Transcriptome Analysis Unveiled the Intricate Interplay between Sugar Metabolism and Lipid Biosynthesis in Symplocos paniculate Fruit
Abstract
:1. Introduction
2. Results
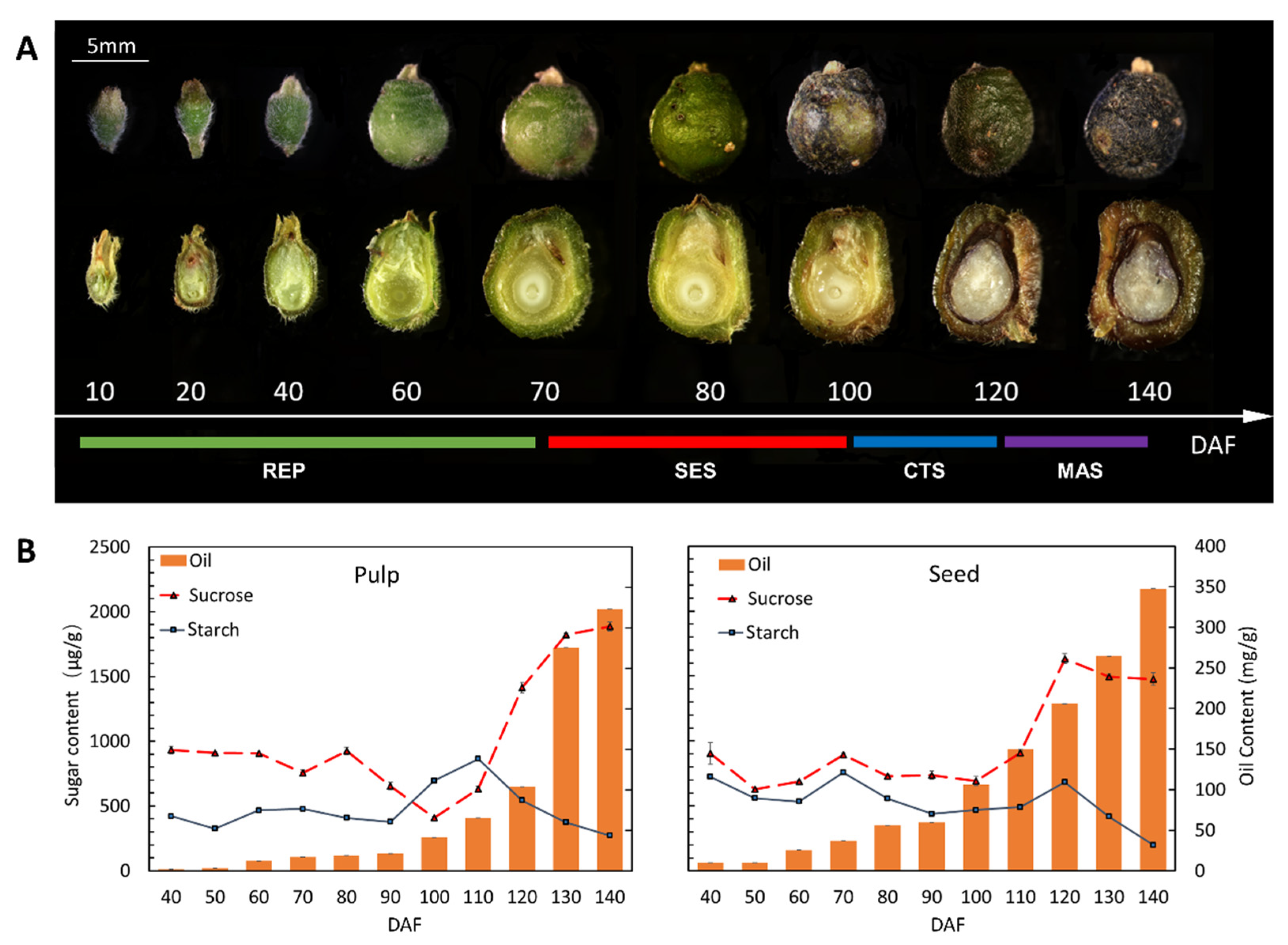
2.1. Temporal Pattern of Metabolite Contents
2.2. Functional Annotation of Sugar Metabolism and Lipid Synthesis Genes in S. paniculata Fruit
2.3. Functional Annotation of Non-Redundant Unigenes
2.4. Functional Annotation of Sugar Metabolism and Oil Synthesis Genes in Symplocos paniculate Fruits
2.5. Analysis of the Expression Pattern of Differentially Expressed Genes in the Pulp and Seeds of Symplocos paniculata
2.6. Real-Time Fluorescence Quantitative PCR Validation
3. Discussion
3.1. The Correlation between the Sugars and Oil Content during Fruit Development
3.2. Key Genes Involved in the Sugar Metabolism Pathway Play a Crucial Role in Regulating Lipid Synthesis
3.3. The Pivotal Genes in the Lipid Metabolism Pathway Facilitate the Biosynthesis of Oil from Symplocos paniculate Fruit
4. Materials and Methods
4.1. Plant Material
4.2. Sucrose and Starch Contents Measurement
4.3. Oil Content Determination
4.4. Fatty Acid Composition Determination
4.5. Transcriptome Sequencing and Analysis
4.6. Validation of RNA-seq by Quantitative PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Q.; Li, C.Z.; Jiang, L.J.; Li, H.; Chen, J.Z.; Yi, X.Y. The oil accumulation of oil plant Symplocos Paniculata. J. Biobased Mater. Bioenergy 2015, 5, 32–36. [Google Scholar] [CrossRef]
- Liu, G.B.; Liu, W.Q.; Huang, C.G.; Du, T.Z.; Huang, Z.; Wen, X.G.; Xia, D.Q.; He, L. Physiochemical properties and preparation of biodiesel by Symplocos paniculata seeds oil. J. Chin. Cereals Oils Assoc. 2011, 26, 64–67. [Google Scholar]
- Guan, Z. Symplocos paniculata. J. Soil Water Conserv. 1991, 8, 44. [Google Scholar]
- Walker, R.P.; Chen, Z.H.; Famiani, F. Gluconeogenesis in Plants: A Key Interface between Organic Acid/Amino Acid/Lipid and Sugar Metabolism. Molecules 2021, 26, 5129. [Google Scholar] [CrossRef]
- Bourgis, F.; Kilaru, A.; Cao, X.; Ngando-Ebongue, G.F.; Drira, N.; Ohlrogge, J.B.; Arondel, V. Comparative transcriptome and metabolite analysis of oil palm and date palm mesocarp that differ dramatically in carbon partitioning. Proc. Natl. Acad. Sci. USA 2011, 108, 12527–12532. [Google Scholar] [CrossRef]
- Hill, L.M.; Morley-Smith, E.R.; Rawsthorne, S. Metabolism of sugars in the endosperm of developing seeds of oilseed rape. Plant Physiol. 2003, 131, 228–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borisjuk, L.; Rolletschek, H.; Radchuk, R.; Weschke, W.; Wobus, U.; Weber, H. Seed development and differentiation: A role for metabolic regulation. Plant Biol. 2004, 6, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.D.; Ohlrogge, J.B. Compartmentation of triacylglycerol accumulation in plants. J. Biol. Chem. 2012, 287, 2288–2294. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, C.; Komatsu, S.; He, M.; Liu, G.; Shen, S. Proteomic analysis of the seed development in Jatropha curcas: From carbon flux to the lipid accumulation. J. Proteom. 2013, 91, 23–40. [Google Scholar] [CrossRef]
- Pommerrenig, B.; Popko, J.; Heilmann, M.; Schulmeister, S.; Dietel, K.; Schmitt, B.; Stadler, R.; Feussner, I.; Sauer, N. SUCROSE TRANSPORTER 5 supplies Arabidopsis embryos with biotin and affects triacylglycerol accumulation. Plant J. 2013, 73, 392–404. [Google Scholar] [CrossRef] [Green Version]
- Borek, S.; Pukacka, S.; Michalski, K. Lipid and protein accumulation in developing seeds of three lupine species: Lupinus luteus L., Lupius albus L., and Lupinus mutabilis Sweet. Exp. Bot. 2009, 60, 3353–3366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, N.; Zhang, Y.; Wang, J.; Liu, X.; Zhao, C.G.; Guo, H.H. Seed Development, Lipid Accumulation and Its relationship with carbohydrates and protein in Xanthoceras sorbifolia Bunge. Bull. Bot. Res. 2015, 35, 133–140. [Google Scholar]
- Kennedy, Y.; Yokoi, S.; Sato, T.; Daimon, H.; Nishida, I.; Takahata, Y. Genetic variation of storage compounds and seed weight in rapeseed (Brassica napus L.) germplasms. Breed. Sci. 2011, 61, 311–315. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, L.D.; Verbeek, R.E.; Draaism, R.B.; Martens, D.E.; Springer, J.; Eggink, G.; Wijffels, R.H. Superior triacylglyerol (TAG) accumulation in starchless mutants of Scenedesmus obliquus: (i) Mutant generation and characterization. Biotechnol. Biofuels 2014, 7, 69. [Google Scholar] [CrossRef]
- Bettey, M.; Smith, A.M. Nature of the effect of the r locus on the lipid content of embryos of peas (Pisum sativum L.). Planta 1990, 180, 420–428. [Google Scholar] [CrossRef]
- Norton, G.; Harris, J.F. Compositional changes in developing rape seed (Brassica napus L.). Planta 1975, 123, 163–174. [Google Scholar] [CrossRef]
- Periappuram, C.; Steinhauer, L.; Barton, D.L.; Taylor, D.C.; Chatson, B.; Zou, J. A plastidic phosphoglucomutase from Arabidopsis. A reversible enzyme reaction with an important role in metabolic control. Plant Physiol. 2000, 122, 1193–1199. [Google Scholar] [CrossRef] [Green Version]
- Vigeolas, H.; Möhlmann, T.; Martini, N.; Neuhaus, H.E.; Geigenberger, P. Embryo-specific reduction of ADP–Glc pyrophosphorylase leads to an inhibition of starch synthesis and a delay in oil accumulation in developing seeds of oilseed rape. Plant Physiol. 2004, 136, 2676–2686. [Google Scholar] [CrossRef] [Green Version]
- Silva, P.M.F.R.D.; Eastmond, P.J.; Hill, L.M.; Rawsthorne, S.S. Starch metabolism in developing embryos of oilseed rape. Planta 1997, 203, 480–487. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Kiryutin, B.; Koonin, E.V.; Krylov, D.M.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; et al. The COG database: An updated version includes eukaryotes. BMC Bioinform. 2003, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- John, E.L.; Elspeth, M.R. New complexities in the synthesis of sucrose. Curr. Opin. Plant Biol. 2003, 6, 208–214. [Google Scholar]
- Zhang, K.; Wu, Z.D.; Tang, D.B.; Luo Kai Lu, H.X.; Liu, Y.Y.; Dong, J.; Wang, X.; Lv, C.W.; Wang, J.C.; Lu, K. Comparative transcriptome analysis reveals critical function of sucrose metabolism related-enzymes in starch accumulation in the storage root of sweet potato. Front. Plant Sci. 2017, 8, 914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.W.; Jia, L.L.; Zhao, J.; Wang, Y.B.; Ren, G.X.; Yang, G.H. Effect of Spraying Biogas Slurry on Apple Yield, Quality and Sucrose Metabolism Enzyme Activity. North. Hortic. 2017, 18, 35–41. [Google Scholar]
- Jiang, H.; Wu, P.; Zhang, S.; Song, C.; Chen, Y.P.; Li, M.R.; Jia, Y.X.; Fang, X.H.; Chen, F.; Wu, G.J. Global analysis of gene expression profiles in developing physic nut (Jatropha curcas L.) seeds. PLoS ONE 2012, 7, 36522. [Google Scholar] [CrossRef] [Green Version]
- Jiang, G.L.; Katuuramu, D.N.; Xu, Y.; Ren, S.; Rutto, L.K. Analysis and comparison of seed protein, oil, and sugars in edamame dried using two oven-drying methods and mature soybeans. J. Sci. Food Agric. 2020, 100, 3987–3994. [Google Scholar] [CrossRef]
- Jia, X.D.; Luo, H.T.; Zhai, M.; Qian, M.H.; Liu, Y.Z.; Li, Y.R.; Guo, Z.R.; Qiao, Y.S. Dynamic changes and correlation analysis of nutrient contents in ‘Pawnee’ pecan (Carya illinoinensis). J. Fruit Sci. 2016, 33, 1120–1130. [Google Scholar]
- Zhang, Y.; Liu, A.Z. The Correlation Between Soluble Carbohydrate Metabolism and Lipid.Accumulation in Castor Seeds. Biotechnol. Bull. 2016, 32, 120–129. [Google Scholar]
- Arthur, G. Sucrose synthase—An enzyme with a central role in the source–sink coordination and carbon flow in trees. New Phytol. 2020, 229, 8–10. [Google Scholar]
- Huang, D.L.; Qin, C.X.; Gui, Y.Y.; Zhao, L.H.; Lakshmanan, P. Role of the SPS gene families in the regulation of sucrose accumulation in sugarcane. Soc. Sugar Res. Promot. 2016, 19, 117–124. [Google Scholar] [CrossRef]
- Yang, Y. Accumulation and Distribution Characteristics and Transport Mechanism of Photosynthetic Products in Camellia oleifera. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2020. [Google Scholar]
- Zuo, Y.L. Studies on carbohydrate catabolism in biochemistry. Chem. Eng. Equip. 2022, 6, 209–210. [Google Scholar]
- Lin, Z.; An, J.; Wang, J.; Niu, J.; Ma, C.; Wang, L.; Yuan, G.; Shi, L.; Liu, L.; Zhang, J.; et al. Integrated analysis of 454 and Illumina transcriptomic sequencing characterizes carbon flux and energy source for fatty acid synthesis in developing Lindera glauca fruits for woody biodiesel. Biotechnol. Biofuels 2017, 10, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selinski, J.; König, N.; Wellmeyer, B.; Hanke, G.T.; Linke, V.; Neuhaus, H.E.; Scheibe, R. The plastid-localized NAD-dependent malate dehydrogenase is crucial for energy homeostasis in developing Arabidopsis thaliana seeds. Mol. Plant 2014, 7, 170–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beeler, S.; Liu, H.C.; Stadler, M.; Schreier, T.; Eicke, S.; Lue, W.L.; Truernit, E.; Zeeman, S.C.; Chen, J.; Kötting, O. Plastidial NAD-dependent malate dehydrogenase is critical for embryo development and heterotrophic metabolism in Arabidopsis. Plant Physiol. 2014, 164, 1175–1190. [Google Scholar] [CrossRef] [Green Version]
- Marillia, E.F.; Micallef, B.J.; Micallef, M.; Weninger, A.; Pedersen, K.K.; Zou, J.; Taylor, D.C. Biochemical and physiological studies of Arabidopsis thaliana transgenic lines with repressed expression of the mitochondrial pyruvate dehydrogenase kinase. J. Exp. Bot. 2003, 54, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Jing, F.; Cantu, D.C.; Tvaruzkova, J.; Chipman, J.P.; Nikolau, B.J.; Yandeau-Nelson, M.D.; Reilly, P.J. Phylogenetic and experimental characterization of an acyl-ACP thioesterase family reveals significant diversity in enzymatic specificity and activity. BMC Biochem. 2011, 12, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.X.; Wu, Y.; Yang, Y.D. Relationship between fatty acid accumulation and FatB gene expression in Cocos nucifera. Guihaia 2021, 41, 1165–1172. [Google Scholar]
- Liu, L.; Wang, Y.M.; Zhao, Y.P.; Wang, D.P.; Zhao, P.P.; Liu, Z.J.; Hua, J.P. Construction of Expression Vectors and a Preliminarily Functional Analysis of Fatty. Cotton Sci. 2016, 28, 527–537. [Google Scholar]
- Lardizabal, K.; Effertz, R.; Levering, C.; Mai, J.; Pedroso, M.C.; Jury, T.; Aasen, E.; Gruys, K.; Bennett, K. Expression of Umbelopsis ramanniana DGAT2A in seed increases oil in soybean. Plant Physiol. 2008, 148, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Gibon, Y.; Blaesing, O.E.; Hannemann, J.; Carillo, P.; Höhne, M.; Hendriks, J.H.M.; Palacios, N.; Cross, J.; Selbig, J.; Stitt, M. A Robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: Comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell 2004, 16, 3304–3325. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Jiang, L.J.; Li, C.Z.; Li, P.W.; Chen, J.Z.; Xu, Q. Investigation and analysis of wild Symplocos paniculata resources in Dawei Mountain. Hunan For. Sci. Technol. 2011, 38, 36–38. [Google Scholar]
- Wiley-Blackwell Launches Wiley Registry, 8th ed.; NIST Mass Spectral Library: Gaithersburg, MD, USA, 2008.
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.R.; Gu, J. Fastp an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Simão, F.A.; Waterhouse, R.M.; Panagiotis, I.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michael, I.L.; Wolfgang, H.; Simon, A. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar]





| Annotated Databases | Number of Unigenes | Percentage of Annotationed Unigenes (%) |
|---|---|---|
| Annotated in Nr | 59,963 | 48.00 |
| Annotated in KEGG | 56,535 | 45.26 |
| Annotated in KOG | 39,085 | 31.29 |
| Annotated in Swissprot | 47,535 | 38.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Jiang, L.; Chen, Y.; Li, C.; Li, P.; Yang, Y.; Chen, J.; Liu, Q. Transcriptome Analysis Unveiled the Intricate Interplay between Sugar Metabolism and Lipid Biosynthesis in Symplocos paniculate Fruit. Plants 2023, 12, 2703. https://doi.org/10.3390/plants12142703
Li W, Jiang L, Chen Y, Li C, Li P, Yang Y, Chen J, Liu Q. Transcriptome Analysis Unveiled the Intricate Interplay between Sugar Metabolism and Lipid Biosynthesis in Symplocos paniculate Fruit. Plants. 2023; 12(14):2703. https://doi.org/10.3390/plants12142703
Chicago/Turabian StyleLi, Wenjun, Lijuan Jiang, Yunzhu Chen, Changzhu Li, Peiwang Li, Yan Yang, Jingzhen Chen, and Qiang Liu. 2023. "Transcriptome Analysis Unveiled the Intricate Interplay between Sugar Metabolism and Lipid Biosynthesis in Symplocos paniculate Fruit" Plants 12, no. 14: 2703. https://doi.org/10.3390/plants12142703
APA StyleLi, W., Jiang, L., Chen, Y., Li, C., Li, P., Yang, Y., Chen, J., & Liu, Q. (2023). Transcriptome Analysis Unveiled the Intricate Interplay between Sugar Metabolism and Lipid Biosynthesis in Symplocos paniculate Fruit. Plants, 12(14), 2703. https://doi.org/10.3390/plants12142703






