Development and Application of a Cleaved Amplified Polymorphic Sequence Marker (Phyto) Linked to the Pc5.1 Locus Conferring Resistance to Phytophthora capsici in Pepper (Capsicum annuum L.)
Abstract
1. Introduction
2. Results
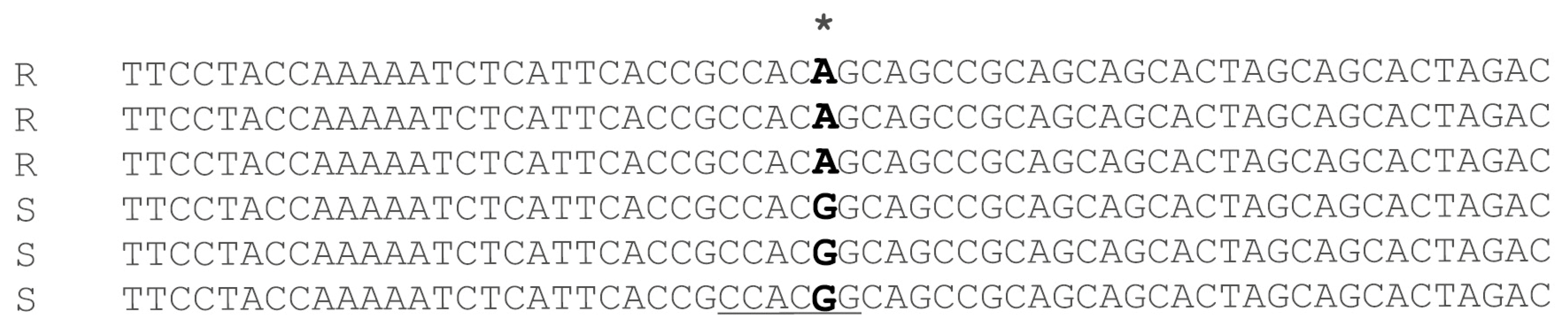
2.1. Development of CAPS Marker
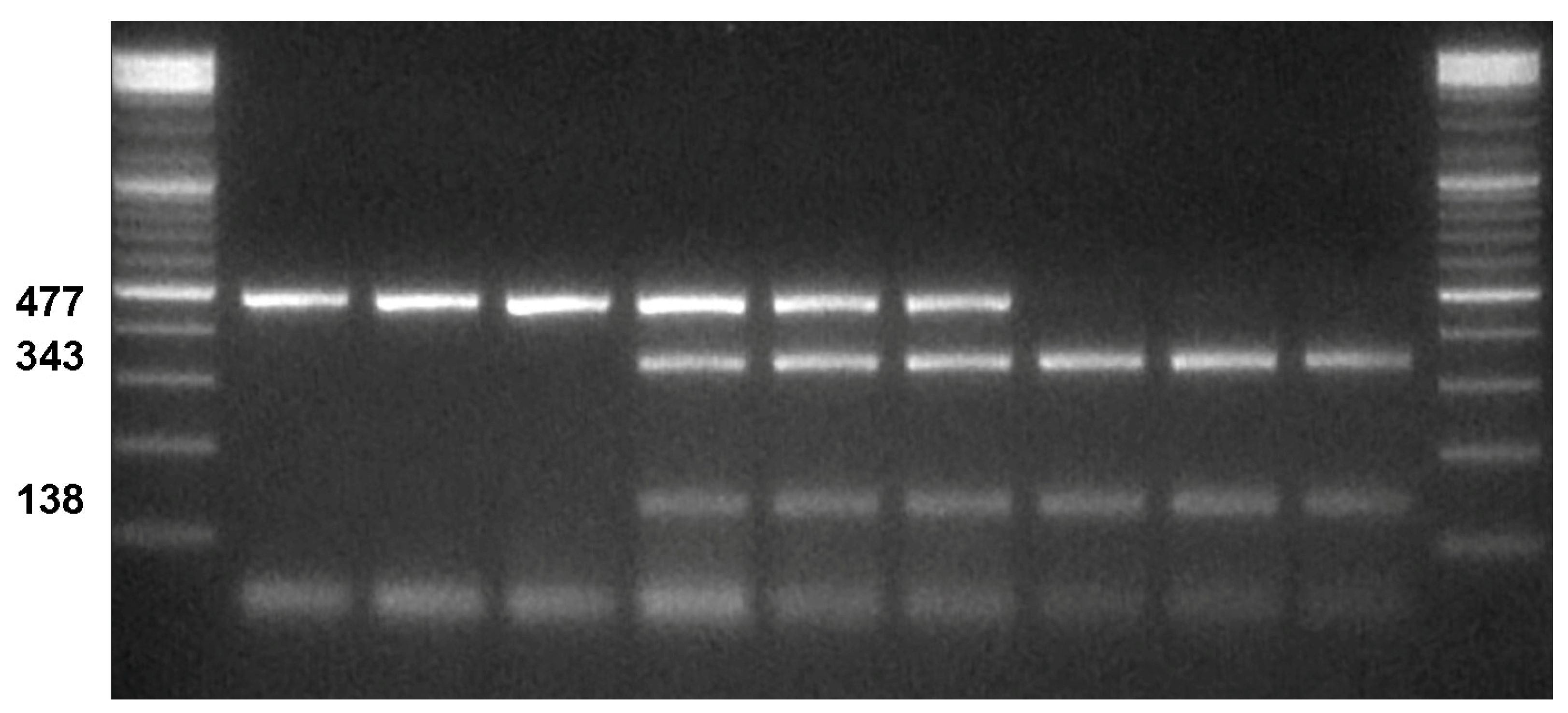
2.2. CAPS Validation
3. Discussion
4. Materials and Methods
4.1. Plant Materials and DNA Extraction
4.2. Marker Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tripodi, P.; Kumar, S. The Capsicum Crop: An introduction. In The Capsicum Genome; Ramchiary, N., Kole, C., Eds.; Springer International Publishing: Basel, Switzerland, 2019; pp. 1–8. [Google Scholar]
- Palombo, N.E.; Carrizo García, C. Geographical Patterns of Genetic Variation in Locoto Chile (Capsicum pubescens) in the Americas Inferred by Genome-Wide Data Analysis. Plants 2022, 11, 2911. [Google Scholar] [CrossRef]
- Barchenger, D.W.; Naresh, P.; Kumar, S. Genetic resources of Capsicum. In The Capsicum Genome; Ramchiary, N., Kole, C., Eds.; Springer International Publishing: Basel, Switzerland, 2019; pp. 9–23. [Google Scholar]
- FAOSTAT. Available online: www.faostat.fao.org (accessed on 1 June 2023).
- Padilha, H.; Barbieri, R. Plant breeding of chili peppers (Capsicum, Solanaceae)—A review. Aust. J. Basic Appl. Sci. 2016, 10, 148–154. [Google Scholar]
- Naves, E.R.; Scossa, F.; Araújo, W.L.; Nunes-Nesi, A.; Fernie, A.R.; Zsögön, A. Heterosis and reciprocal effects for agronomic and fruit traits in Capsicum pepper hybrids. Sci. Hortic. 2022, 295, 110821. [Google Scholar] [CrossRef]
- Srivastava, A.; Mangal, M. Capsicum breeding: History and development. In The Capsicum Genome; Ramchiary, N., Kole, C., Eds.; Springer International Publishing: Basel, Switzerland, 2019; pp. 25–55. [Google Scholar]
- Mandal, S.K.; Rath, S.K.; Logesh, R.; Mishra, S.K.; Devkota, H.P.; Das, N. Capsicum annuum L. and its bioactive constituents: A critical review of a traditional culinary spice in terms of its modern pharmacological potentials with toxicological issues. Phytother. Res. 2023, 37, 965–1002. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pérez, T.; Gómez-García, M.d.R.; Valverde, M.E.; Paredes-López, O. Capsicum annuum (hot pepper): An ancient Latin-American crop with outstanding bioactive compounds and nutraceutical potential. A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2972–2993. [Google Scholar] [CrossRef] [PubMed]
- Majid, M.U.; Awan, M.F.; Fatima, K.; Tahir, M.S.; Ali, Q.; Rasid, B.; Rao, A.Q.; Nasit, I.A.; Husnain, T. Phytophthora capsici on chili pepper (Capsicum annuum L.) and its management through genetic and bio-control: A review. Zemdirbyste 2016, 103, 419–430. [Google Scholar] [CrossRef]
- Parisi, M.; Alioto, D.; Tripodi, P. Overview of Biotic Stresses in Pepper (Capsicum spp.): Sources of Genetic Resistance, Molecular Breeding and Genomics. Int. J. Mol. Sci. 2020, 21, 2587. [Google Scholar] [CrossRef]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; APS Press, American Phytopathological Society: St. Paul, MI, USA, 1996; p. 592. ISBN 0-89054-212-0. [Google Scholar]
- Black, L.L.; Green, S.K.; Hartman, G.L.; Poulos, M. Pepper Diseases: A Field Guide; CTA: Wageningen, The Netherlands, 1991; p. 98. [Google Scholar]
- Biles, C.L.; Brunton, B.D.; Wall, M.M.; Rivas, M. Phytophthora capsici zoospore infection of pepper fruit in various physical environments. Proc. Okla. Acad. Sci. 1995, 75, 1–5. [Google Scholar]
- Oelke, L.M.; Bosland, P.W.; Steiner, R. Differentiation of race specific resistance to Phytophthora root rot and foliar blight in Capsicum Annuum. J. Am. Soc. Hortic. Sci. 2003, 128, 213–218. [Google Scholar] [CrossRef]
- Granke, L.L.; Quesada-Ocampo, L.; Lamour, K.; Hausbeck, M.K. Advances in research on Phytophthora capsici on vegetable crops in the United States. Plant Dis. 2012, 96, 1588–1600. [Google Scholar] [CrossRef]
- Barksdale, T.H.; Papavizas, G.C.; Johnston, S.A. Resistance to foliar blight and crown rot of pepper caused by Phytophthora capsici. Plant Dis. 1984, 68, 506–509. [Google Scholar] [CrossRef]
- Sy, O.; Steiner, R.; Bosland, P.W. Inheritance of Phytophthora stem blight resistance as compared to Phytophthora root rot and Phytophthora foliar blight resistance in Capsicum annuum L. J. Am. Hortic. Soc. 2005, 130, 75–78. [Google Scholar] [CrossRef]
- Foster, J.M.; Hausbeck, M.K. Managing Phytophthora Crown and Root Rot in Bell Pepper Using Fungicides and Host Resistance. Plant Dis. 2010, 94, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Barchenger, D.W.; Lamour, K.H.; Bosland, P.W. Challenges and strategies for breeding resistance in Capsicum annuum to the multifarious pathogen, Phytophthora capsici. Front. Plant Sci. 2018, 9, 628. [Google Scholar] [CrossRef]
- Monroy-Barbosa, A.; Bosland, P.W. Genetic analysis of Phytophthora root rot race-specific resistance in chile pepper. J. Am. Hortic. Sci. 2008, 133, 825–829. [Google Scholar] [CrossRef]
- Sanogo, S.; Ji, P. Integrated Management of Phytophthora capsica on Solanaceous and Cucurbitaceous Crops: Current Status, Gaps in Knowledge and Research Needs. Can. J. Plant Pathol. 2012, 34, 479–492. [Google Scholar] [CrossRef]
- Hausbeck, M.K.; Lamour, K.H. Phytophthora capsici on vegetable crops: Research progress and management challenges. Plant Dis. 2004, 88, 1292–1303. [Google Scholar] [CrossRef]
- Lamour, K.H.; Hausbeck, M.K. Effect of crop rotation on the survival of Phytophthora capsici in Michigan. Plant Dis. 2003, 87, 841–845. [Google Scholar] [CrossRef]
- Babadoost, M.; Pavón, C. Survival of Oospores of Phytophthora capsici in Soil. Plant Dis. 2013, 97, 1478–1483. [Google Scholar] [CrossRef]
- Lamour, K.H.; Hausbeck, M.K. Mefenoxam Insensitivity and the Sexual Stage of Phytophthora capsici in Michigan Cucurbit Fields. Phytopathology 2000, 90, 396–400. [Google Scholar] [CrossRef]
- Sanogo, S.; Lamour, K.; Kousik, S.; Lozada, D.N.; Parada Rojas, C.H.; Quesada-Ocampo, L.; Wyenandt, C.A.; Babadoost, M.; Hausbeck, M.K.; Hansen, Z.; et al. Phytophthora capsici, 100 Years Later: Research Mile Markers from 1922 to 2022. Phytopathology, 2022; in press. [Google Scholar]
- Foster, J.M.; Hausbeck, M.K. Resistance of Pepper to Phytophthora Crown, Root, and Fruit Rot Is Affected by Isolate Virulence. Plant Dis. 2010, 94, 24–30. [Google Scholar] [CrossRef]
- Barchenger, D.W.; Sheu, Z.M.; Kumar, S.; Lin, S.W.; Burlakoti, R.R.; Bosland, P.W. Race characterization of Phytophthora root rot on Capsicum in Taiwan as a basis for anticipatory resistance breeding. Phytopathology 2018, 108, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; Palloix, A. Both epistatic and additive effects of QTLs are involved in polygenic induced resistance to disease: A case study, the interaction pepper-Phytophthora capsici Leonian. Theor. Appl. Genet. 1996, 93, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Thabuis, A.; Palloix, A.; Pflieger, S.; Daubeze, A.M.; Caranta, C.; Lefebvre, V. Comparative mapping of Phytophthora resistance loci in pepper germplasm: Evidence for conserved resistance loci across Solanaceae and for a large genetic diversity. Theor. Appl. Genet. 2003, 106, 1473–1485. [Google Scholar] [CrossRef]
- Thabuis, A.; Lefebvre, V.; Bernard, G.; Daubeze, A.M.; Phaly, T.; Pochard, E.; Palloix, A. Phenotypic and molecular evaluation of a recurrent selection program for a polygenic resistance to Phytophthora capsici in pepper. Theor. Appl. Genet. 2004, 109, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Ogundiwin, E.A.; Berke, T.F.; Massoudi, M.; Black, L.L.; Huestis, G.; Choi, D.; Lee, S.; Prince, J.P. Construction of 2 intraspecific linkage maps and identification of resistance QTLs for Phytophthora capsici root-rot and foliar-blight diseases of pepper (Capsicum annuum L.). Genome 2005, 48, 698–711. [Google Scholar] [CrossRef]
- Minamiyama, Y.; Tsuro, M.; Kubo, T.; Hirai, M. QTL analysis for resistance to Phytophthora capsica in pepper using a high density SSR-based map. Breed. Sci. 2007, 57, 129–134. [Google Scholar] [CrossRef]
- Quirin, E.A.; Ogundiwin, E.A.; Prince, J.P.; Mazourek, M.; Briggs, M.O.; Chlada, T.S.; Kim, K.T.; Falise, M.; Kang, B.C.; Jahn, M.M. Development of sequence characterized amplified region (SCAR) primers for the detection of Phyto.5.2, a major QTL for resistance to Phytophthora capsici Leon in pepper. Theor. Appl. Genet. 2005, 110, 605–612. [Google Scholar] [CrossRef]
- Sugita, T.; Yamaguchi, K.; Kinoshita, T.; Yuji, K.; Sugimura, Y.; Nagata, R.; Kawasaki, S.; Todoroki, A. QTL analysis for resistance to Phytophthora blight (Phytophthora capsici Leon.) using an intraspecific doubled-haploid population of Capsicum annuum. Breed. Sci. 2006, 56, 137–145. [Google Scholar] [CrossRef]
- Bonnet, J.; Danan, S.; Boudet, C.; Barchi, L.; Sage-Palloix, A.-M.; Caromel, B.; Palloix, A.; Lefebvre, V. Are the polygenic architectures of resistance to Phytophthora capsici and P. parasitica independent in pepper? Theor. Appl. Genet. 2007, 115, 253–264. [Google Scholar] [CrossRef]
- Barchi, L.; Bonnet, J.; Boudet, C.; Signoret, P.; Nagy, I.; Lanteri, S.; Palloix, A.; Lefebvre, V. A High-Resolution, Intraspecific Linkage Map of Pepper (Capsicum annuum L.) and Selection of Reduced Recombinant Inbred Line Subsets for Fast Mapping. Genome 2007, 50, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Nahm, S.H.; Lee, H.R.; Yoon, G.B.; Kim, K.T.; Kang, B.C.; Choi, D.; Kweon, O.Y.; Cho, M.C.; Kwon, J.K. BAC-derived markers converted from RFLP linked to Phytophthora capsici resistance in pepper (Capsicum annuum L.). Theor. Appl. Genet. 2008, 118, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.P.; Lee, J.; Han, J.H.; Kang, B.C.; Yoon, J.B. Validity test for molecular markers associated with resistance to Phytophthora root rot in chili pepper (Capsicum annuum L.). Korean J. Hortic. Sci. Technol. 2012, 30, 64–72. [Google Scholar] [CrossRef][Green Version]
- Truong, H.T.H.; Kim, J.H.; Cho, M.C.; Chae, S.Y.; Lee, H.E. Identification and development of molecular markers linked to Phytophthora root rot resistance in pepper (Capsicum annuum L.). Eur. J. Plant Pathol. 2013, 135, 289–297. [Google Scholar] [CrossRef]
- Mallard, S.; Cantet, M.; Massire, A.; Bachellez, A.; Ewert, S.; Lefebvre, V. A key QTL cluster is conserved among accessions and exhibits broad-spectrum resistance to Phytophthora capsici: A valuable locus for pepper breeding. Mol. Breed. 2013, 32, 349–364. [Google Scholar] [CrossRef]
- Rehrig, W.Z.; Ashrafi, H.; Hill, T.; Prince, J.; Van Deynze, A. CaDMR1 cosegregates with QTL Pc5.1for resistance to Phytophthora capsici in pepper (Capsicum annuum). Plant Genome 2014, 7, 1–12. [Google Scholar] [CrossRef]
- Liu, W.Y.; Kang, J.H.; Jeong, H.S.; Choi, H.J.; Yang, H.B.; Kim, K.T.; Choi, D.; Choi, G.J.; Jahn, M.; Kang, B.C. Combined used of bulk-segregated analysis of microarrays reveals SNP markers pinpointing a major QTL for resistance to Phytophthora capsica in pepper. Theor. Appl. Genet. 2014, 127, 2503–2513. [Google Scholar] [CrossRef]
- Wang, P.; Wang, L.; Guo, J.; Yang, W.; Shen, H. Molecular mapping of a gene conferring resistance to Phytophthora capsici Leonian race 2 in pepper line PI201234 (Capsicum annuum L.). Mol. Breed. 2016, 36, 66. [Google Scholar] [CrossRef]
- Siddique, M.I.; Lee, H.-Y.; Ro, N.-Y.; Han, K.; Venkatesh, J.; Solomon, A.M.; Patil, A.S.; Changkwian, A.; Kwon, J.-K.; Kang, B.-C. Identifying candidate genes for Phytophthora capsici resistance in pepper (Capsicum annuum) via genotyping-by-sequencing-based QTL mapping and genome-wide association study. Sci. Rep. 2019, 9, 9962. [Google Scholar] [CrossRef]
- Du, J.S.; Hang, L.F.; Hao, Q.; Yang, H.T.; Ali, S.; Ezaat, R.; Xu, X.Y.; Tan, H.Q.; Su, L.H.; Li, H.X.; et al. The dissection of R genes and locus Pc5.1 in Phytophthora capsici infection provides a novel view of disease resistance in peppers. BMC Genom. 2021, 22, 372. [Google Scholar] [CrossRef]
- Li, Y.-F.; Zhang, S.-C.; Yang, X.-M.; Wang, C.-P.; Huang, Q.-Z.; Huang, R.-Z. Generation of a High-Density Genetic Map of Pepper (Capsicum annuum L.) by SLAF-seq and QTL Analysis of Phytophthora capsici Resistance. Horticulturae 2021, 7, 92. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, Y.; Wang, Y.; Yu, H.; Wu, H.; Liu, J.; An, D.; Zhu, Y.; Feng, X.; Wang, L. Development and validation of KASP markers for resistance to Phytophthora capsici in Capsicum annuum L. Mol. Breed. 2023, 43, 20. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chao, J.; Cheng, X.; Wang, R.; Sun, B.; Wang, H.; Luo, S.; Xu, X.; Wu, T.; Li, Y. Mapping of a Novel Race Specific Resistance Gene to Phytophthora Root Rot of Pepper (Capsicum annuum) Using Bulked Segregant Analysis Combined with Specific Length Amplified Fragment Sequencing Strategy. PLoS ONE 2016, 11, e0151401. [Google Scholar] [CrossRef] [PubMed]
- Ro, N.; Haile, M.; Hur, O.; Geum, B.; Rhee, J.; Hwang, A.; Kim, B.; Lee, J.; Hahn, B.S.; Lee, J.; et al. Genome-wide association study of resistance to Phytophthora capsici in the pepper (Capsicum spp.) collection. Front. Plant Sci. 2022, 20, 902464. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kang, W.H.; Lee, J.; Yeom, S.I. Development of clustered resistance gene analogs-based markers of resistance to Phytophthora capsici in chili pepper. BioMed Res. Int. 2018, 2018, 8637598. [Google Scholar]
- Bartual, R.; Carbonell, E.A.; Marsal, J.I.; Tello, J.C.; Campos, T. Gene action in the resistance of peppers (Capsicum annuum) to Phytophthora stem blight (Phytophthora capsici L.). Euphytica 1991, 54, 195–200. [Google Scholar] [CrossRef]
- Walker, S.J.; Bosland, P.W. Inheritance of Phytophthora root rot and foliar blight resistance in pepper. J. Am. Soc. Hortic. Sci. 1999, 124, 14–18. [Google Scholar] [CrossRef]
- Yeom, S.I.; Baek, H.K.; Oh, S.K.; Kang, W.H.; Lee, S.J.; Lee, J.M.; Seo, E.; Rose, J.K.; Kim, B.D.; Choi, D. Use of a secretion trap screen in pepper following Phytophthora capsici infection reveals novel functions of secreted plant proteins in modulating cell death. Mol. Plant-Microbe Interact. 2011, 24, 671–684. [Google Scholar] [CrossRef]
- Agarwal, M.; Shrivastava, N.; Padh, H. Advances in molecular marker techniques and their application in plant sciences. Plant Cell Rep. 2008, 27, 617–631. [Google Scholar] [CrossRef]
- Shavrukov, Y.N. CAPS markers in plant biology. Russ. J. Genet. Appl. Res. 2016, 6, 279–287. [Google Scholar] [CrossRef]
- Wiesner-Hanks, T.; Nelson, R. Multiple disease resistance in plants. Annu. Rev. Phytopathol. 2016, 54, 229–252. [Google Scholar] [CrossRef]
- Ribaut, J.M.; Hoisington, D. Marker-assisted selection: New tools and strategies. Trends Plant Sci. 1998, 3, 236–239. [Google Scholar] [CrossRef]
- Cobb, J.N.; Juma, R.U.; Biswas, P.S.; Arbelaez, J.D.; Rutkoski, J.; Atlin, G.; Hagen, T.; Quinn, M.; Ng, E.H. Enhancing the rate of genetic gain in public-sector plant breeding programs: Lessons from the breeder’s equation. Theor. Appl. Genet. 2019, 132, 627–645. [Google Scholar] [CrossRef]
- Barka, G.D.; Lee, J. Molecular Marker Development and Gene Cloning for Diverse Disease Resistance in Pepper (Capsicum annuum L.): Current Status and Prospects. Plant Breed. Biotechnol. 2020, 8, 89–113. [Google Scholar] [CrossRef]
- Quesada-Ocampo, L.M.; Parada-Rojas, C.H.; Hansen, Z.; Vogel, G.; Smart, C.; Hausbeck, M.K.; Carmo, R.M.; Huitema, E.; Naegele, R.P.; Kousik, C.S.; et al. Phytophthora capsici: Recent Progress on Fundamental Biology and Disease Management 100 Years After Its Description. Annu. Rev. Phytopathol. 2023, 61, 1. [Google Scholar] [CrossRef]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 1–17. [Google Scholar] [CrossRef]
- Collard, B.C.Y.; Mackill, D.J. Marker–assisted selection: An approach for precision plant breeding in the 21st century. Philos. Trans. R. Soc. B Rev. 2008, 363, 557–572. [Google Scholar] [CrossRef]
- Langridge, P.; Chalmers, K. The Principle: Identification and Application of Molecular Markers. In Molecular Marker Systems in Plant Breeding and Crop Improvement; Springer: Heidelberg, Germany, 2004; pp. 3–22. [Google Scholar]
- Sharp, P.J.; Johnston, S.; Brown, G.; McIntosh, R.A.; Pallotta, M.; Carter, M.; Bariana, H.S.; Khatkar, S.; Lagudah, E.S.; Singh, R.P.; et al. Validation of molecular markers for wheat breeding. Aust. J. Agric. Res. 2001, 52, 1357–1366. [Google Scholar] [CrossRef]
- Vincze, T.; Posfai, J.; Roberts, R.J. NEBcutter: A program to cleave DNA with restriction enzymes. Nucleic Acids Res. 2003, 31, 3688–3691. [Google Scholar] [CrossRef]
- Fernandez-Pozo, N.; Menda, N.; Edwards, J.D.; Saha, S.; Tecle, I.Y.; Strickler, S.R.; Bombarely, A.; Fisher-York, T.; Pujar, A.; Foerster, H.; et al. The Sol Genomics Network (SGN)—From genotype to phenotype to breeding. Nucleic Acids Res. 2015, 43, D1036–D1041. [Google Scholar] [CrossRef]
| Primer Set | Sequence (5′-3′) | Product Size (bp) |
|---|---|---|
| Phyto_for_1 | AGCTGATCAACACTCAATTTCCT | 98 |
| Phyto_rev_1 | CCGTTGGGTAGTGGACTTGG | |
| Phyto_for_1 | AGCTGATCAACACTCAATTTCCT | 182 |
| Phyto_rev_2 | TGTGCTGGAATTGCTGCTTT | |
| Phyto_for_1 | AGCTGATCAACACTCAATTTCCT | 373 |
| Phyto_rev_3 | CTTCCGTCAAATCCTTCGCC | |
| Phyto_for_2 | CCTCGAATCCCCTTGCAGTA | 393 |
| Phyto_rev_1 | CCGTTGGGTAGTGGACTTGG | |
| Phyto_for_2 | CCTCGAATCCCCTTGCAGTA | 477 |
| Phyto_rev_2 | TGTGCTGGAATTGCTGCTTT | |
| Phyto_for_2 | CCTCGAATCCCCTTGCAGTA | 668 |
| Phyto_rev_3 | CTTCCGTCAAATCCTTCGCC | |
| Phyto_for_3 | ATCTCACAAGTGGGGTCTGG | 923 |
| Phyto_rev_1 | CCGTTGGGTAGTGGACTTGG | |
| Phyto_for_3 | ATCTCACAAGTGGGGTCTGG | 1007 |
| Phyto_rev_2 | TGTGCTGGAATTGCTGCTTT | |
| Phyto_for_3 | ATCTCACAAGTGGGGTCTGG | 1198 |
| Phyto_rev_3 | CTTCCGTCAAATCCTTCGCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bongiorno, G.; Di Noia, A.; Ciancaleoni, S.; Marconi, G.; Cassibba, V.; Albertini, E. Development and Application of a Cleaved Amplified Polymorphic Sequence Marker (Phyto) Linked to the Pc5.1 Locus Conferring Resistance to Phytophthora capsici in Pepper (Capsicum annuum L.). Plants 2023, 12, 2757. https://doi.org/10.3390/plants12152757
Bongiorno G, Di Noia A, Ciancaleoni S, Marconi G, Cassibba V, Albertini E. Development and Application of a Cleaved Amplified Polymorphic Sequence Marker (Phyto) Linked to the Pc5.1 Locus Conferring Resistance to Phytophthora capsici in Pepper (Capsicum annuum L.). Plants. 2023; 12(15):2757. https://doi.org/10.3390/plants12152757
Chicago/Turabian StyleBongiorno, Giacomo, Annamaria Di Noia, Simona Ciancaleoni, Gianpiero Marconi, Vincenzo Cassibba, and Emidio Albertini. 2023. "Development and Application of a Cleaved Amplified Polymorphic Sequence Marker (Phyto) Linked to the Pc5.1 Locus Conferring Resistance to Phytophthora capsici in Pepper (Capsicum annuum L.)" Plants 12, no. 15: 2757. https://doi.org/10.3390/plants12152757
APA StyleBongiorno, G., Di Noia, A., Ciancaleoni, S., Marconi, G., Cassibba, V., & Albertini, E. (2023). Development and Application of a Cleaved Amplified Polymorphic Sequence Marker (Phyto) Linked to the Pc5.1 Locus Conferring Resistance to Phytophthora capsici in Pepper (Capsicum annuum L.). Plants, 12(15), 2757. https://doi.org/10.3390/plants12152757







