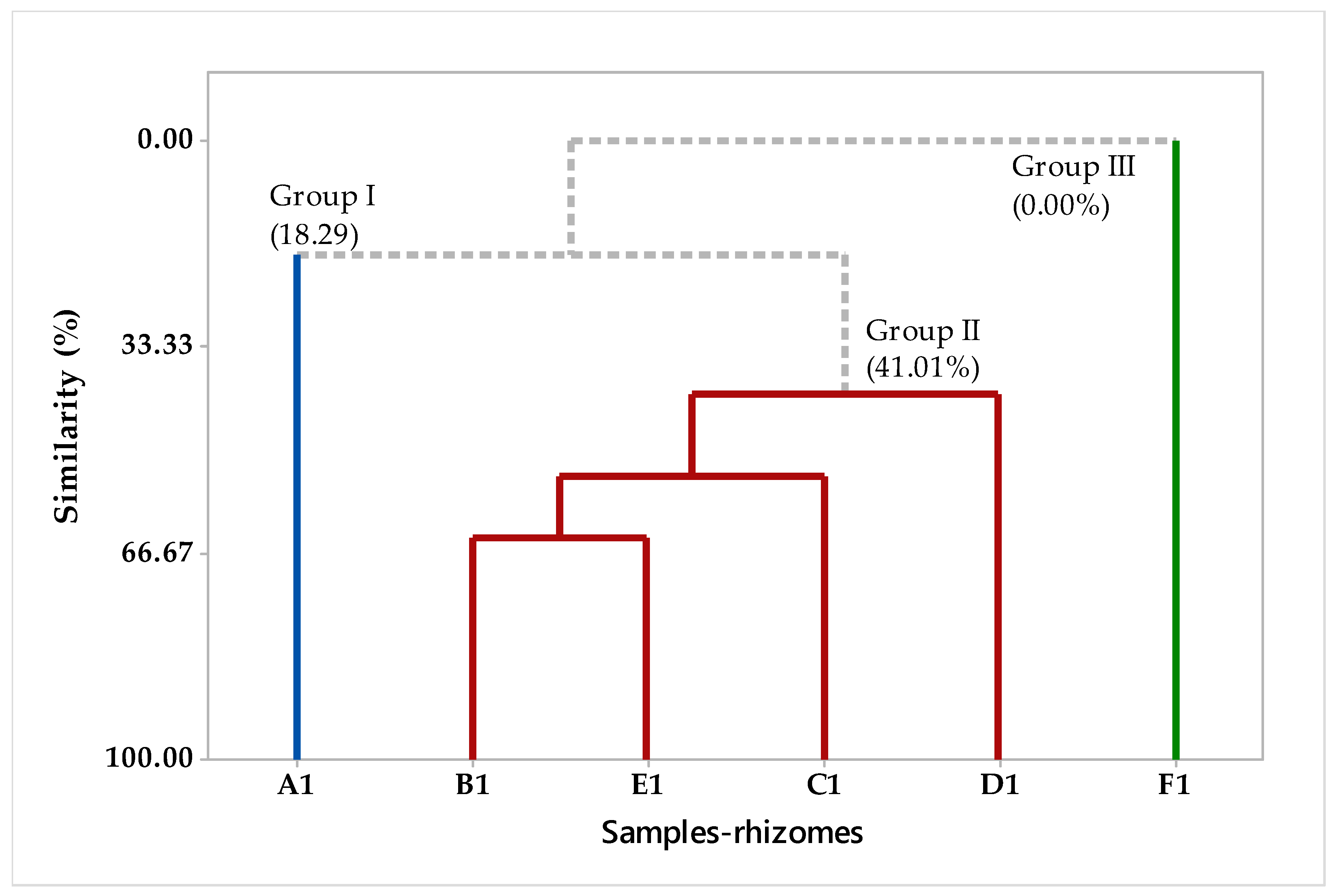
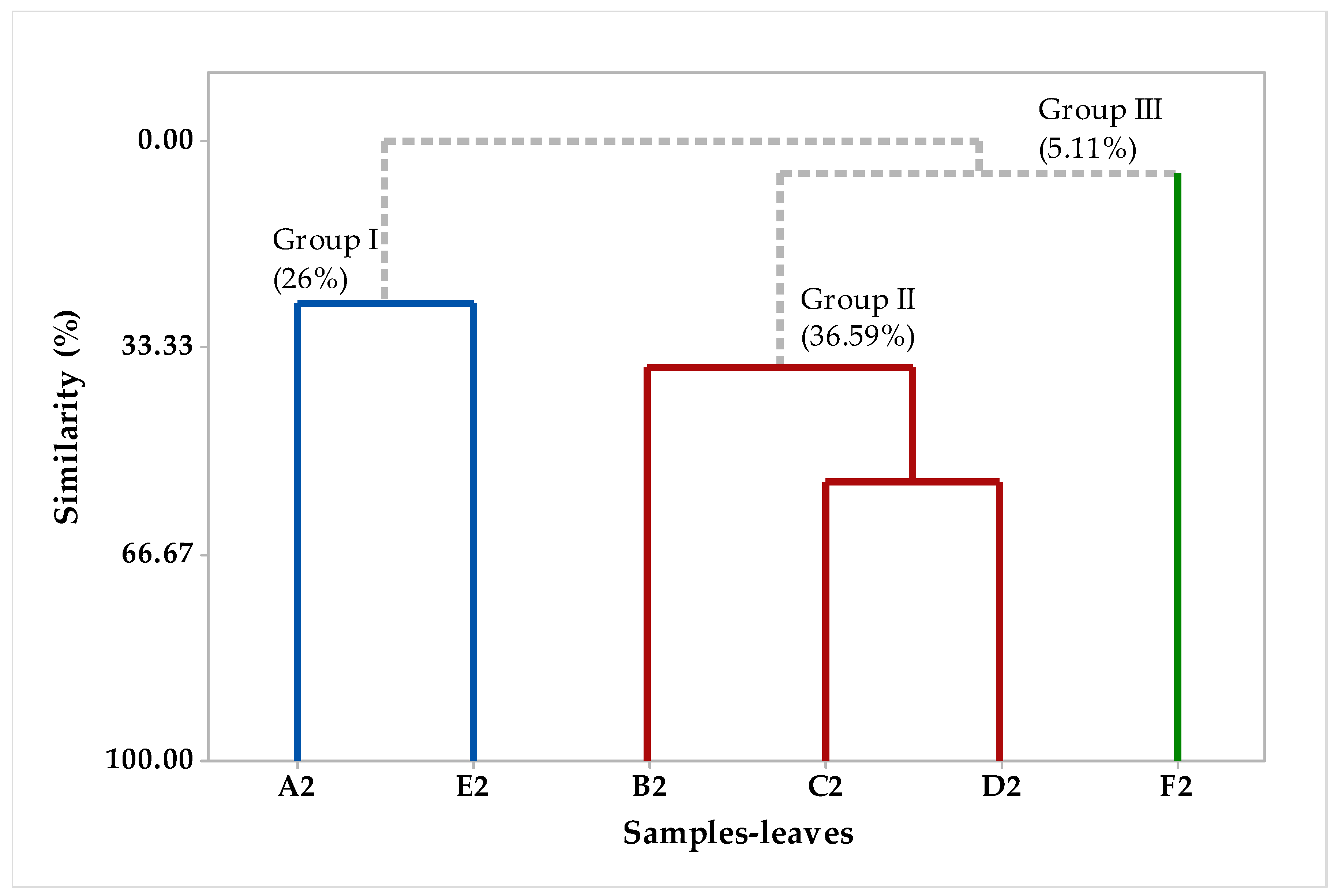
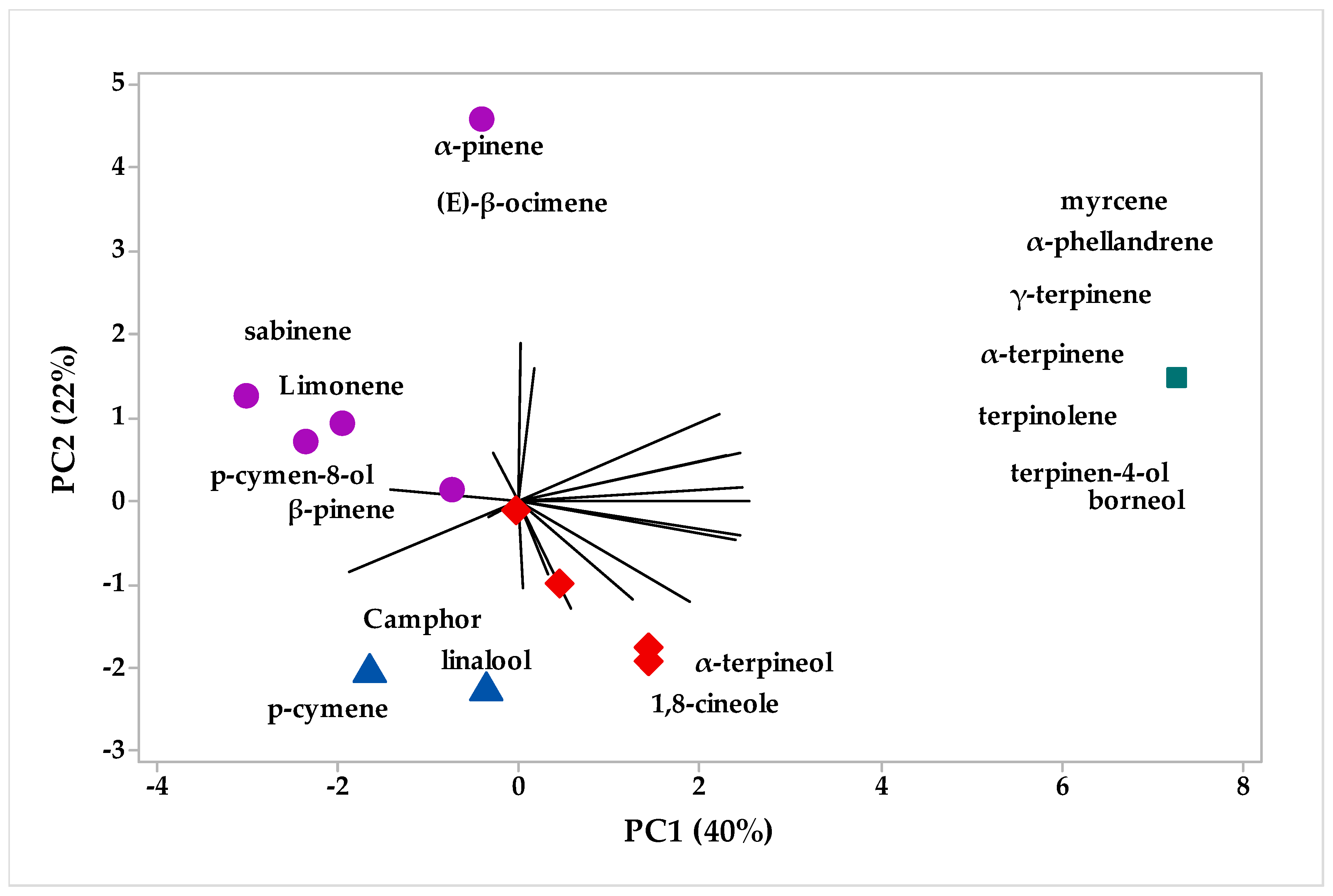
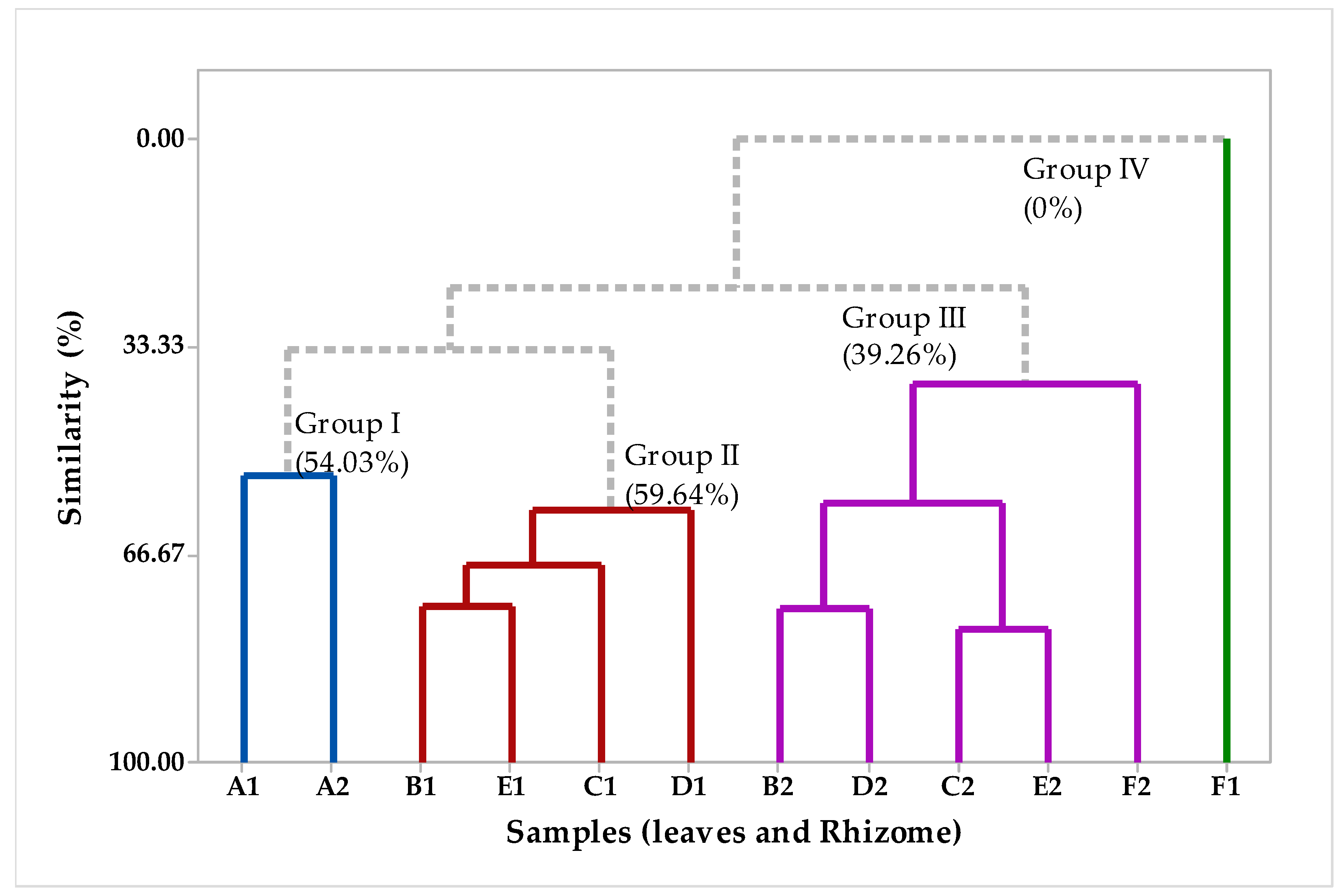
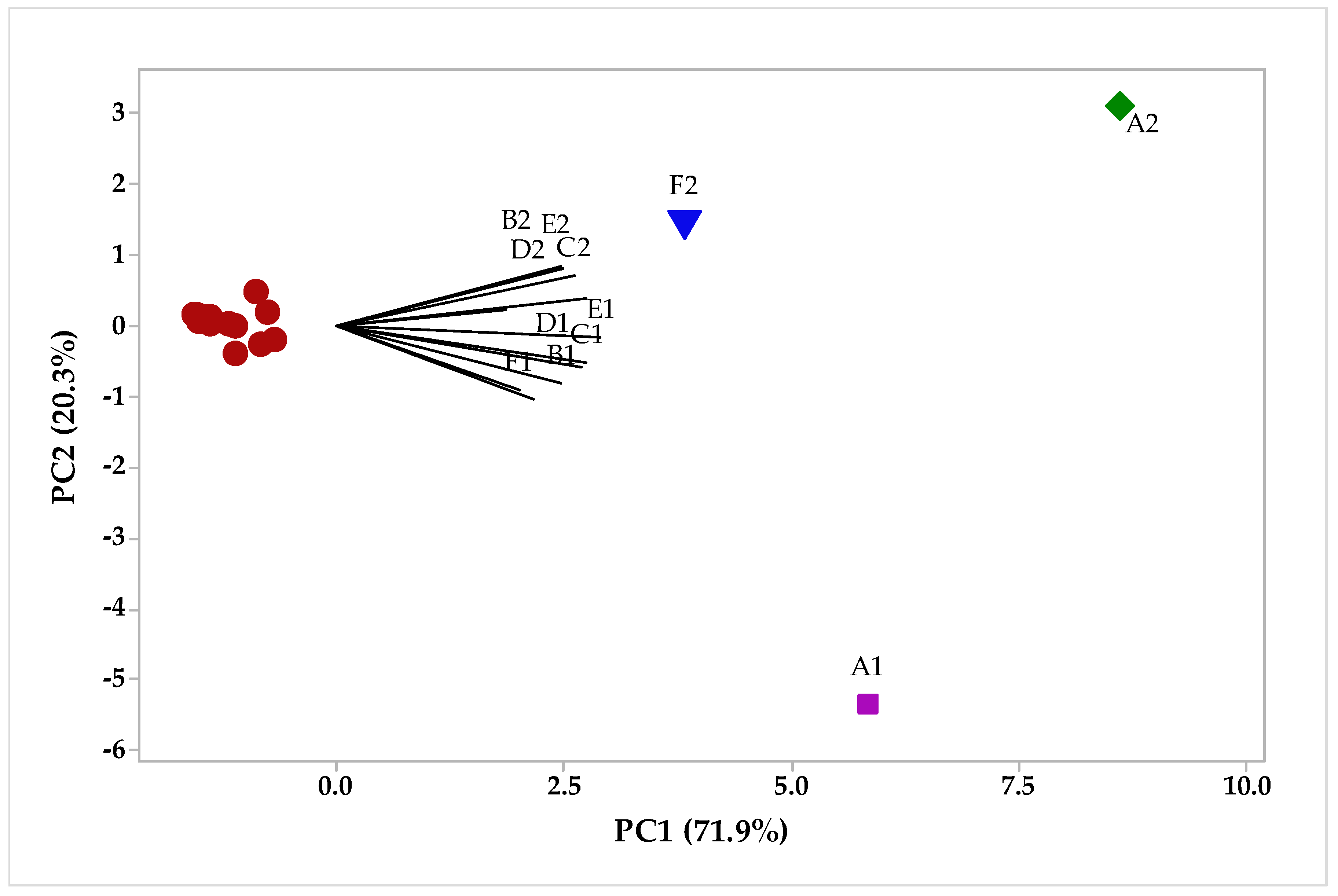
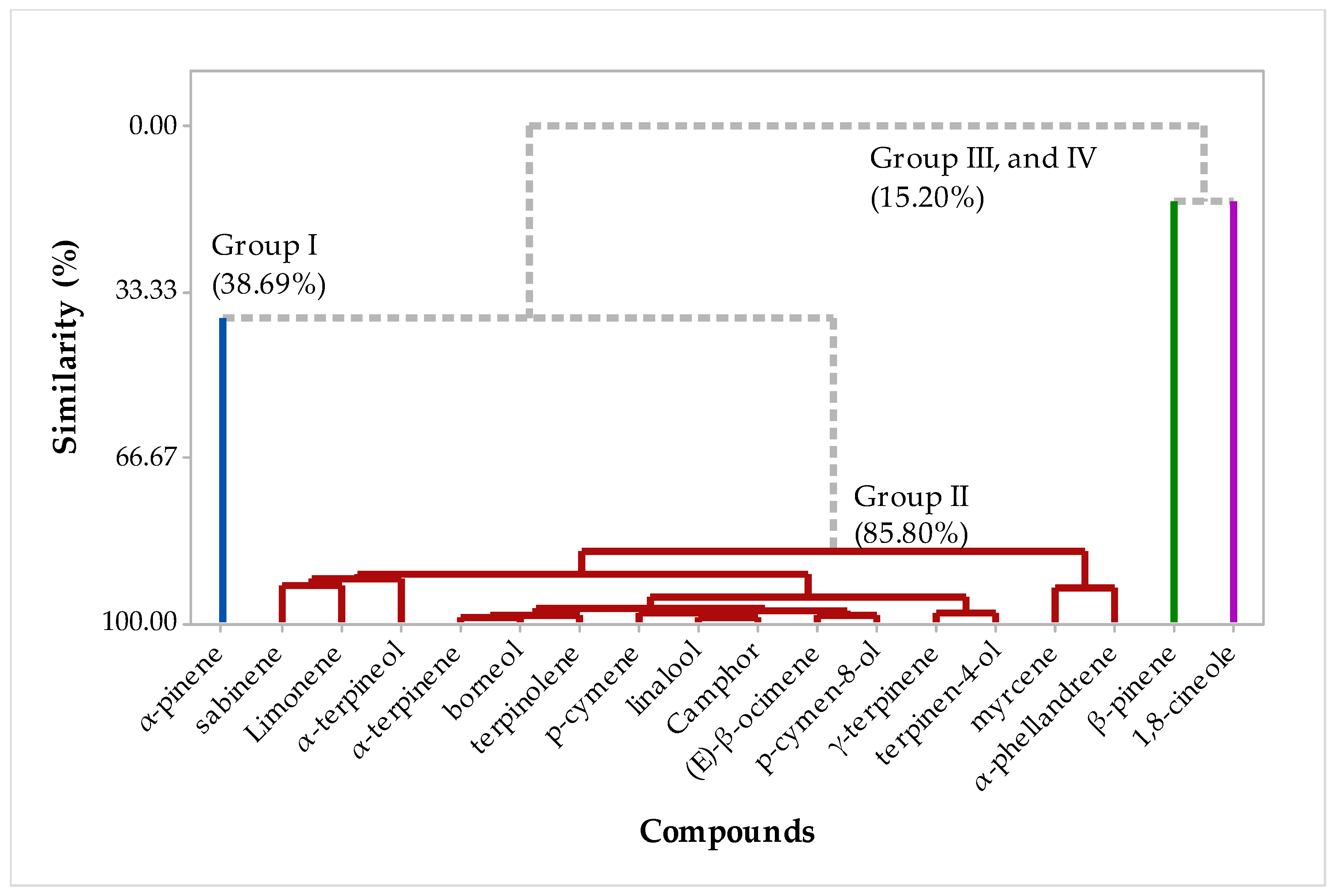
2.2. Multivariate Analysis
2.3. In Silico ADMET Analysis
3. Materials and Methods
3.1. Material
3.2. Preparation of the Botanical Material
3.3. Extraction of Volatile Compounds
3.4. Analysis of the Volatiles
3.5. ADMET Analyses
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cascaes, M.M.; Carneiro, O.D.S.; Do Nascimento, L.D.; de Moraes, Â.A.B.; de Oliveira, M.S.; Cruz, J.N.; Guilhon, G.M.S.P.; Andrade, E.H.D.A. Essential Oils from Annonaceae Species from Brazil: A Systematic Review of Their Phytochemistry, and Biological Activities. Int. J. Mol. Sci. 2021, 22, 12140. [Google Scholar] [CrossRef] [PubMed]
- André, T. Zingiberaceae in Flora e Funga Do Brasil. Jardim Botânico Do Rio de Janeiro. Available online: https://floradobrasil.jbrj.gov.br/FB110730 (accessed on 1 January 2023).
- Muzammil, S.; Neves Cruz, J.; Mumtaz, R.; Rasul, I.; Hayat, S.; Khan, M.A.; Khan, A.M.; Ijaz, M.U.; Lima, R.R.; Zubair, M. Effects of Drying Temperature and Solvents on In Vitro Diabetic Wound Healing Potential of Moringa Oleifera Leaf Extracts; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2023; Volume 28, p. 710. [Google Scholar]
- da Silva Júnior, O.S.; Franco, C.D.J.P.; de Moraes, A.A.B.; Cruz, J.N.; da Costa, K.S.; do Nascimento, L.D.; Andrade, E.H.D.A. In Silico Analyses of Toxicity of the Major Constituents of Essential Oils from Two Ipomoea L. Species. Toxicon 2021, 195, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Wong, S.K. Phytochemistry and Pharmacology of Ornamental Gingers, Hedychium coronarium and Alpinia Purpurata: A Review. J. Integr. Med. 2015, 13, 368–379. [Google Scholar] [CrossRef]
- Simmonds, M.S.J.; van Valkenburg, J.L.C.H.; Bunyapraphatsara, N. Plant Resources of South East Asia No 12 (2) Medicinal and Poisonous Plants 2; Pudoc: Wageningen, The Netherlands, 2002; Volume 57, ISBN 9057820420. [Google Scholar]
- Jäger, S.; Beffert, M.; Hoppe, K.; Nadberezny, D.; Frank, B.; Scheffler, A. Preparation of Herbal Tea as Infusion or by Maceration at Room Temperature Using Mistletoe Tea as an Example. Sci. Pharm. 2011, 79, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhong, C.X.; Wang, L.; Lu, C.; Li, X.L.; Wang, P.J. Anti-Inflammation Activity and Chemical Composition of Flower Essential Oil from Hedychium coronarium. Afr. J. Biotechnol. 2009, 8, 5373–5377. [Google Scholar]
- Ferreira, O.O.; Neves da Cruz, J.; de Jesus Pereira Franco, C.; Silva, S.G.; da Costa, W.A.; de Oliveira, M.S.; de Aguiar Andrade, E.H. First Report on Yield and Chemical Composition of Essential Oil Extracted from Myrcia Eximia DC (Myrtaceae) from the Brazilian Amazon. Molecules 2020, 25, 783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pachurekar, P.; Dixit, A. A Review on Pharmacognostical Phytochemical and Ethnomedicinal Properties of Hedychium coronarium J. Koenig an Endangered Medicine. Int. J. Chin. Med. 2017, 1, 49–61. [Google Scholar] [CrossRef]
- Ferreira, O.O.; Cruz, J.N.; de Moraes, Â.A.B.; Franco, C.D.J.P.; Lima, R.R.; Dos Anjos, T.O.; Siqueira, G.M.; Do Nascimento, L.D.; Cascaes, M.M.; de Oliveira, M.S.; et al. Essential Oil of the Plants Growing in the Brazilian Amazon: Chemical Composition, Antioxidants, and Biological Applications. Molecules 2022, 27, 4373. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Jena, S.; Dash, B.; Kar, B.; Halder, T.; Chatterjee, T.; Ghosh, B.; Panda, P.C.; Nayak, S.; Mahapatra, N. Chemical Diversity, Antioxidant and Antimicrobial Activities of the Essential Oils from Indian Populations of Hedychium coronarium Koen. Ind. Crop. Prod. 2018, 112, 353–362. [Google Scholar] [CrossRef]
- Costa, R.O.; José, C.M.; Grombone-Guaratini, M.T.; Silva Matos, D.M. Chemical Characterization and Phytotoxicity of the Essential Oil from the Invasive Hedychium coronarium on Seeds of Brazilian Riparian Trees. Flora Morphol. Distrib. Funct. Ecol. Plants 2019, 257, 151411. [Google Scholar] [CrossRef]
- Miranda, C.A.S.F.; Cardoso, M.G.; Mansanares, M.E.; Gomes, M.S.; Marcussi, S. Preliminary Assessment of Hedychium coronarium Essential Oil on Fibrinogenolytic and Coagulant Activity Induced by Bothrops and Lachesis Snake Venoms. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 39. [Google Scholar] [CrossRef] [Green Version]
- Do Nascimento, L.D.; de Moraes, A.A.B.; da Costa, K.S.; Galúcio, J.M.P.; Taube, P.S.; Costa, C.M.L.; Cruz, J.N.; Andrade, E.H.d.A.; de Faria, L.J.G. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Noriega, P.; Guerrini, A.; Sacchetti, G.; Grandini, A.; Ankuash, E.; Manfredini, S. Chemical Composition and Biological Activity of Five Essential Oils from the Ecuadorian Amazon Rain Forest. Molecules 2019, 24, 1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miguel, M.G. Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rout, S.; Tambe, S.; Deshmukh, R.K.; Mali, S.; Cruz, J.; Srivastav, P.P.; Amin, P.D.; Gaikwad, K.K.; Andrade, E.H.D.A.; Oliveira, M.S.D. Recent Trends in the Application of Essential Oils: The next Generation of Food Preservation and Food Packaging. Trends Food Sci. Technol. 2022, 129, 421–439. [Google Scholar] [CrossRef]
- Ho, J.C. Antimicrobial, Mosquito Larvicidal and Antioxidant Properties of the Leaf and Rhizome of Hedychium coronarium. J. Chin. Chem. Soc. 2011, 58, 563–567. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.S.; Costa Junior, H.N.P.; Costa-Junior, L.M.; Monteiro, O.S.; Maia, J.G.S.; da Rocha, C.Q. Anthelmintic Effect of Essential Rhizome Oil from Hedychium coronarium Koenig (Zingiberaceae) Introduced in Northeastern Brazil. Acta Trop. 2021, 218, 105912. [Google Scholar] [CrossRef]
- Figueiredo, P.L.B.; Pinto, L.C.; da Costa, J.S.; da Silva, A.R.C.; Mourão, R.H.V.; Montenegro, R.C.; da Silva, J.K.R.; Maia, J.G.S. Composition, Antioxidant Capacity and Cytotoxic Activity of Eugenia Uniflora L. Chemotype-Oils from the Amazon. J. Ethnopharmacol. 2019, 232, 30–38. [Google Scholar] [CrossRef]
- Liao, Z.; Huang, Q.; Cheng, Q.; Khan, S.; Yu, X. Seasonal Variation in Chemical Compositions of Essential Oils Extracted from Lavandin Flowers in the Yun-Gui Plateau of China. Molecules 2021, 26, 5639. [Google Scholar] [CrossRef]
- Feitosa-Alcantara, R.B.; Bacci, L.; Blank, A.F.; Alves, P.B.; Silva, I.M.D.A.; Soares, C.A.; Sampaio, T.S.; Nogueira, P.C.D.L.; Arrigoni-Blank, M.D.F. Essential Oils of Hyptis Pectinata Chemotypes: Isolation, Binary Mixtures and Acute Toxicity on Leaf-Cutting Ants. Molecules 2017, 22, 621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Cruz, E.D.N.S.; Peixoto, L.D.S.; da Costa, J.S.; Mourão, R.H.V.; Do Nascimento, W.M.O.; Maia, J.G.S.; Setzer, W.N.; da Silva, J.K.; Figueiredo, P.L.B. Seasonal Variability of a Caryophyllane Chemotype Essential Oil of Eugenia Patrisii Vahl Occurring in the Brazilian Amazon. Molecules 2022, 27, 2417. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.L.; Cury, J.A.; Rosalen, P.L.; Alencar, S.M.; Ikegaki, M.; Duarte, S.; Koo, H. Própolis Do Sudeste e Nordeste Do Brasil: Influência Da Sazonalidade Na Atividade Antibacteriana e Composição Fenólica. Quim. Nova 2007, 30, 1512–1516. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.G.; Figueiredo, P.L.B.; Nascimento, L.D.; Costa, W.A.; Maia, J.G.S.; Andrade, E.H.A. Planting and Seasonal and Circadian Evaluation of a Thymol-Type Oil from Lippia Thymoides Mart. & Schauer. Chem. Cent. J. 2018, 12, 113. [Google Scholar] [CrossRef]
- Ray, A.; Dash, B.; Sahoo, A.; Nasim, N.; Panda, P.C.; Patnaik, J.; Ghosh, B.; Nayak, S.; Kar, B. Assessment of the Terpenic Composition of Hedychium coronarium Oil from Eastern India. Ind. Crop. Prod. 2017, 97, 49–55. [Google Scholar] [CrossRef]
- Prakash, O.; Rajput, M.; Kumar, M.; Pant, A.K. Chemical Composition and Antibacterial Activity of Rhizome Oils from Hedychium coronarium Koenig and Hedychium Spicatum Buch-Ham. J. Essent. Oil-Bear. Plants 2010, 13, 250–259. [Google Scholar] [CrossRef]
- Van Thanh, B.; Dai, D.N.; Thang, T.D.; Binh, N.Q.; Anh, L.D.N.; Ogunwande, I.A. Composition of Essential Oils of Four Hedychium Species from Vietnam. Chem. Cent. J. 2014, 8, 54. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, D.F.; Arenas Velásquez, A.M.; Cavaleiro, C.; Salgueiro, L.; Martins, G.Z.; Magalhães, N.O.; Martins, M.B.G.; Cicarelli, R.M.B.; Moreira, R.R.D. Chemical Composition and Trypanocidal Activity of the Essential Oils from Hedychium coronarium J. Koenig (Zingiberaceae). ISRN Infect. Dis. 2013, 2013, 639275. [Google Scholar] [CrossRef] [Green Version]
- Murata, S.; Shiragami, R.; Kosugi, C.; Tezuka, T.; Yamazaki, M.; Hirano, A.; Yoshimura, Y.; Suzuki, M.; Shuto, K.; Ohkohchi, N.; et al. Antitumor Effect of 1, 8-Cineole against Colon Cancer. Oncol. Rep. 2013, 30, 2647–2652. [Google Scholar] [CrossRef] [Green Version]
- Vilela, G.R.; de Almeida, G.S.; D’Arce, M.A.B.R.; Moraes, M.H.D.; Brito, J.O.; da Silva, M.F.D.G.F.; Silva, S.C.; de Stefano Piedade, S.M.; Calori-Domingues, M.A.; da Gloria, E.M. Activity of Essential Oil and Its Major Compound, 1,8-Cineole, from Eucalyptus Globulus Labill., against the Storage Fungi Aspergillus Flavus Link and Aspergillus Parasiticus Speare. J. Stored Prod. Res. 2009, 45, 108–111. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joy, B.; Rajan, A.; Abraham, E. Antimicrobial Activity and Chemical Composition of Essential Oil from Hedychium coronarium. Phytother. Res. 2007, 21, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.R.; De Melo, T.S.; Carvalho, K.M.M.B.; De Oliveira, Í.B.; Arruda, B.R.; De Castro Brito, G.A.; Rao, V.S.; Santos, F.A. 1,8-Cineole (Eucalyptol) Ameliorates Cerulein-Induced Acute Pancreatitis via Modulation of Cytokines, Oxidative Stress and NF-ΚB Activity in Mice. Life Sci. 2013, 92, 1195–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Z.M.; Peng, J.Q.; Chen, Y.; Tao, L.; Zhang, Y.Y.; Fu, L.Y.; Long, Q.D.; Shen, X.C. 1,8-Cineole: A Review of Source, Biological Activities, and Application. J. Asian Nat. Prod. Res. 2021, 23, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Baghalpour, N.; Cho, W.C.; Sharifi-Rad, J. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [Green Version]
- Magwa, M.L.; Gundidza, M.; Gweru, N.; Humphrey, G. Chemical Composition and Biological Activities of Essential Oil from the Leaves of Sesuvium Portulacastrum. J. Ethnopharmacol. 2006, 103, 85–89. [Google Scholar] [CrossRef]
- Kovač, J.; Šimunović, K.; Wu, Z.; Klančnik, A.; Bucar, F.; Zhang, Q.; Možina, S.S. Antibiotic Resistance Modulation and Modes of Action of (-)-α-Pinene in Campylobacter Jejuni. PLoS ONE 2015, 10, e0122871. [Google Scholar] [CrossRef] [Green Version]
- Weston-Green, K.; Clunas, H.; Jimenez Naranjo, C. A Review of the Potential Use of Pinene and Linalool as Terpene-Based Medicines for Brain Health: Discovering Novel Therapeutics in the Flavours and Fragrances of Cannabis. Front. Psychiatry 2021, 12, 583211. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. Β-Caryophyllene and Β-Caryophyllene Oxide—Natural Compounds of Anticancer and Analgesic Properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Ghelardini, C.; Galeotti, N.; Di Cesare Mannelli, L.; Mazzanti, G.; Bartolini, A. Local Anaesthetic Activity of β-Caryophyllene. Farmaco 2001, 56, 387–389. [Google Scholar] [CrossRef]
- Legault, J.; Pichette, A. Potentiating Effect of β-Caryophyllene on Anticancer Activity of α-Humulene, Isocaryophyllene and Paclitaxel. J. Pharm. Pharmacol. 2010, 59, 1643–1647. [Google Scholar] [CrossRef]
- Scandiffio, R.; Geddo, F.; Cottone, E.; Querio, G.; Antoniotti, S.; Pia Gallo, M.; Maffei, M.E.; Bovolin, P. Protective Effects of (E)-β-Caryophyllene (Bcp) in Chronic Inflammation. Nutrients 2020, 12, 3273. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.C.D.; Oliveira, C.V.D.; Grigoletto, J.; Ribeiro, L.R.; Funck, V.R.; Grauncke, A.C.B.; Souza, T.L.D.; Souto, N.S.; Furian, A.F.; Menezes, I.R.A.; et al. Anticonvulsant Activity of β-Caryophyllene against Pentylenetetrazol-Induced Seizures. Epilepsy Behav. 2016, 56, 26–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, N.J.; Mosaddik, A.; Moon, J.Y.; Jang, K.C.; Lee, D.S.; Ahn, K.S.; Cho, S.K. Cytotoxic Activity of β-Caryophyllene Oxide Isolated from Jeju Guava (Psidium Cattleianum Sabine) Leaf. Rec. Nat. Prod. 2011, 5, 242–246. [Google Scholar]
- Tung, Y.T.; Chua, M.T.; Wang, S.Y.; Chang, S.T. Anti-Inflammation Activities of Essential Oil and Its Constituents from Indigenous Cinnamon (Cinnamomum Osmophloeum) Twigs. Bioresour. Technol. 2008, 99, 3908–3913. [Google Scholar] [CrossRef] [PubMed]
- Bartikova, H.; Hanusova, V.; Skalova, L.; Ambroz, M.; Bousova, I. Antioxidant, Pro-Oxidant and Other Biological Activities of Sesquiterpenes. Curr. Top. Med. Chem. 2014, 14, 2478–2494. [Google Scholar] [CrossRef]
- Hammami, S.; Jmii, H.; Mokni, R.E.; Khmiri, A.; Faidi, K.; Dhaouadi, H.; Aouni, M.H.E.; Aouni, M.; Joshi, R.K. Antiviral Activities of Teucrium Pseudochamaepitys Growing Spontaneously in Tunisia. Molecules 2015, 20, 20426–20433. [Google Scholar] [CrossRef] [Green Version]
- Singh, T.P.; Singh, R.K.; Malik, P. Analgesic and Anti-Inflammatory Activities of Annona Squamosa Linn Bark. J. Sci. Innov. Res. 2014, 3, 60–64. [Google Scholar] [CrossRef]
- Adams, R.P.; Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Adams, R.P., Ed.; Allured Pub Corp: Carol Stream, IL, USA, 2017; Volume 8, ISBN 1932633219. [Google Scholar]
- de Oliveira, M.S.; Cruz, J.N.; Ferreira, O.O.; Pereira, D.S.; Pereira, N.S.; Oliveira, M.E.C.; Venturieri, G.C.; Guilhon, G.M.S.P.; Souza Filho, A.P.D.S.; Andrade, E.H.D.A. Chemical Composition of Volatile Compounds in Apis Mellifera Propolis from the Northeast Region of Pará State, Brazil. Molecules 2021, 26, 3462. [Google Scholar] [CrossRef]
- Lima, A.R.J.A.D.M.; Siqueira, A.S.; Möller, M.L.S.; Souza, R.C.D.; Cruz, J.N.; Lima, A.R.J.A.D.M.; Silva, R.C.D.; Aguiar, D.C.F.; Junior, J.L.D.S.G.V.; Gonçalves, E.C. In Silico Improvement of the Cyanobacterial Lectin Microvirin and Mannose Interaction. J. Biomol. Struct. Dyn. 2020, 40, 1064–1073. [Google Scholar] [CrossRef]
- Almeida, V.M.; Dias, Ê.R.; Souza, B.C.; Cruz, J.N.; Santos, C.B.R.; Leite, F.H.A.; Queiroz, R.F.; Branco, A. Methoxylated Flavonols from Vellozia Dasypus Seub Ethyl Acetate Active Myeloperoxidase Extract: In Vitro and in Silico Assays. J. Biomol. Struct. Dyn. 2022, 40, 7574–7583. [Google Scholar] [CrossRef] [PubMed]
- Rego, C.M.A.; Francisco, A.F.; Boeno, C.N.; Paloschi, M.V.; Lopes, J.A.; Silva, M.D.S.; Santana, H.M.; Serrath, S.N.; Rodrigues, J.E.; Lemos, C.T.L.; et al. Inflammasome NLRP3 Activation Induced by Convulxin, a C-Type Lectin-like Isolated from Crotalus Durissus Terrificus Snake Venom. Sci. Rep. 2022, 12, 4706. [Google Scholar] [CrossRef] [PubMed]
- Pandya, C.V.; Jadeja, A.J.; Golakiya, B.A. Antifungal Activity of Crude Extracts of Hedychium coronarium. Int. J. Res. Phytochem. Pharmacol. 2014, 4, 4–6. [Google Scholar]
- Pollastri, M.P. Overview on the Rule of Five. Curr. Protoc. Pharmacol. 2010, 49, 9.12.1–9.12.8. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.; Mahawer, S.K.; Karakoti, H.; Kumar, R.; Prakash, O.; Kumar, S.; Latwal, M.; Panday, G.; Srivastava, R.M.; de Oliveira, M.S.; et al. Study of the Variability of the Chemical Profile, and Biological Activity Approaches of Hedychium coronarium J. Koenig Essential Oil from Different Habitats of Uttarakhand, India. J. Food Qual. 2023, 2023, 7335134. [Google Scholar] [CrossRef]
- Franco, C.D.J.P.; Ferreira, O.O.; Cruz, J.N.; Varela, E.L.P.; de Moraes, Â.A.B.; Nascimento, L.D.D.; Cascaes, M.M.; Souza Filho, A.P.D.S.; Lima, R.R.; Percário, S.; et al. Phytochemical Profile and Herbicidal (Phytotoxic), Antioxidants Potential of Essential Oils from Calycolpus Goetheanus (Myrtaceae) Specimens, and in Silico Study. Molecules 2022, 27, 4678. [Google Scholar] [CrossRef]
- van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Santos, C.B.R.; Santos, K.L.B.; Cruz, J.N.; Leite, F.H.A.; Borges, R.S.; Taft, C.A.; Campos, J.M.; Silva, C.H.T.P. Molecular Modeling Approaches of Selective Adenosine Receptor Type 2A Agonists as Potential Anti-Inflammatory Drugs. J. Biomol. Struct. Dyn. 2021, 39, 3115–3127. [Google Scholar] [CrossRef]
- Galucio, N.C.D.R.; Moysés, D.D.A.; Pina, J.R.S.; Marinho, P.S.B.; Gomes Júnior, P.C.; Cruz, J.N.; Vale, V.V.; Khayat, A.S.; Marinho, A.M.D.R. Antiproliferative, Genotoxic Activities and Quantification of Extracts and Cucurbitacin B Obtained from Luffa Operculata (L.) Cogn. Arab. J. Chem. 2022, 15, 103589. [Google Scholar] [CrossRef]
- Santana de Oliveira, M.; Pereira da Silva, V.M.; Cantão Freitas, L.; Gomes Silva, S.; Nevez Cruz, J.; de Aguiar Andrade, E.H. Extraction Yield, Chemical Composition, Preliminary Toxicity of Bignonia Nocturna (Bignoniaceae) Essential Oil and in Silico Evaluation of the Interaction. Chem. Biodivers 2021, 18, cbdv.202000982. [Google Scholar] [CrossRef]
- Mali, S.N.; Pandey, A. Balanced QSAR and Molecular Modeling to Identify Structural Requirements of Imidazopyridine Analogues as Anti-Infective Agents Against Trypanosomiases. J. Comput. Biophys. Chem. 2022, 21, 83–114. [Google Scholar] [CrossRef]

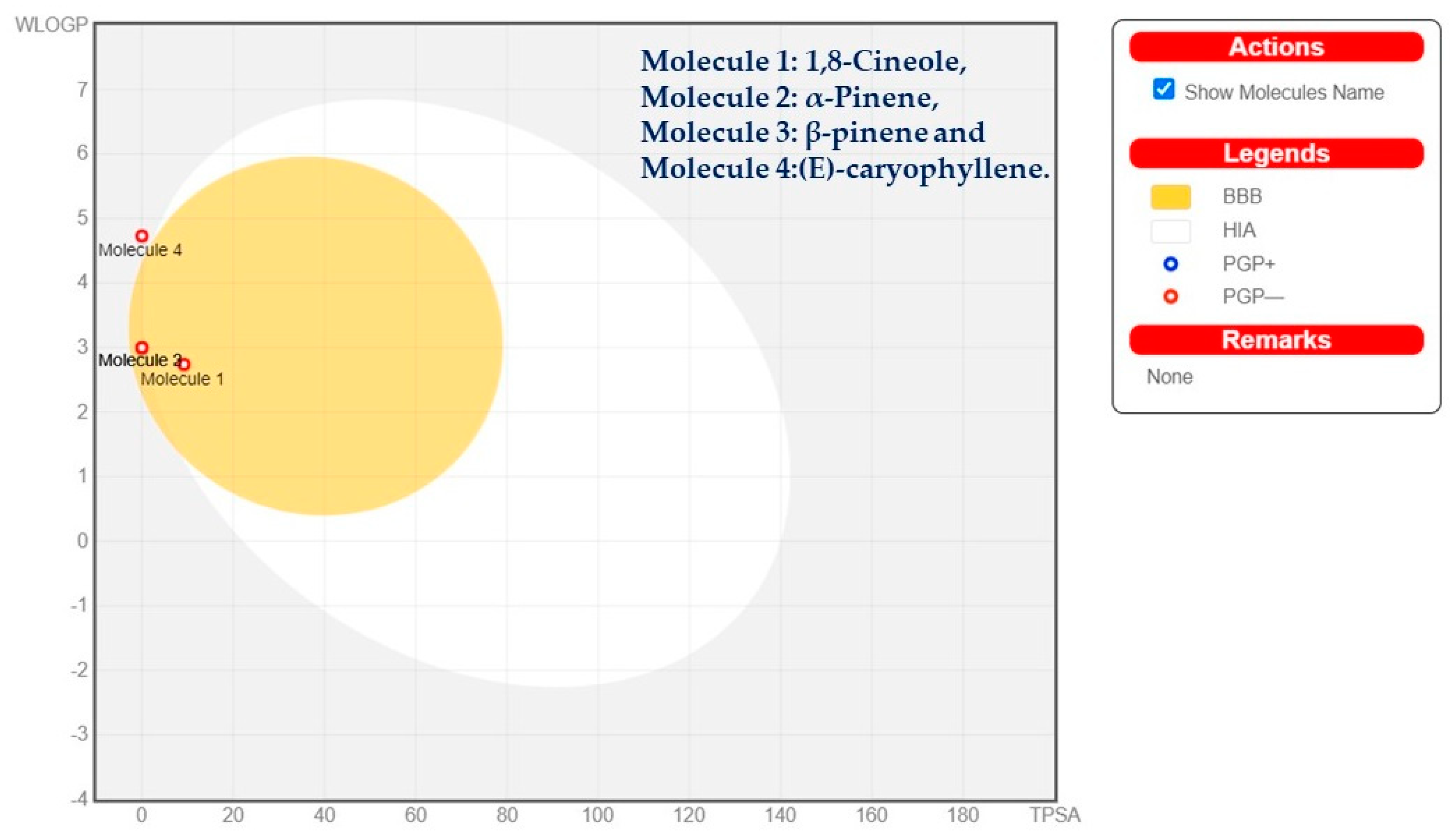

| Constituents | 1,8-Cineole | α-Pinene | β-Pinene | (E)-Caryophyllene |
|---|---|---|---|---|
| TPSA * (Å2) | 9.23 | 0.00 | 0.00 | 0.00 |
| Consensus log Po/w | 2.67 | 3.44 | 3.44 | 4.24 |
| Mol wt. (g/mol) | 154.25 | 136.23 | 136.23 | 204.35 |
| nRB | 0 | 0 | 0 | 0 |
| nOHA | 1 | 0 | 0 | 0 |
| nOND | 0 | 0 | 0 | 0 |
| WLOGP | 2.74 | 3.00 | 3.00 | 4.73 |
| Water solubility | Soluble | Soluble # | Soluble # | Soluble # |
| GI absorption ** | High | Low | Low | Low |
| BBB permeant ** | Yes | Yes | Yes | No |
| P-gp substrate ** | No | No | No | No |
| CYP1A2 inhibitor ** | No | No | No | No |
| CYP2C19 inhibitor ** | No | No | No | Yes |
| CYP2C9 inhibitor ** | No | Yes | Yes | Yes |
| CYP2D6 inhibitor ** | No | No | No | No |
| CYP3A4 inhibitor | No | No | No | No |
| Log Kp (cm/s) (skin permeation) | −5.30 | −3.95 | −3.95 | −4.44 |
| Lipinski *** | Yes | Yes | Yes | Yes |
| Lipinski violation | 0 | 1 | 1 | 1 |
| Bioavailability score *** | 0.55 | 0.55 | 0.55 | 0.55 |
| Hepatotoxicity **** | No | No | No | No |
| Carcinogenicity **** | No | No | No | No |
| Cytotoxicity **** | No | No | No | No |
| Immunotoxicity **** | No | No | No | Yes |
| Mutagenicity **** | No | No | No | No |
| Predicted **** LD50 (mg/kg) | 2480 | 3700 | 3700 | 5300 |
| Toxicity class **** | V | V | V | V |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, J.N.; Oliveira, M.S.d.; Cascaes, M.; Mali, S.N.; Tambe, S.; Santos, C.B.R.d.; Zoghbi, M.d.G.B.; Andrade, E.H.d.A. Variation in the Chemical Composition of Endemic Specimens of Hedychium coronarium J. Koenig from the Amazon and In Silico Investigation of the ADME/Tox Properties of the Major Compounds. Plants 2023, 12, 2626. https://doi.org/10.3390/plants12142626
Cruz JN, Oliveira MSd, Cascaes M, Mali SN, Tambe S, Santos CBRd, Zoghbi MdGB, Andrade EHdA. Variation in the Chemical Composition of Endemic Specimens of Hedychium coronarium J. Koenig from the Amazon and In Silico Investigation of the ADME/Tox Properties of the Major Compounds. Plants. 2023; 12(14):2626. https://doi.org/10.3390/plants12142626
Chicago/Turabian StyleCruz, Jorddy Neves, Mozaniel Santana de Oliveira, Marcia Cascaes, Suraj N. Mali, Srushti Tambe, Cleydson Breno Rodrigues dos Santos, Maria das Graças Bichara Zoghbi, and Eloisa Helena de Aguiar Andrade. 2023. "Variation in the Chemical Composition of Endemic Specimens of Hedychium coronarium J. Koenig from the Amazon and In Silico Investigation of the ADME/Tox Properties of the Major Compounds" Plants 12, no. 14: 2626. https://doi.org/10.3390/plants12142626











