Topography of UV-Melanized Thalli of Lobaria pulmonaria (L.) Hoffm
Abstract
:1. Introduction
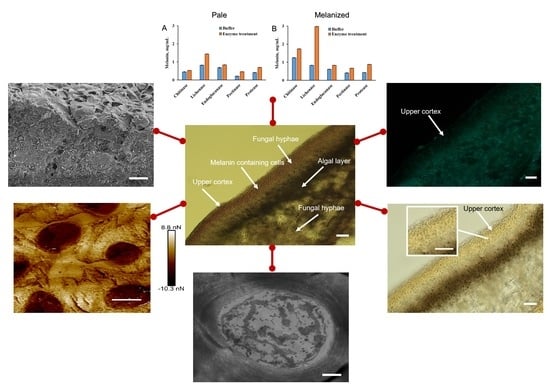
2. Results
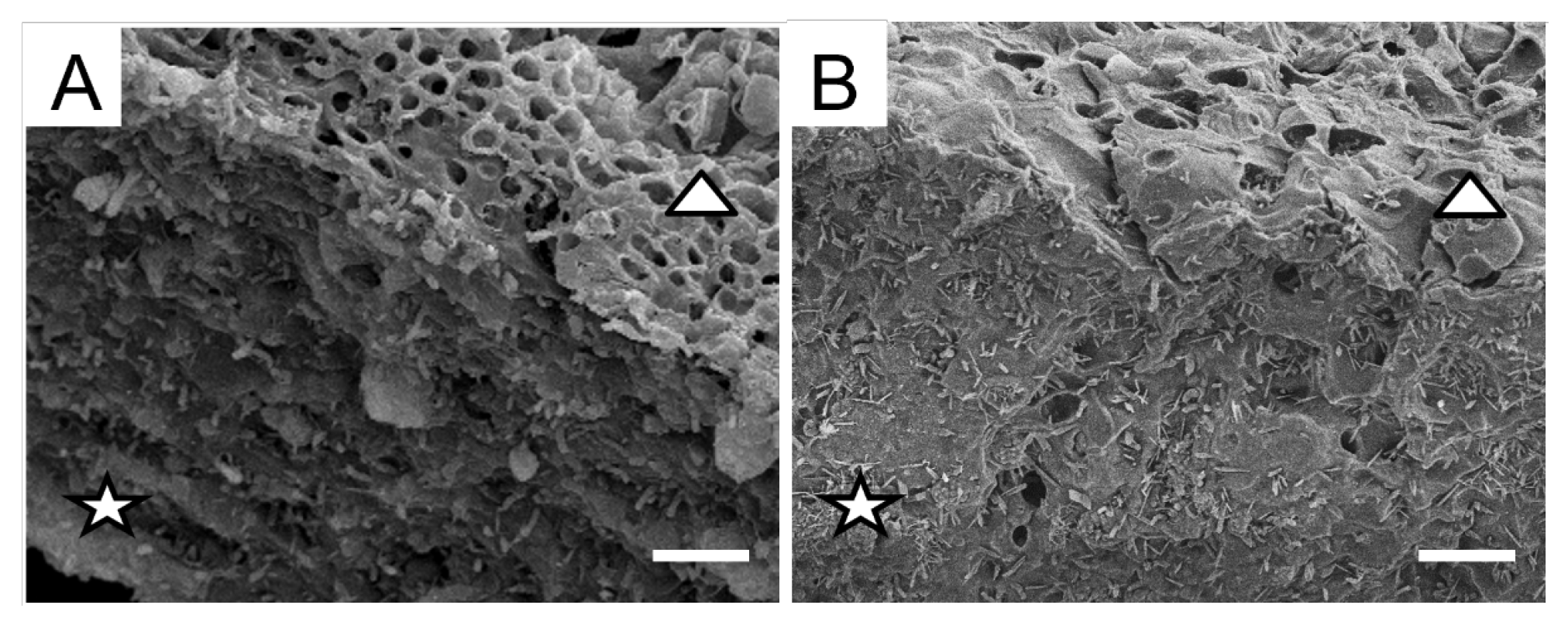
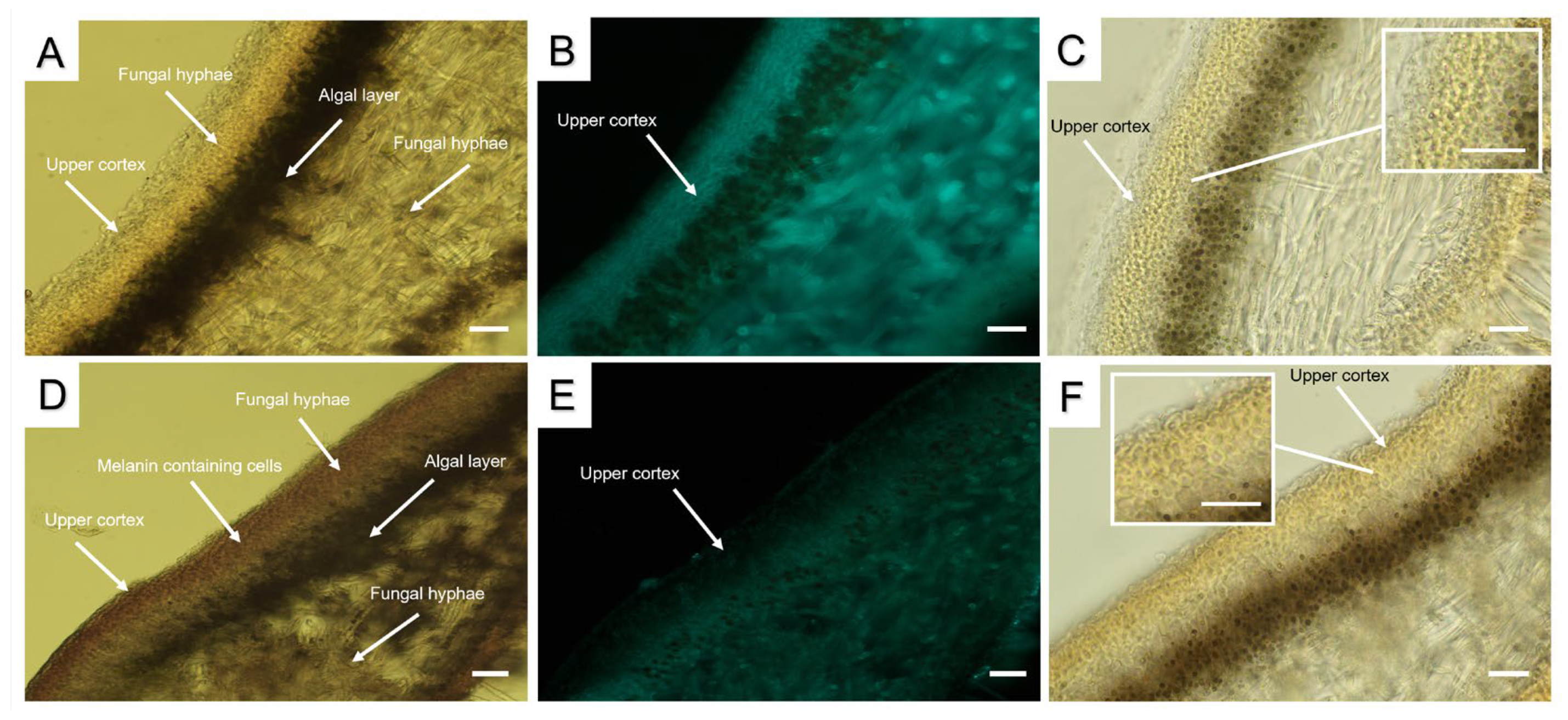
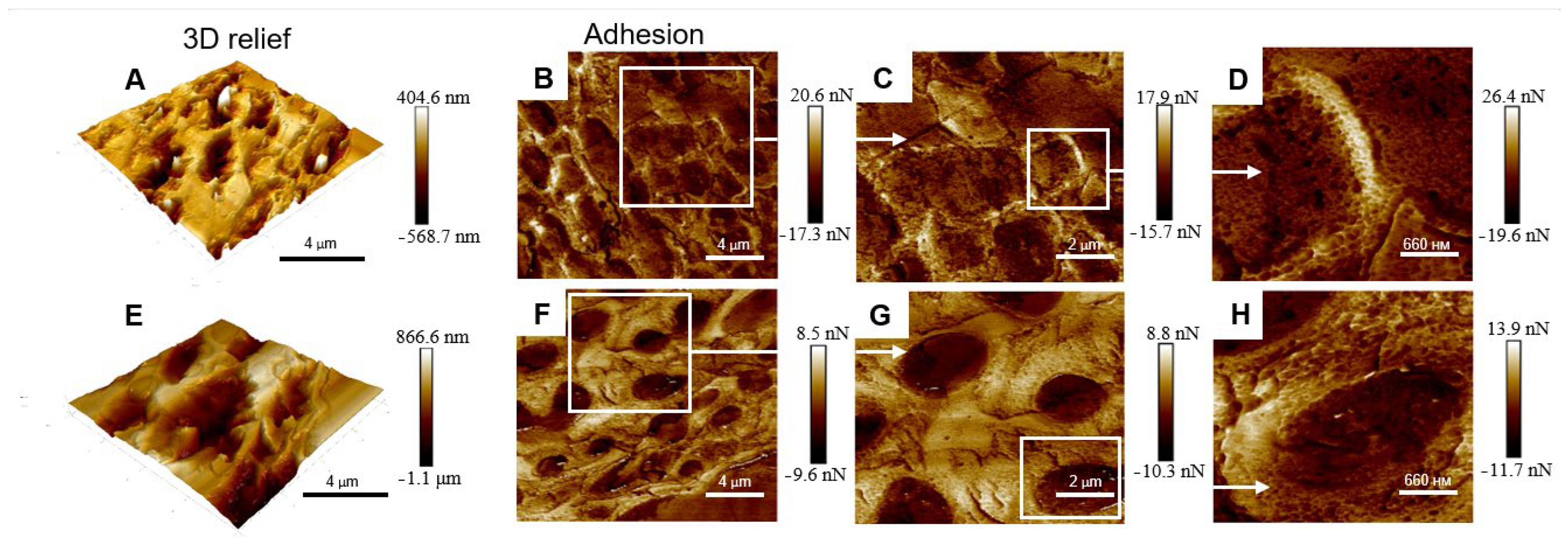
2.1. Morphology
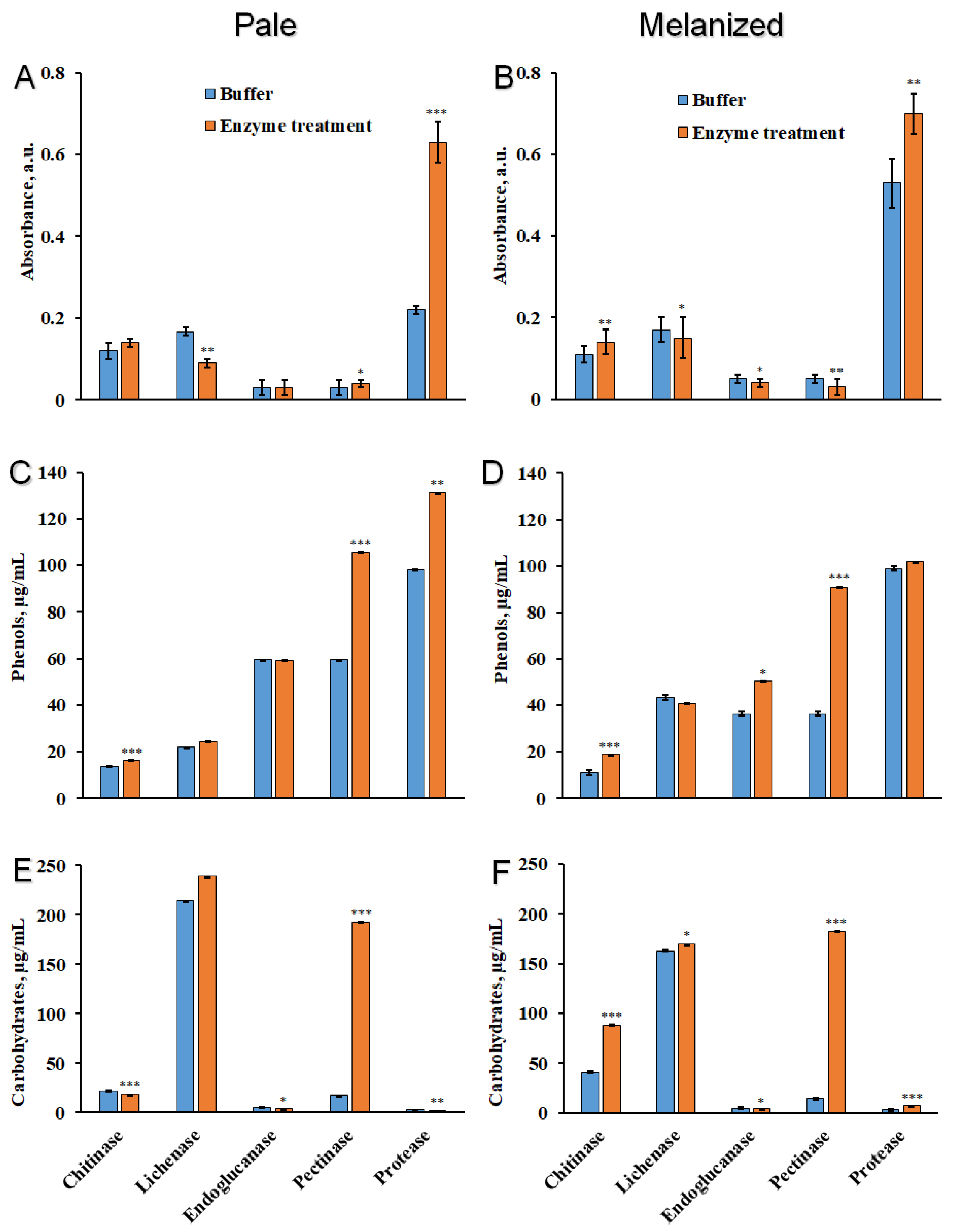
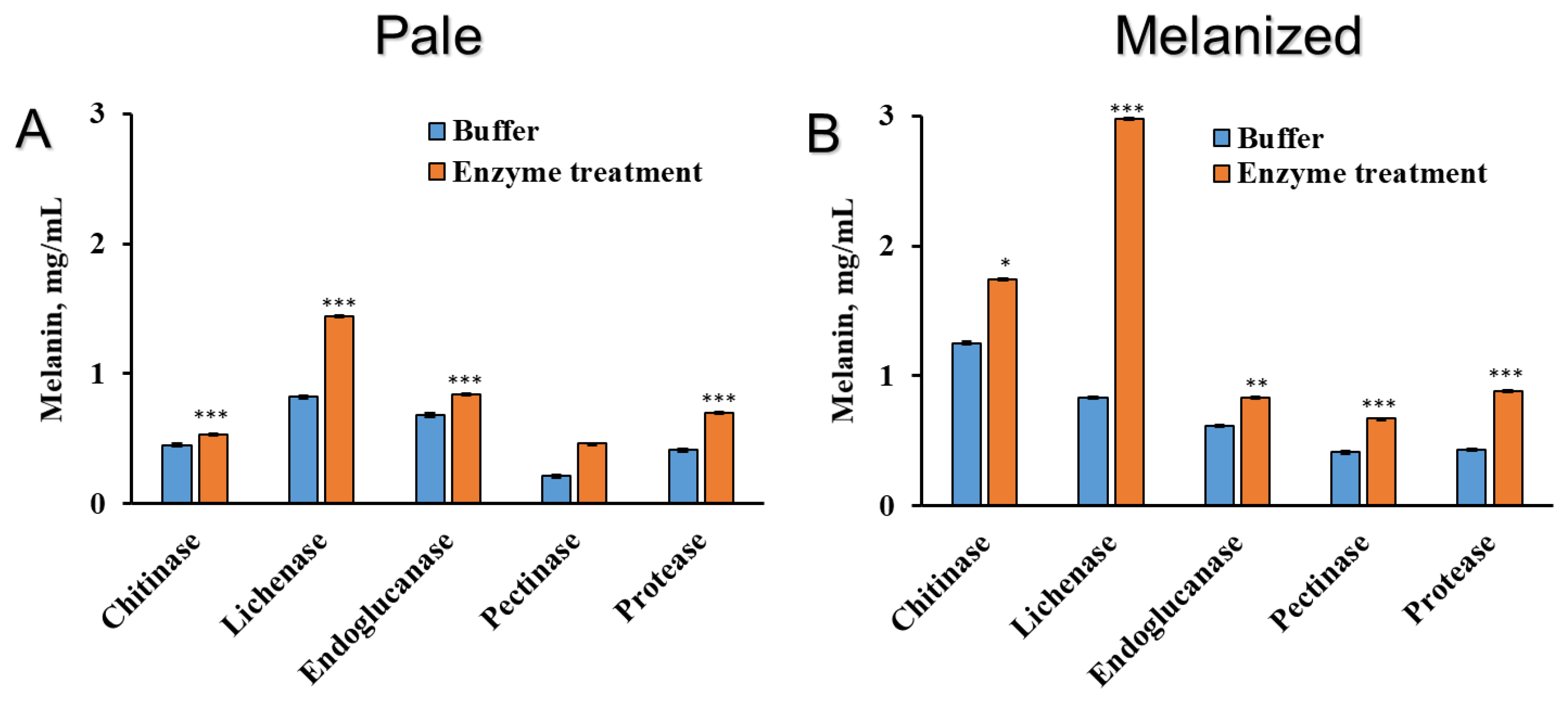
2.2. The Effect of Enzyme Treatment on the Release of Components
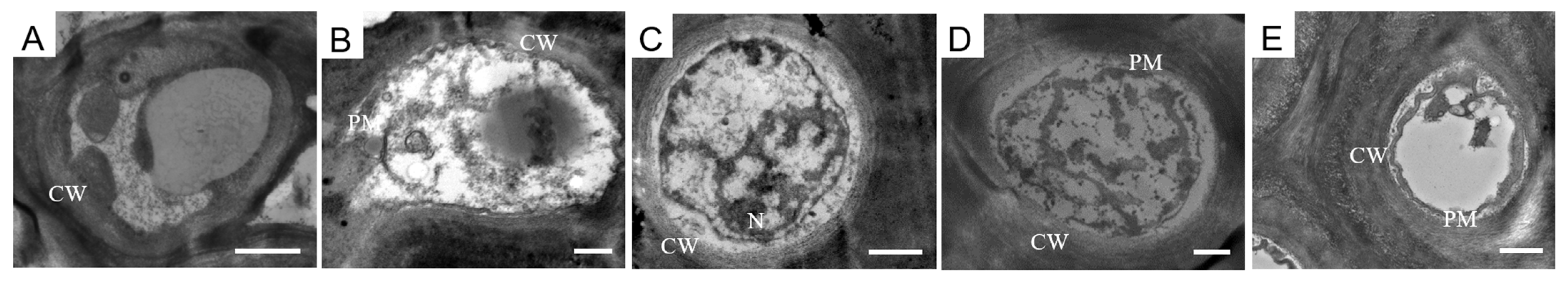
2.3. The Ultrastructural Characteristics of the Cross Sections of Melanized Lichen Thalli
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Scanning Electron Microscopy
4.3. Atomic Force Microscopy
4.4. Light Microscopy
4.5. Enzyme Treatments
4.6. Determination of the Content of Compounds Released into Incubation Solutions
4.7. Content of Melanin in Lichen Thalli
4.8. Transmission Electron Microscopy
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawksworth, D.L.; Grube, M. Lichens redefined as complex ecosystems. New Phyt. 2020, 227, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Spribille, T. Relative symbiont input and the lichen symbiotic outcome. Curr. Opin. Plant Biol. 2018, 44, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Pichler, G.; Muggia, L.; Carniel, F.C.; Grube, M.; Kranner, I. How to build a lichen: From metabolite release to symbiotic interplay. New Phytol. 2023, 238, 1362–1378. [Google Scholar] [CrossRef] [PubMed]
- Honegger, R. Functional aspects of the lichen symbiosis. Annu. Rev. Plant Biol. 1991, 42, 553–578. [Google Scholar] [CrossRef]
- Chowdhury, D.P.; Solhaug, K.A.; Gauslaa, Y. Ultraviolet radiation reduces lichen growth rates. Symbiosis 2017, 73, 27–34. [Google Scholar] [CrossRef]
- Mafole, T.C.; Solhaug, K.A.; Minibayeva, F.V.; Beckett, R.P. Tolerance to photoinhibition within a lichen species is higher in melanised thalli. Photosynthetica 2019, 57, 96–102. [Google Scholar] [CrossRef]
- Ndhlovu, N.T.; Solhaug, K.A.; Minibayeva, F.; Beckett, R.P. Melanisation in boreal lichens is accompanied by variable changes in non-photochemical quenching. Plants 2022, 11, 2726. [Google Scholar] [CrossRef]
- Mafole, T.C.; Solhaug, K.A.; Minibayeva, F.V.; Beckett, R.P. Occurrence and possible roles of melanic pigments in lichenized ascomycetes. Fungal Biol. Rev. 2019, 33, 159–165. [Google Scholar] [CrossRef]
- Caesar-Tonthat, T.; Van Ommen, K.F.; Geesey, G.G.; Henson, J.M. Melanin production by a filamentous soil fungus in response to copper and localization of copper sulfide by sulfide-silver staining. Appl. Environ. Microbiol. 1995, 61, 1968–1975. [Google Scholar] [CrossRef] [Green Version]
- Daminova, A.G.; Rogov, A.M.; Rassabina, A.E.; Beckett, R.P.; Minibayeva, F.V. Effect of melanization on thallus microstructure in the lichen Lobaria pulmonaria. J. Fungi. 2022, 8, 791. [Google Scholar] [CrossRef]
- Babitskaya, V.G.; Scherba, V.V.; Ikonnikova, N.V.; Bisko, N.A.; Mitropolskaya, N.Y. Melanin complex from medicinal mushroom Inonotus obliquus (Pers.: Fr.) Pilat (Chaga)(Aphyllophoromycetidae). Int. J. Med. Mushrooms 2002, 4, 139–145. [Google Scholar]
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, R.L.; Double, K.L.; Gerber, J.P. Using Sepia melanin as a PD model to describe the binding characteristics of neuromelanin–A critical review. J. Chem. Neuroanat. 2015, 64, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Fujikawa, K.; Zucca, F.A.; Zecca, L.; Ito, S. The structure of neuromelanin as studied by chemical degradative methods. J. Neurochem. 2003, 86, 1015–1023. [Google Scholar] [CrossRef]
- Butler, M.J.; Day, A.W. Destruction of fungal melanins by ligninases of Phanerochaete chrysosporium and other white rot fungi. Int. J. Plant Sci. 1998, 159, 989–995. [Google Scholar] [CrossRef]
- Camacho, E.; Vij, R.; Chrissian, C.; Prados-Rosales, R.; Gil, D.; O’Meally, R.N.; Cordero, R.J.B.; Cole, R.N.; McCaffery, J.M.; Stark, R.E.; et al. The structural unit of melanin in the cell wall of the fungal pathogen Cryptococcus neoformans. J. Biol. Chem. 2019, 294, 10471–10489. [Google Scholar] [CrossRef]
- Bull, A.T. Chemical composition of wild-type and mutant Aspergillus nidulans cell walls. The nature of polysaccharide and melanin constituents. J. Gen. Microbiol. 1970, 63, 75–94. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zheng, L.; Hauser, M.; Becker, J.M.; Szaniszlo, P.J. WdChs4p, a homolog of chitin synthase 3 in Saccharomyces cerevisiae, alone cannot support growth of Wangiella (Exophiala) dermatitidis at the temperature of infection. Infect. Immun. 1999, 67, 6619–6630. [Google Scholar] [CrossRef]
- Walker, C.A.; Gómez, B.L.; Mora-Montes, H.M.; Mackenzie, K.S.; Munro, C.A.; Brown, A.J.; Gow, N.A.; Kibbler, C.C.; Odds, F.C. Melanin externalization in Candida albicans depends on cell wall chitin structures. Eukaryot. Cell 2010, 9, 1329–1342. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, E.S.; Ikeda, R. Effect of melanization upon porosity of the cryptococcal cell wall. Med. Mycol. J. 2005, 43, 327–333. [Google Scholar] [CrossRef] [Green Version]
- Chrissian, C.; Camacho, E.; Fu, M.S.; Prados-Rosales, R.; Chatterjee, S.; Cordero, R.J.B.; Lodge, J.K.; Casadevall, A.; Stark, R.E. Melanin deposition in two Cryptococcus species depends on cell-wall composition and flexibility. J. Biol. Chem. 2020, 295, 1815–1828. [Google Scholar] [CrossRef] [PubMed]
- Rassabina, A.; Khabibrakhmanova, V.; Babaev, V.; Daminova, A.; Minibayeva, F. Melanins from the lichens Lobaria pulmonaria and Lobaria retigera as eco-friendly adsorbents of synthetic dyes. Int. J. Mol. Sci. 2022, 23, 15605. [Google Scholar] [CrossRef] [PubMed]
- Rassabina, A.E.; Gurjanov, O.P.; Beckett, R.P.; Minibayeva, F.V. Melanin from the lichens Cetraria islandica and Pseudevernia furfuracea: Structural features and physicochemical properties. Biochemistry 2020, 85, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Liu, X.; Xiang, K.; Chen, L.; Wu, X.; Lin, W.; Zheng, M.; Fu, J. Characterization of the physicochemical properties and extraction optimization of natural melanin from Inonotus hispidus mushroom. Food Chem. 2019, 277, 533–542. [Google Scholar] [CrossRef]
- Zhang, F.; Xue, F.; Xu, H.; Yuan, Y.; Wu, X.; Zhang, J.; Fu, J. Optimization of solid-state fermentation extraction of Inonotus hispidus fruiting body melanin. Foods 2021, 10, 2893. [Google Scholar] [CrossRef]
- Cornejo, C.; Scheidegger, C. New morphological aspects of cephalodium formation in the lichen Lobaria pulmonaria (Lecanorales, Ascomycota). Lichenologist 2013, 45, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Gauslaa, Y.; Solhaug, K.A. Fungal melanins as a sun screen for symbiotic green algae in the lichen Lobaria pulmonaria. Oecologia 2001, 126, 462–471. [Google Scholar] [CrossRef]
- McEvoy, M.; Gauslaa, Y.; Solhaug, K.A. Changes in pools of depsidones and melanins, and their function, during growth and acclimation under contrasting natural light in the lichen Lobaria pulmonaria. New Phytol. 2007, 175, 271–282. [Google Scholar] [CrossRef]
- Matee, L.P.; Beckett, R.P.; Solhaug, K.A.; Minibayeva, F.V. Characterization and role of tyrosinases in the lichen Lobaria pulmonaria (L.) Hoffm. Lichenologist 2016, 48, 311–322. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Chollet-Krugler, M.; Gouault, N.; Tomasi, S. UV-protectant metabolites from lichens and their symbiotic partners. Nat. Prod. Rep. 2013, 30, 1490–1508. [Google Scholar] [CrossRef]
- Beilinson, Y.; Rassabina, A.; Lunev, I.; Faizullin, D.; Greenbaum, A.; Salnikov, V.; Zuev, Y.; Minibayeva, F.; Feldman, Y. Dielectric response of hydrated water as a structural signature of nanoconfined lichen melanins. Phys. Chem. Chem. Phys. 2022, 24, 22624–22633. [Google Scholar] [CrossRef] [PubMed]
- Toledo, A.V.; Franco, M.E.E.; Lopez, S.M.Y.; Troncozo, M.I.; Saparrat, M.C.N.; Balatti, P.A. Melanins in fungi: Types, localization and putative biological roles. Physiol. Mol. Plant Pathol. 2017, 99, 2–6. [Google Scholar] [CrossRef]
- Bell, A.A.; Wheeler, M.H. Biosynthesis and functions of fungal melanins. Ann. Rev. Phytopathol. 1986, 24, 411–451. [Google Scholar] [CrossRef]
- Zajac, G.W.; Gallas, J.M.; Cheng, J.; Eisner, M.; Moss, S.C.; Alvarado-Swaisgood, A.E. The fundamental unit of synthetic melanin: A verification by tunneling microscopy of X-ray scattering results. Biochim. Biophys. Acta 1994, 1199, 271–278. [Google Scholar] [CrossRef]
- Eisenman, H.C.; Nosanchuk, J.D.; Webber, J.B.W.; Emerson, R.J.; Camesano, T.A.; Casadevall, A. Microstructure of cell wall-associated melanin in the human pathogenic fungus Cryptococcus neoformans. Biochemistry 2005, 44, 3683–3693. [Google Scholar] [CrossRef]
- Tudor, D.; Robinson, S.C.; Krigstin, T.L.; Cooper, P.A. Microscopic investigation on fungal pigment formation and its morphology in wood substrates. Open Microbiol. J. 2014, 8, 174–186. [Google Scholar] [CrossRef]
- Abo Elsoud, M.M.; El Kady, E.M. Current trends in fungal biosynthesis of chitin and chitosan. Bull. Natl. Res. Cent. 2019, 43, 59. [Google Scholar] [CrossRef] [Green Version]
- Brown, H.E.; Esher, S.K.; Alspaugh, J.A. Chitin: A "Hidden figure" in the fungal cell wall. Curr. Top. Microbiol. Immunol. 2020, 425, 83–111. [Google Scholar]
- Banks, S.C.; Lindenmayer, D.B.; Ward, S.J.; Taylor, A.C. The effects of habitat fragmentation via forestry plantation establishment on spatial genotypic structure in the small marsupial carnivore, Antechinus agilis. Mol. Ecol. 2005, 14, 1667–1680. [Google Scholar] [CrossRef]
- Walton, F.J.; Idnurm, A.; Heitman, J. Novel gene functions required for melanization of the human pathogen Cryptococcus neoformans. Mol. Microbiol. 2005, 57, 1381–1396. [Google Scholar] [CrossRef]
- Baker, L.G.; Specht, C.A.; Donlin, M.J.; Lodge, J.K. Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryot. Cell 2007, 6, 855–867. [Google Scholar] [CrossRef] [Green Version]
- Nosanchuk, J.D.; Stark, R.E.; Casadevall, A. Fungal melanin: What do we know about structure? Front. Microbiol. 2015, 6, 1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Routier-Kierzkowska, A.L.; Smith, R.S. Measuring the mechanics of morphogenesis. Curr. Opin. Plant Biol. 2013, 16, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Petrova, A.A.; Kozlova, L.V.; Gaifullina, I.Z.; Ananchenko, B.A.; Martinson, E.A.; Mikshina, P.V.; Gorshkova, T.A. AFM analysis reveals polymorphism of purified flax rhamnogalacturonans I of distinct functional types. Carbohyd. Polym. 2019, 216, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.M.; Simon, J.D. Ultrastructural organization of eumelanin from Sepia officinalis measured by atomic force microscopy. Biochemistry 2001, 40, 13353–13360. [Google Scholar] [CrossRef]
- Watt, A.A.R.; Bothma, J.P.; Meredith, P. The supramolecular structure of melanin. Soft Matter 2009, 19, 3754–3760. [Google Scholar] [CrossRef]
- Callow, M.E.; Callow, J.A.; Ista, L.K.; Coleman, S.E.; Nolasco, A.C.; López, G.P. Use of self-assembled monolayers of different wettabilities to study surface selection and primary adhesion processes of green algal (Enteromorpha) zoospores. Appl. Environ. Microbiol. 2000, 66, 3249–3254. [Google Scholar] [CrossRef] [Green Version]
- Yagüe, E.; Orus, M.I.; Estevez, M.P. Extracellular polysaccharidases synthesized by the epiphytic lichen Evernia prunastri (L.) Ach. Planta 1984, 160, 212–216. [Google Scholar] [CrossRef]
- Beckett, R.P.; Zavarzina, A.G.; Liers, C. Oxidoreductases and cellulases in lichens: Possible roles in lichen biology and soil organic matter turnover. Fungal Biol. 2013, 117, 431–438. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, X.; Sun, S.; Zhang, L.; Shan, S.; Zhu, H. Production of natural melanin by Auricularia auricula and study on its molecular structure. Food Chem. 2016, 190, 801–807. [Google Scholar] [CrossRef]
- Pralea, I.E.; Moldovan, R.C.; Petrache, A.M.; Ilieș, M.; Hegheș, S.C.; Ielciu, I.; Nicoară, R.; Moldovan, M.; Ene, M.; Radu, M.; et al. From extraction to advanced analytical methods: The challenges of melanin analysis. Int. J. Mol. Sci. 2019, 20, 3943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olafsdottir, E.S.; Ingólfsdottir, K. Polysaccharides from lichens: Structural characteristics and biological activity. Planta Med. 2001, 67, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Mattson, J.C.; Borgerding, P.J.; Craft, D.L. Fixation of platelets for scanning and transmission electron microscopy. Stain Technol. 1977, 52, 151–158. [Google Scholar] [CrossRef]
- Petrova, A.; Kozlova, L. Characterizing mechanical properties of primary cell wall in living plant organs using atomic force microscopy. J. Vis. Exp. 2022, 183, e63904. [Google Scholar]
- Nikolaeva, V.; Kamalov, M.; Abdullin, T.I.; Salakhieva, D.; Chasov, V.; Rogov, A.; Zoughaib, M. Evaluation of GHK peptide–heparin interactions in multifunctional liposomal covering. J. Liposome Res. 2023, 1–13. [Google Scholar] [CrossRef]
- Akhatova, F.; Ishmukhametov, I.; Fakhrullina, G.; Fakhrullin, R. Nanomechanical atomic force microscopy to probe cellular microplastics uptake and distribution. Int. J. Mol. Sci. 2022, 23, 806. [Google Scholar] [CrossRef]
- Yajima, H.; Morita, M.; Hashimoto, M.; Sashiwa, H.; Kikuchi, T.; Ishii, T. Complex formation of chitosan with iodine and its structure and spectroscopic properties--molecular assembly and thermal hysteresis behavior. Int. J. Thermophys. 2001, 22, 1265–1283. [Google Scholar] [CrossRef]
- Henríquez, C.; Almonacid, S.; Chiffelle, I.; Valenzuela, T.; Araya, M.; Cabezas, L.; Simpson, R.; Speisky, H. Determination of antioxidant capacity, total phenolic content and mineral composition of different fruit tissue of five apple cultivars grown in Chile. Chil. J. Agric. Res. 2010, 70, 523–536. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Nazarova, A.V. Polysaccharides from Arctostaphyllos uva-ursi. Chem. Nat. Compd. 2009, 45, 702–704. [Google Scholar] [CrossRef]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [Green Version]
| Enzyme | Specific Activity | Source | Buffer | Units mL−1 |
|---|---|---|---|---|
| Chitinase 1 | Hydrolysis of N-acetyl- β-D-glucosaminide (1→4)-β-linkages in chitin and chitodextrins | Trichoderma viride | 50 mM sodium phosphate, pH 6.0 | 0.5 |
| Lichenase 2 | Hydrolysis of (1,4)-β-D-glucosidic linkages in β-D-glucans containing (1,3)- and (1,4)-bonds | Bacillus subtilis | 10 mM sodium phosphate, pH 6.0 | 5 |
| Endoglucanase 2 | Hydrolysis of (1,3)-β-D-glucosidic linkages in (1,3)-β-D-glucans | Trichoderma sp. | 100 mM sodium acetate, pH 4.5 | 0.5 |
| Pectinase 1 | Hydrolysis of pectin-containing substances | Aspergillus aculeatus | 100 mM sodium acetate, pH 4.5 | 1 |
| Protease 1 | Hydrolysis of peptide bonds in proteins with conversion to shorter polypeptides and amino acids | Bacillus licheniformis | 100 mM sodium phosphate, pH 7.4 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daminova, A.G.; Rassabina, A.E.; Khabibrakhmanova, V.R.; Beckett, R.P.; Minibayeva, F.V. Topography of UV-Melanized Thalli of Lobaria pulmonaria (L.) Hoffm. Plants 2023, 12, 2627. https://doi.org/10.3390/plants12142627
Daminova AG, Rassabina AE, Khabibrakhmanova VR, Beckett RP, Minibayeva FV. Topography of UV-Melanized Thalli of Lobaria pulmonaria (L.) Hoffm. Plants. 2023; 12(14):2627. https://doi.org/10.3390/plants12142627
Chicago/Turabian StyleDaminova, Amina G., Anna E. Rassabina, Venera R. Khabibrakhmanova, Richard P. Beckett, and Farida V. Minibayeva. 2023. "Topography of UV-Melanized Thalli of Lobaria pulmonaria (L.) Hoffm" Plants 12, no. 14: 2627. https://doi.org/10.3390/plants12142627
APA StyleDaminova, A. G., Rassabina, A. E., Khabibrakhmanova, V. R., Beckett, R. P., & Minibayeva, F. V. (2023). Topography of UV-Melanized Thalli of Lobaria pulmonaria (L.) Hoffm. Plants, 12(14), 2627. https://doi.org/10.3390/plants12142627