A Chromosome-Level Genome of ‘Xiaobaixing’ (Prunus armeniaca L.) Provides Clues to Its Domestication and Identification of Key bHLH Genes in Amygdalin Biosynthesis
Abstract
:1. Introduction
2. Results
2.1. Genome Sequencing and Assembly
2.2. Genome Annotation
2.3. Phylogenetic Analysis
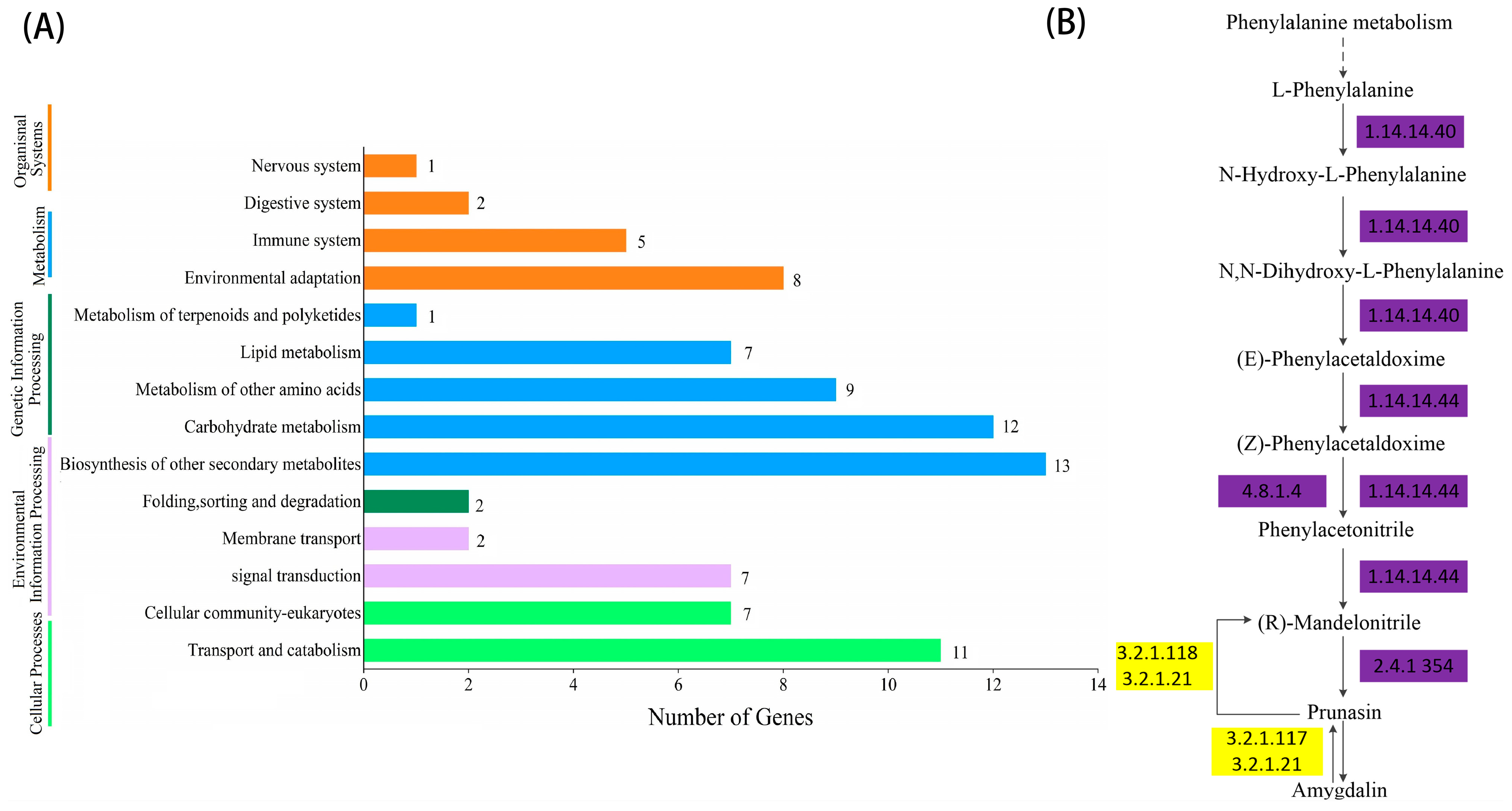
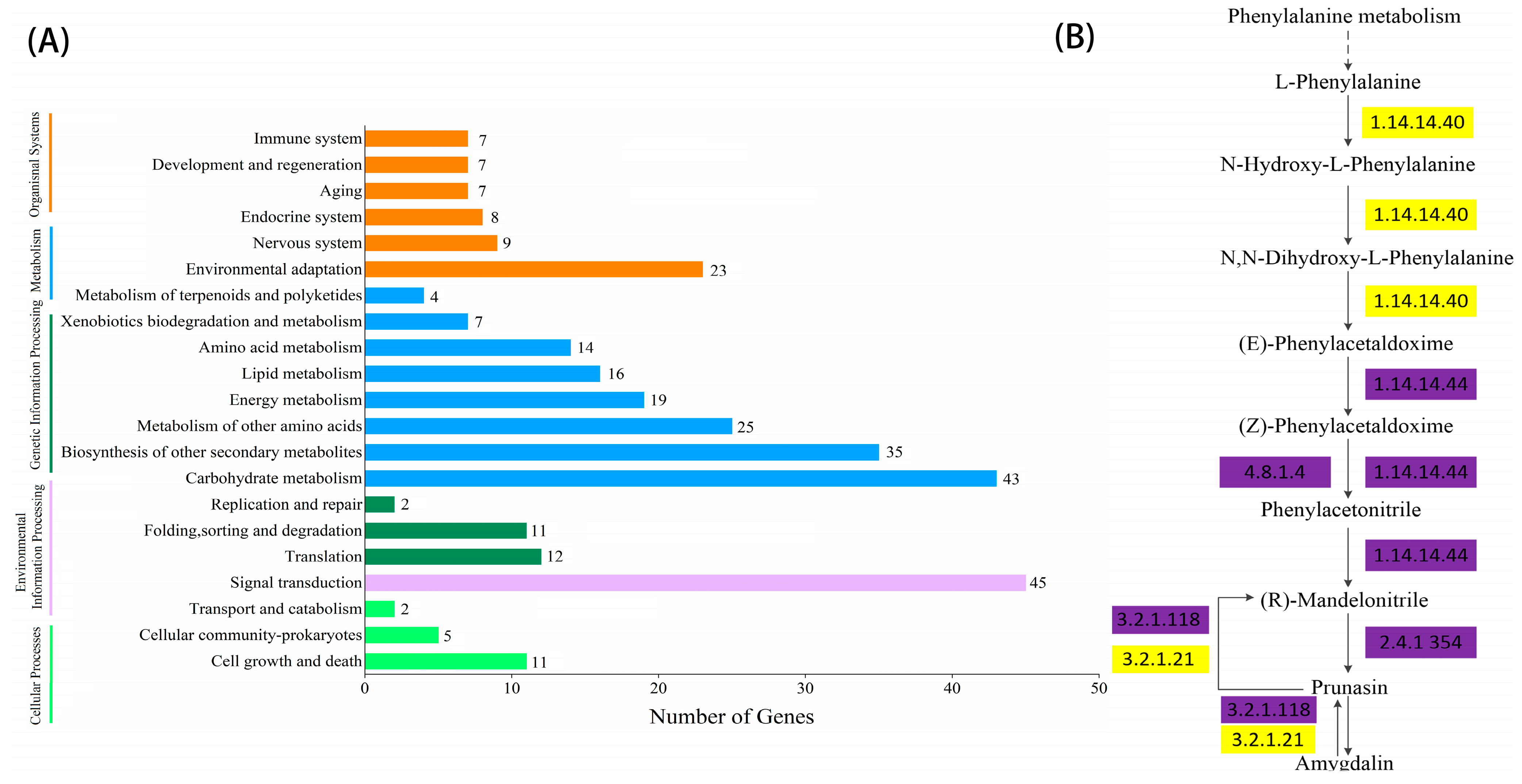
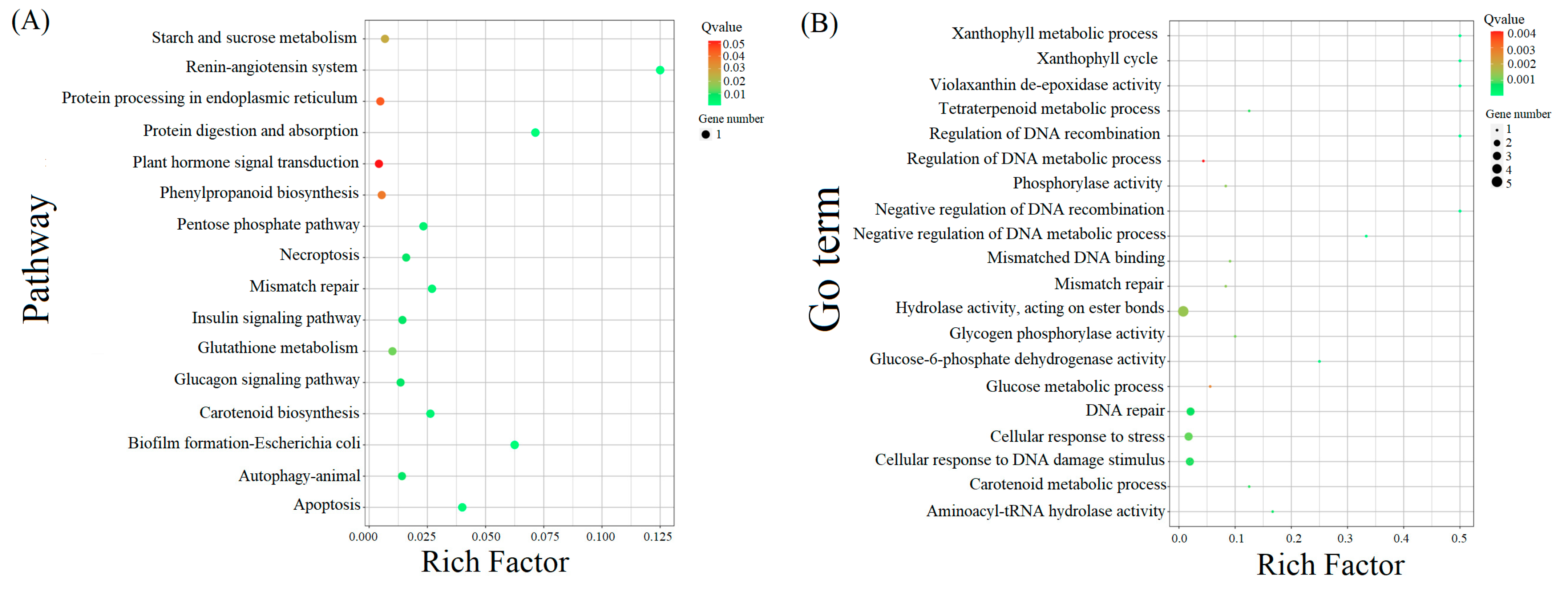
2.4. KEGG Enrichment and Metabolic Pathway Analysis
2.5. Collinear Analysis
2.6. Analysis of the bHLH Gene
2.6.1. The Analysis of the Nucleotide Sequence for the bHLH Gene in Apricot
2.6.2. Analysis of the Amino Acids Encoded by the bHLH Gene Family in Apricot
2.6.3. Phylogenetic Analysis
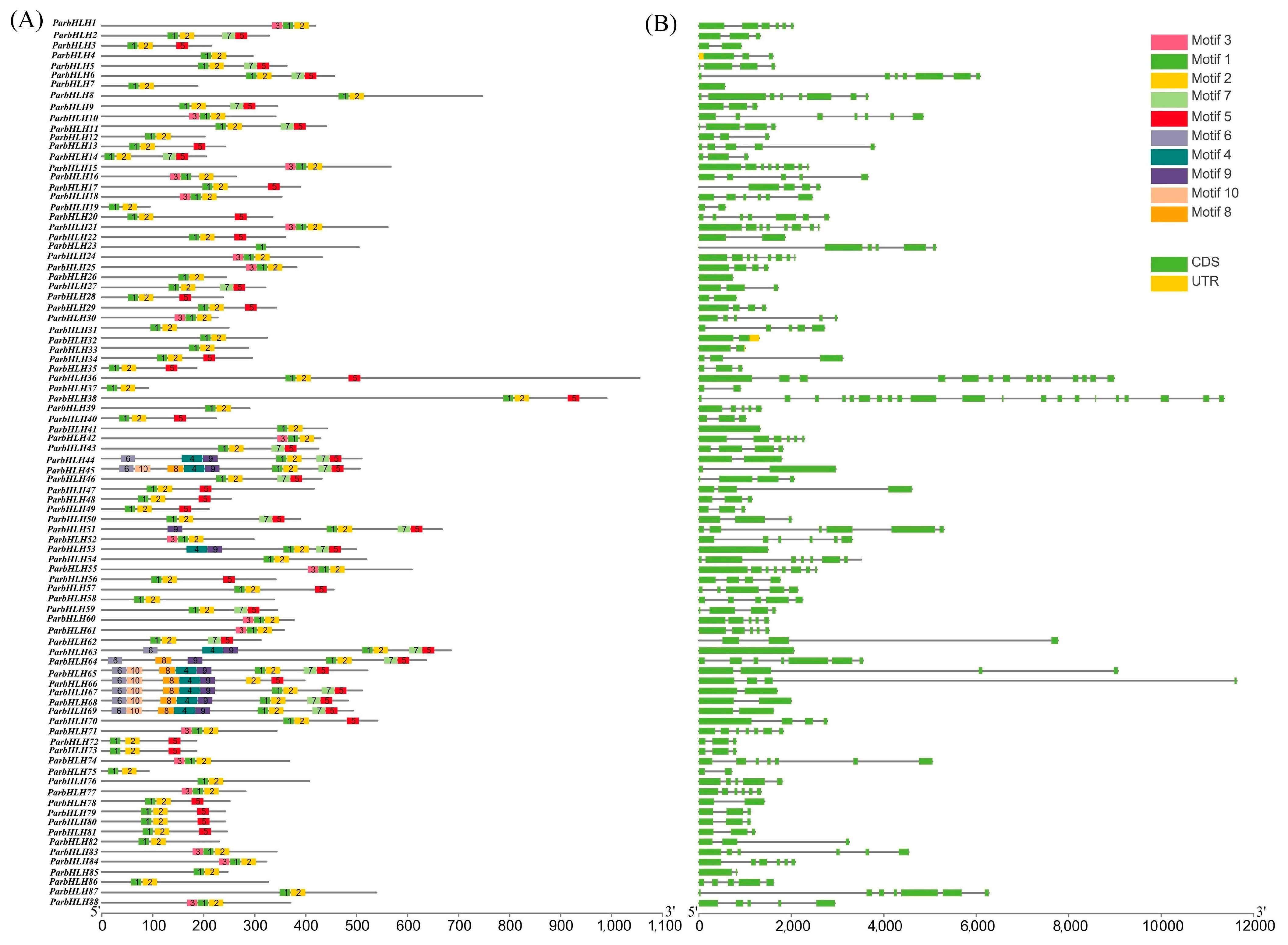
2.6.4. Analysis of the bHLH Family Gene Structure and Conserved Motifs in ‘Xiaobaixing’
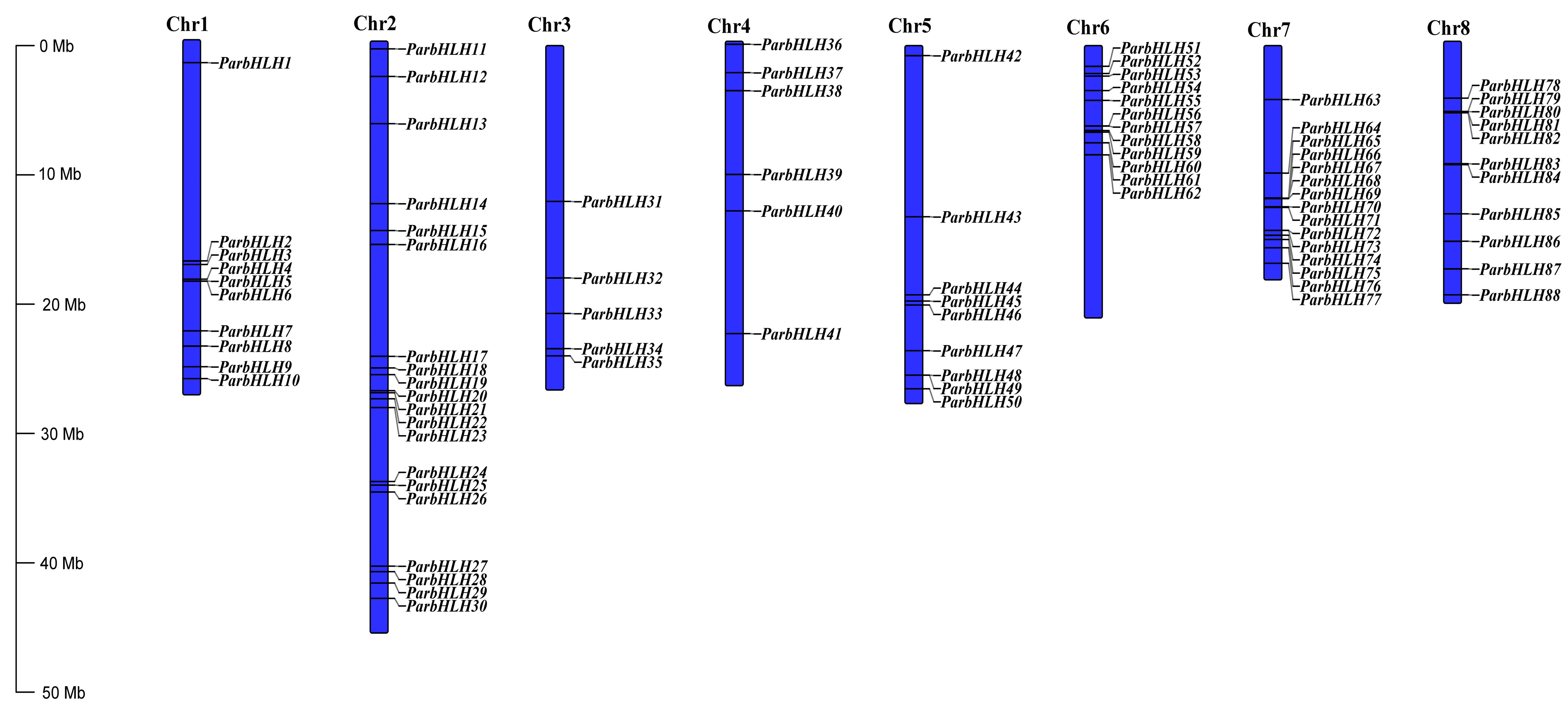
2.6.5. Chromosomal Location and Gene Co-Linearity Analysis of bHLH Family Genes in Apricot
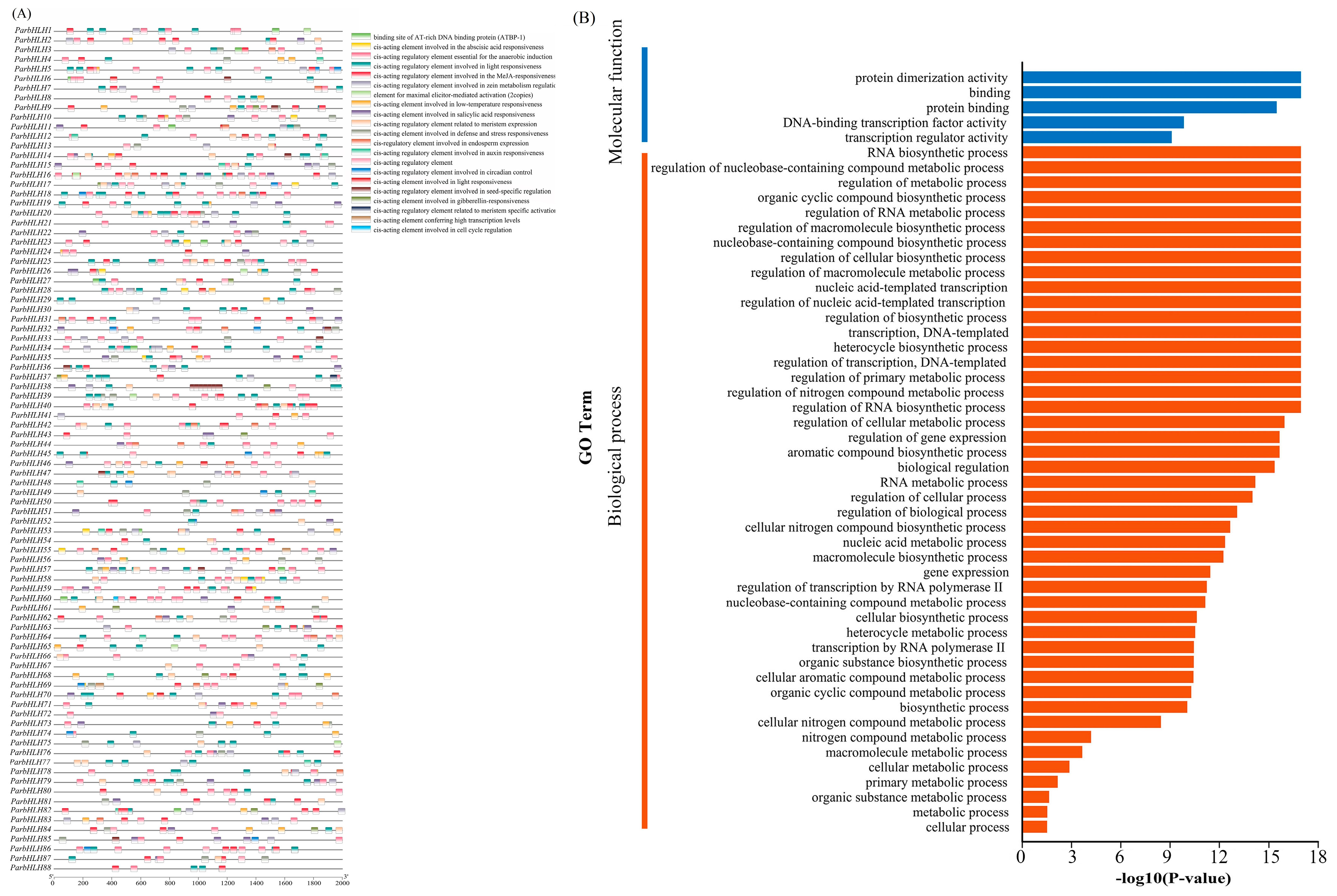
2.6.6. Analysis of Cis-Regulatory Elements of the bHLH Gene Family Promoter
2.6.7. GO Annotation and Protein Interactions of the bHLH Family Genes of Apricot
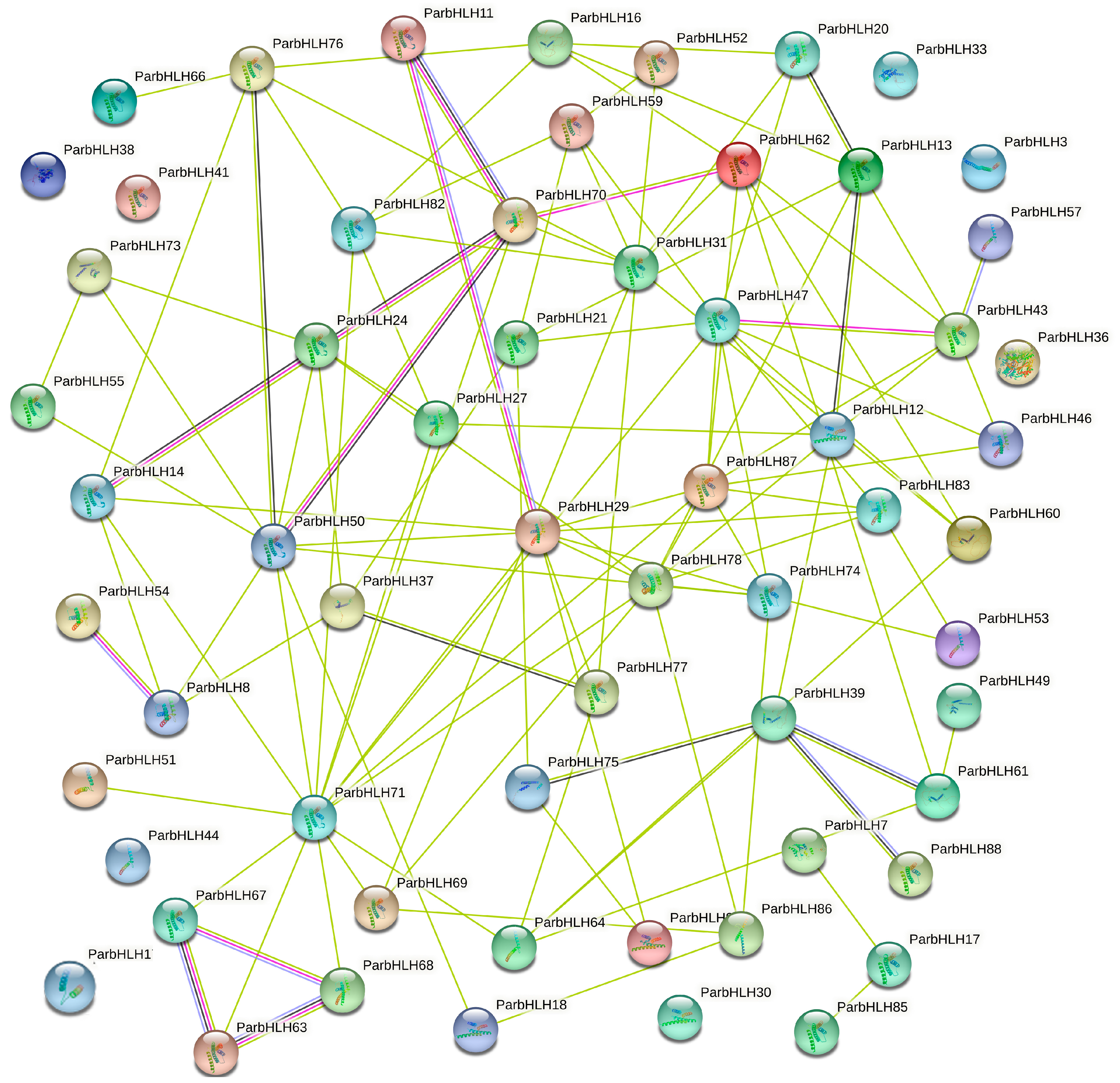
2.6.8. Analysis of the Prediction of Protein Interaction Networks for ParbHLH
3. Discussion
3.1. Genome Evolution
3.2. bHLH Transcription Factor Allowed Apricot Domestication
4. Materials and Methods
4.1. Plant Material and Genome Sequencing
4.2. Genome Assembly and Annotation
4.3. Analysis of Phylogeny and Estimation of Divergence Times
4.4. Expansion and Contraction Analysis, Positive Selection Analysis and Covariance Analysis on Gene Families
4.5. Analysis of the bHLH Gene Family in Apricot
4.5.1. Identification of the bHLH Gene
4.5.2. Bioinformatics Analysis of the bHLH Gene Family
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Zhang, Q.; Li, W.; Zhang, S.; Xi, W. Identification of key genes and regulators associated with carotenoid metabolism in apricot (Prunus armeniaca) fruit using weighted gene coexpression network analysis. BMC Genom. 2019, 20, 876. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Liu, L.; Wang, Y.; Zhang, Q.; Fan, G.; Zhang, S.; Wang, Y.; Liao, K. Genetic diversity, population structure, and relationships of apricot (Prunus) based on restriction site-associated DNA sequencing. Hortic. Res. 2020, 7, 69. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, X.; Xu, X.; Li, Z.; Zhang, J. Variation in amygdalin content in kernels of six almond species (Prunus spp. L.) distributed in China. Front. Plant Sci. 2022, 28, 753151. [Google Scholar] [CrossRef]
- Guo, X. Cultivation and evaluation of Xinjiang ‘Xiaobaixing’ introduced in Chaoyang District, Liaoning Province. Spec. Econ. Anim. Plants 2022, 25, 137–138. [Google Scholar] [CrossRef]
- Hu, D.; Huang, L.; Gao, J.; Wang, J. Research on countermeasures for high-quality development of xinjiang xiaobai apricot industry. China For. Econ. 2021, 6, 76–78. [Google Scholar] [CrossRef]
- Ghorab, H.; Lammi, C.; Arnoldi, A.; Kabouche, Z.; Aiello, G. Proteomic analysis of sweet algerian apricot kernels (Prunus armeniaca L.) by combinatorial peptide ligand libraries and LC–MS/MS. Food Chem. 2018, 239, 935–945. [Google Scholar] [CrossRef]
- Cervellati, C.; Paetz, C.; Dondini, L.; Tartarini, S.; Bassi, D.; Schneider, B.; Masia, A. A qNMR approach for bitterness phenotyping and QTL identification in an F1 apricot progeny. J. Biotechnol. 2012, 159, 312–319. [Google Scholar] [CrossRef]
- Makovi, C.M.; Parker, C.H.; Zhang, K. Determination of amygdalin in apricot kernels and almonds using LC-MS/MS. J. AOAC Int. 2023, 106, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Tsaur, I.; Thomas, A.; Monecke, M.; Zugelder, M.; Rutz, J.; Grein, T.; Maxeiner, S.; Xie, H.; Chun, F.K.; Rothweiler, F.; et al. Amygdalin exerts antitumor activity in taxane-resistant prostate cancer cells. Cancers 2022, 14, 3111. [Google Scholar] [CrossRef] [PubMed]
- El-Desouky, M.A.; Fahmi, A.A.; Nasraldin, K.M. The postulated mechanism of action of amygdalin (vitamin B17) on cancer cells. Anticancer Agents Med. Chem. 2023, 23, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Cui, B.; Zhu, H.; Phommakoun, B.; Zhang, D.; Li, Y.; Zhao, F.; Zhao, Z. Accumulation pattern of amygdalin and Prunasin and its correlation with fruit and kernel agronomic characteristics during apricot (Prunus armeniaca L.) kernel development. Foods 2021, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Jaszczak-Wilke, E.; Polkowska, Ż.; Koprowski, M.; Owsianik, K.; Mitchell, A.E.; Bałczewski, P. Amygdalin: Toxicity, anticancer activity and analytical procedures for its determination in plant seeds. Molecules 2021, 26, 2253. [Google Scholar] [CrossRef] [PubMed]
- Savic, I.M.; Nikolic, V.D.; Savic-Gajic, I.M.; Nikolic, L.B.; Ibric, S.R.; Gajic, D.G. Optimization of technological procedure for amygdalin isolation from plum seeds (Pruni domesticae semen). Front. Plant Sci. 2015, 6, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, J.Z.; Deng, J.G. Research progress in pharmacological effects of amygdalin. Drugs Clin. 2012, 27, 530–535. [Google Scholar]
- Femenia, A.; Rossello, C.; Mulet, A.; Canellas, J. Chemical composition of bitter and sweet apricot kernels. J. Agric. Food Chem. 1995, 43, 356–361. [Google Scholar] [CrossRef]
- Asma, B.M.; Ozturk, K. Analysis of morphological, pomological and yield characteristics of some apricot germplasm in Turkey. Genet. Resour. Crop Evol. 2005, 52, 305–313. [Google Scholar] [CrossRef]
- Krichen, L.; Jean-Marc, A.; Neila, T. Variability of morphological characters among Tunisian apricot germplasm. Sci. Hortic. 2014, 179, 328–339. [Google Scholar] [CrossRef]
- Wani, A.A.; Zargar, S.A.; Malik, A.H.; Kashtwari, M.; Nazir, M.; Khuroo, A.A.; Ahmad, F.; Dar, T.A. Assessment of variability in morphological characters of apricot germplasm of Kashmir, India. Sci. Hortic. 2017, 225, 630–637. [Google Scholar] [CrossRef]
- Huang, X.; Han, A.; Wu, T.; Guo, L. Comparison and analysis of amino acids in kernel of apricot core germplasm in In Southern Xinjiang. J. Huazhong Agric. Univ. 2021, 40, 133–140. [Google Scholar] [CrossRef]
- Sánchez-Pérez, R. Bitternes in almond. Acta Hortic. 2014, 28, 73–76. [Google Scholar] [CrossRef]
- Karsavuran, N.; Charehsaz, M.; Celik, H.; Asmad, B.M.; Yakıncıa, C.; Aydın, A. Amygdalin in bitter and sweet seeds of apricots. Toxicol. Environ. Chem. 2014, 96, 1564–1570. [Google Scholar] [CrossRef]
- Naryal, A.; Bhardwaj, P.; Stobdan, T.; Kant, A. Altitude and seed phenotypic effect on amygdalin content in apricot (Prunus armeniaca L.) Kernel. Pharmacogn. J. 2019, 11, 332–337. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Yamamoto, K.; Asano, Y. Identification and characterization of CYP79D16 and CYP71AN24 catalyzing the first and second steps in L-phenylalanine-derived cyanogenic glycoside biosynthesis in the Japanese apricot, Prunus mume Sieb. Et Zucc. Plant Mol. Biol. 2014, 86, 215–223. [Google Scholar] [CrossRef]
- Hao, Y.; Zong, X.; Ren, P.; Qian, Y.; Fu, A. Basic Helix-Loop-Helix (bHLH) transcription factors regulate a wide range of functions in arabidopsis. Int. J. Mol. Sci. 2021, 22, 7152. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, J.; Zhang, S.; Yu, Q.; Lan, H. Investigation of the nature of CgCDPK and CgbHLH001 interaction and the function of bHLH transcription factor in stress tolerance in Chenopodium glaucum. Front. Plant Sci. 2021, 11, 603298. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xu, Q.; Zhao, S.; Xia, X.; Yan, X.; An, Y.; Mi, X.; Guo, L.; Samarina, L.; Wei, C. Comprehensive co-expression analysis provides novel insights into temporal variation of flavonoids in fresh leaves of the tea plant (Camellia sinensis). Plant Sci. 2020, 290, 110306. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Perez, R.; Pavan, S.; Mazzeo, R.; Moldovan, C.; Aiese Cigliano, R.; Del Cueto, J.; Ricciardi, F.; Lotti, C.; Ricciardi, L.; Dicenta, F.; et al. Mutation of a bHLH transcription factor allowed almond domestication. Science 2019, 364, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Li, B.; Anees, M.; Zhu, H.; Zhao, S.; He, N. Fine-mapping reveals that the bHLH gene Cla011508 regulates the bitterness of watermelon fruit. Sci. Hortic. 2022, 292, 110626. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, H.; Zhong, Y.; Jiang, N.; Zhong, X.; Zhang, Q.; Chai, S.; Li, H.; Zhang, Z. Comparative genomics analysis of bHLH genes in cucurbits identifies a novel gene regulating cucurbitacin biosynthesis. Hortic. Res. 2022, 19, 33. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, J.; Wang, S.; Yang, L.; Luo, Y.; Gao, S.; Zhang, M.; Wu, S.; Hu, S.; Sun, H.; et al. The apricot (Prunus armeniaca L.) genome elucidates Rosaceae evolution and beta-carotenoid synthesis. Hortic. Res. 2019, 6, 128. [Google Scholar] [CrossRef] [Green Version]
- Yuan, K.; Ge, F.; Niu, Q. De novo apricot (Prunus armeniaca) genome assembly and evolutionary analysis. Plant Physiol. J. 2020, 56, 2187–2200. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, D.; Yu, K.; Ji, J.; Liu, N.; Zhang, Y.; Xu, M.; Zhang, Y.; Ma, X.; Liu, S.; et al. Frequent germplasm exchanges drive the high genetic diversity of Chinese-cultivated common apricot germplasm. Hortic. Res. 2021, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, H.; Gou, N.; Huang, M.; Xu, W.; Zhu, X.; Yin, M.; Bai, H.; Wang, L.; Wuyun, T.N. AprGPD: The apricot genomic and phenotypic database. Plant Methods 2021, 17, 98. [Google Scholar] [CrossRef]
- Xie, T.; Zeng, L.; Chen, X.; Rong, H.; Wu, J.; Batley, J.; Jiang, J.; Wang, Y. Genome-wide analysis of the lateral organ boundaries domain gene family in Brassica napus. Genes 2020, 11, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.; Liu, Y.; Li, L.; Meng, H.; Yang, Y.; Dong, Z.; Wang, L.; Wu, G. Genome-wide identification and characterization of bHLH transcription factors related to anthocyanin biosynthesis in red walnut (Juglans regia L.). Front. Genet. 2021, 12, 632509. [Google Scholar] [CrossRef]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D.; et al. The genome of the domesticated apple (Malus × domestica Borkh.). Nat. Genet. 2010, 42, 833–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Kui, L.; Zhang, J.; Wang, L.; Yan, Y.; Wang, N.; Xu, J.; Li, C.; Wang, W.; van Nocker, S.; et al. Improved hybrid de novo genome assembly of domesticated apple (Malus × domestica). Gigascience 2016, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Daccord, N.; Celton, J.M.; Linsmith, G.; Becker, C.; Choisne, N.; Schijlen, E.; van de Geest, H.; Bianco, L.; Micheletti, D.; Velasco, R.; et al. High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat. Genet. 2017, 49, 1099–1106. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, J.; Yang, L.; Jiang, F.; Zhang, M.; Wang, Y. Fruit scientific research in New China in the past 70 years: Apricot. J. Fruit Sci. 2019, 36, 1302–1319. [Google Scholar] [CrossRef]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.H. Evolution of gene duplication in Plants. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef] [Green Version]
- Toledo-Ortiz, G.; Huq, E.; Quail, P.H. The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell 2003, 15, 1749–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Chen, J.; Liang, C.; Liu, F.; Hou, X.; Zou, X. Genome-wide identification and characterization of the bHLH transcription factor family in pepper (Capsicum annuum L.). Front. Genet. 2020, 11, 570156. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Xie, F.; Huang, X.; Luo, Z. A Chromosome-Level Genome of ‘Xiaobaixing’ (Prunus armeniaca L.) Provides Clues to Its Domestication and Identification of Key bHLH Genes in Amygdalin Biosynthesis. Plants 2023, 12, 2756. https://doi.org/10.3390/plants12152756
Guo L, Xie F, Huang X, Luo Z. A Chromosome-Level Genome of ‘Xiaobaixing’ (Prunus armeniaca L.) Provides Clues to Its Domestication and Identification of Key bHLH Genes in Amygdalin Biosynthesis. Plants. 2023; 12(15):2756. https://doi.org/10.3390/plants12152756
Chicago/Turabian StyleGuo, Ling, Fangjie Xie, Xue Huang, and Zhengrong Luo. 2023. "A Chromosome-Level Genome of ‘Xiaobaixing’ (Prunus armeniaca L.) Provides Clues to Its Domestication and Identification of Key bHLH Genes in Amygdalin Biosynthesis" Plants 12, no. 15: 2756. https://doi.org/10.3390/plants12152756




