Spectral Reflectance Indexes Reveal Differences in the Physiological Status of Brassica oleracea with Contrasting Glucosinolate Content under Biotic Stress
Abstract
:1. Introduction
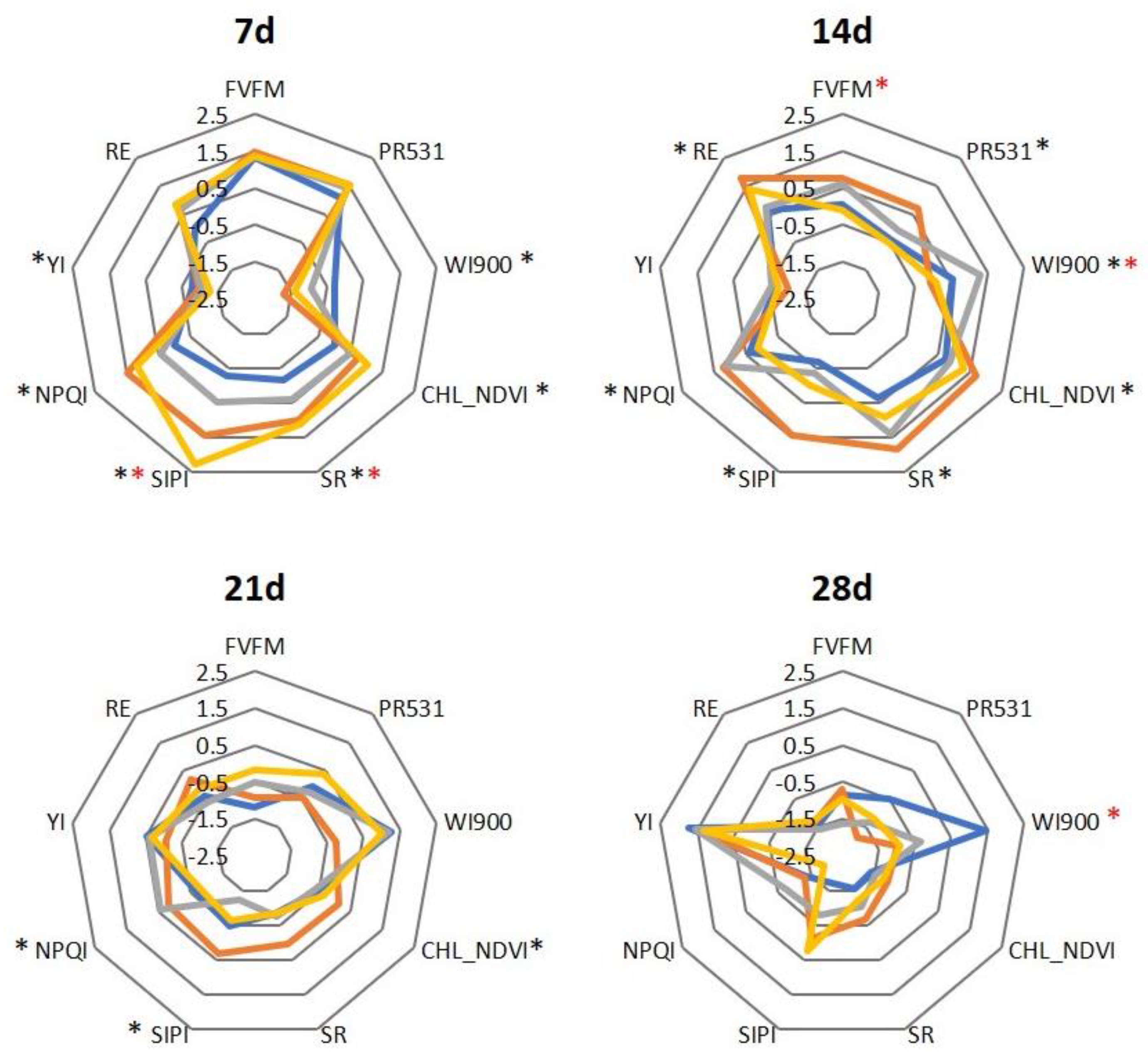
2. Results
3. Discussion
4. Materials and Methods
4.1. Pathogens and Plant Culture
4.2. Inoculation and Physiological Evaluation under Control and Biotic Stress Conditions
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fenwick, G.R.; Heaney, R.K. Glucosinolates and their breakdown products in cruciferous crops, foods and feedingstuffs. Food Chem. 1983, 11, 249–271. [Google Scholar] [CrossRef]
- Zukalová, H.; Vašák, J. The Role and Effects of Glucosinolates of Brassica Species—A Review. Rostl. Vyrob. 2002, 48, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Brader, G.; Mikkelsen, M.D.; Halkier, B.A.; Tapio Palva, E. Altering Glucosinolate Profiles Modulates Disease Resistance in Plants. Plant J. 2006, 46, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Buxdorf, K.; Yaffe, H.; Barda, O.; Levy, M. The Effects of Glucosinolates and Their Breakdown Products on Necrotrophic Fungi. PLoS ONE 2013, 8, e70771. [Google Scholar] [CrossRef] [Green Version]
- Calmes, B.; N’Guyen, G.; Dumur, J.; Brisach, C.A.; Campion, C.; Lacomi, B.; PignÉ, S.; Dias, E.; Macherel, D.; Guillemette, T.; et al. Glucosinolate-Derived Isothiocyanates Impact Mitochondrial Function in Fungal Cells and Elicit an Oxidative Stress Response Necessary for Growth Recovery. Front. Plant Sci. 2015, 6, 414. [Google Scholar] [CrossRef] [Green Version]
- Sotelo, T.; Lema, M.; Soengas, P.; Cartea, M.E.; Velasco, P. In Vitro Activity of Glucosinolates and Their Degradation Products against Brassica-Pathogenic Bacteria and Fungi. Appl. Environ. Microbiol. 2015, 81, 432–440. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Ullah, C.; Reichelt, M.; Beran, F.; Yang, Z.L.; Gershenzon, J.; Hammerbacher, A.; Vassão, D.G. The Phytopathogenic Fungus Sclerotinia sclerotiorum Detoxifies Plant Glucosinolate Hydrolysis Products via an Isothiocyanate Hydrolase. Nat. Commun. 2020, 11, 3090. [Google Scholar] [CrossRef]
- Madloo, P.; Lema, M.; Francisco, M.; Soengas, P. Role of Major Glucosinolates in the Defense of Kale against Sclerotinia sclerotiorum and Xanthomonas campestris pv. campestris. Phytopathology 2019, 109, 1246–1256. [Google Scholar] [CrossRef]
- Piślewska-Bednarek, M.; Nakano, R.T.; Hiruma, K.; Pastorczyk, M.; Sanchez-Vallet, A.; Singkaravanit-Ogawa, S.; Ciesiołka, D.; Takano, Y.; Molina, A.; Schulze-Lefert, P.; et al. Glutathione Transferase U13 Functions in Pathogen-Triggered Glucosinolate Metabolism. Plant Physiol. 2018, 176, 538–551. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Huai, D.; Yang, Q.; Cheng, Y.; Ma, M.; Kliebenstein, D.J.; Zhou, Y. Overexpression of Three Glucosinolate Biosynthesis Genes in Brassica napus Identifies Enhanced Resistance to Sclerotinia sclerotiorum and Botrytis cinerea. PLoS ONE 2015, 10, e0140491. [Google Scholar] [CrossRef] [Green Version]
- Sotelo, T.; Velasco, P.; Soengas, P.; Rodríguez, V.M.; Cartea, M.E. Modification of Leaf Glucosinolate Contents in Brassica oleracea by Divergent Selection and Effect on Expression of Genes Controlling Glucosinolate Pathway. Front. Plant Sci. 2016, 7, 1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth-Defense Tradeoffs in Plants: A Balancing Act to Optimize Fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Zhao, B.; Fan, Z.; Yang, D.; Guo, X.; Wu, Q.; Yu, B.; Zhou, S.; Wang, H. Systematic Identification of Genes Associated with Plant Growth–Defense Tradeoffs under JA Signaling in Arabidopsis. Planta 2020, 251, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hua, J.; Zhang, J.; Luo, S. Negative Interactions Balance Growth and Defense in Plants Confronted with Herbivores or Pathogens. J. Agric. Food Chem. 2022, 70, 12723–12732. [Google Scholar] [CrossRef]
- Bekaert, M.; Edger, P.P.; Hudson, C.M.; Pires, J.C.; Conant, G.C. Metabolic and Evolutionary Costs of Herbivory Defense: Systems Biology of Glucosinolate Synthesis. New Phytol. 2012, 196, 596–605. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, L. Technical Focus: Visible and near-Infrared Reflectance Techniques for Diagnosing Plant Physiological Status. Trends Plant Sci. 1998, 3, 151–156. [Google Scholar] [CrossRef]
- Pineda, M.; Pérez-Bueno, M.L.; Barón, M. Novel Vegetation Indices to Identify Broccoli Plants Infected With Xanthomonas campestris pv. campestris. Front. Plant Sci. 2022, 13, 790268. [Google Scholar] [CrossRef]
- Mawlong, I.; Sujith Kumar, M.S.; Gurung, B.; Singh, K.H.; Singh, D. A Simple Spectrophotometric Method for Estimating Total Glucosinolates in Mustard De-Oiled Cake. Int. J. Food Prop. 2017, 20, 3274–3281. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, M.; Ngo, V.D.; Islam, M.N.; Ali, M.; Islam, S.; Rasool, K.; Park, S.U.; Chung, S.O. Estimation of Glucosinolates and Anthocyanins in Kale Leaves Grown in a Plant Factory Using Spectral Reflectance. Horticulturae 2021, 7, 56. [Google Scholar] [CrossRef]
- Francisco, M.; Joseph, B.; Caligagan, H.; Li, B.; Corwin, J.A.; Lin, C.; Kerwin, R.; Burow, M.; Kliebenstein, D.J. The Defense Metabolite, Allyl Glucosinolate, Modulates Arabidopsis thaliana Biomass Dependent upon the Endogenous Glucosinolate Pathway. Front. Plant Sci. 2016, 7, 774. [Google Scholar] [CrossRef] [Green Version]
- Jensen, L.M.; Jepsen, H.S.K.; Halkier, B.A.; Kliebenstein, D.J.; Burow, M. Natural Variation in Cross-Talk between Glucosinolates and Onset of Flowering in Arabidopsis. Front. Plant Sci. 2015, 6, 697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeschke, V.; Weber, K.; Moore, S.S.; Burow, M. Coordination of Glucosinolate Biosynthesis and Turnover Under Different Nutrient Conditions. Front. Plant Sci. 2019, 10, 1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, E.; Nisani, S.; Yadav, B.S.; Woldemariam, M.G.; Shai, B.; Obolski, U.; Ehrlich, M.; Shani, E.; Jander, G.; Chamovitz, D.A. The Glucosinolate Breakdown Product Indole-3-Carbinol Acts as an Auxin Antagonist in Roots of Arabidopsis thaliana. Plant J. 2015, 82, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, R.E.; Jimenez-Gomez, J.M.; Fulop, D.; Harmer, S.L.; Maloof, J.N.; Kliebensteina, D.J. Network Quantitative Trait Loci Mapping of Circadian Clock Outputs Identifies Metabolic Pathway-to-Clock Linkages in Arabidopsis. Plant Cell 2011, 23, 471–485. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Ballesta, M.D.C.; Muries, B.; Moreno, D.Á.; Dominguez-Perles, R.; García-Viguera, C.; Carvajal, M. Involvement of a Glucosinolate (Sinigrin) in the Regulation of Water Transport in Brassica oleracea Grown under Salt Stress. Physiol. Plant 2014, 150, 145–160. [Google Scholar] [CrossRef]
- Urbancsok, J.; Bones, A.M.; Kissen, R. Glucosinolate-Derived Isothiocyanates Inhibit Arabidopsis Growth and the Potency Depends on Their Side Chain Structure. Int. J. Mol. Sci. 2017, 18, 2372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Zhang, W.; Stanley, B.A.; Assmann, S.M. Functional Proteomics of Arabidopsis thaliana Guard Cells Uncovers New Stomatal Signaling Pathways. Plant Cell 2008, 20, 3210–3226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khokon, M.A.R.; Jahan, M.S.; Rahman, T.; Hossain, M.A.; Muroyama, D.; Minami, I.; Munemasa, S.; Mori, I.C.; Nakamura, Y.; Murata, Y. Allyl Isothiocyanate (AITC) Induces Stomatal Closure in Arabidopsis. Plant Cell Environ. 2011, 34, 1900–1906. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Yuan, J.J.; Xiao, C.C.; Li, G.X.; Yan, J.Y.; Zheng, S.J.; Ding, Z.J. RING-Box Proteins Regulate Leaf Senescence and Stomatal Closure via Repression of ABA Transporter Gene ABCG40. J. Integr. Plant Biol. 2022, 64, 979–994. [Google Scholar] [CrossRef]
- Vik, D.; Mitarai, N.; Wulff, N.; Halkier, B.A.; Burow, M. Dynamic Modeling of Indole Glucosinolate Hydrolysis and Its Impact on Auxin Signaling. Front. Plant Sci. 2018, 9, 550. [Google Scholar] [CrossRef] [Green Version]
- Poveda, J.; Velasco, P.; de Haro, A.; Johansen, T.J.; McAlvay, A.C.; Möllers, C.; Mølmann, J.A.B.; Ordiales, E.; Rodríguez, V.M. Agronomic and Metabolomic Side-Effects of a Divergent Selection for Indol-3-Ylmethylglucosinolate Content in Kale (Brassica oleracea var. acephala). Metabolites 2021, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Abdulridha, J.; Batuman, O.; Ampatzidis, Y. UAV-Based Remote Sensing Technique to Detect Citrus Canker Disease Utilizing Hyperspectral Imaging and Machine Learning. Remote Sens 2019, 11, 1373. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Feng, X.; Wu, Q.; Yang, G.; Tao, M.; Yang, Y.; He, Y. Rice Bacterial Blight Resistant Cultivar Selection Based on Visible/near-Infrared Spectrum and Deep Learning. Plant Methods 2022, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, P.; Jedryczka, M.; Mazurek, W.; Babula-Skowronska, D.; Siedliska, A.; Kaczmarek, J. Hyperspectral and Thermal Imaging of Oilseed Rape (Brassica napus) Response to Fungal Species of the Genus Alternaria. PLoS ONE 2015, 10, e0122913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauricio, R.; Rausher, M.D. Experimental Manipulation of Putative Selective Agents Provides Evidence for the Role of Natural Enemies in the Evolution of Plant Defense. Evolution 1997, 51, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Mauricio, R. Costs of Resistance to Natural Enemies in Field Populations of the Annual Plant Arabidopsis Thaliana. Am. Nat. 1998, 151, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.L.; Philpot, W.D.; Norvell, W.A. Yellowness Index: An Application of Spectral Second Derivatives to Estimate Chlorosis of Leaves in Stressed Vegetation. Int. J. Remote Sens. 1999, 20, 3663–3675. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soengas, P.; Madloo, P.; Lema, M. Spectral Reflectance Indexes Reveal Differences in the Physiological Status of Brassica oleracea with Contrasting Glucosinolate Content under Biotic Stress. Plants 2023, 12, 2698. https://doi.org/10.3390/plants12142698
Soengas P, Madloo P, Lema M. Spectral Reflectance Indexes Reveal Differences in the Physiological Status of Brassica oleracea with Contrasting Glucosinolate Content under Biotic Stress. Plants. 2023; 12(14):2698. https://doi.org/10.3390/plants12142698
Chicago/Turabian StyleSoengas, Pilar, Pari Madloo, and Margarita Lema. 2023. "Spectral Reflectance Indexes Reveal Differences in the Physiological Status of Brassica oleracea with Contrasting Glucosinolate Content under Biotic Stress" Plants 12, no. 14: 2698. https://doi.org/10.3390/plants12142698






