Chemical Characterization, Bioactivity and Toxicity of European Flora Plant Extracts in Search for Potential Natural Origin Preservatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Profiles in Phenolic Compounds
2.2. Bioactive Properties
2.3. In Vitro and In Vivo Toxicity
2.3.1. Cytotoxicity
2.3.2. In Vivo Acute Toxicity
3. Materials and Methods
3.1. Plant Material
3.2. Hydroethanolic Extracts
3.3. Chemical Characterization
3.4. Antioxidant Activity
3.4.1. Inhibition of Lipid Peroxidation by Thiobarbituric Acid Reactive Species (TBARS)
3.4.2. Inhibition of Oxidative Hemolysis (OxHLIA)
3.4.3. Diphenyl-1-picrylhydrazyl Radicals Scavenging (DPPH)
3.5. Antimicrobial Activity—Microdilution Method
3.6. Cytotoxicity in Tumor and Non-Tumor Cell Lines
3.7. Artemia Franciscana Acute Toxicity Test
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Staszowska-Karkut, M.; Materska, M. Phenolic composition, mineral content, and beneficial bioactivities of leaf extracts from black currant (Ribes nigrum L.), raspberry (Rubus idaeus), and aronia (Aronia melanocarpa). Nutrients 2020, 12, 463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cucu, A.-A.; Baci, G.-M.; Cucu, A.-B.; Dezsi, Ş.; Lujerdean, C.; Hegeduş, I.C.; Bobiş, O.; Moise, A.R.; Dezmirean, D.S. Calluna vulgaris as a Valuable Source of Bioactive Compounds: Exploring Its Phytochemical Profile, Biological Activities and Apitherapeutic Potential. Plants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Schulz, M.; Chim, J.F. Nutritional and bioactive value of Rubus berries. Food Biosci. 2019, 31, 100438. [Google Scholar] [CrossRef]
- Chepel, V.; Lisun, V.; Skrypnik, L. Changes in the content of some groups of phenolic compounds and biological activity of extracts of various parts of heather (Calluna vulgaris (L.) Hull) at different growth stages. Plants 2020, 9, 926. [Google Scholar] [CrossRef] [PubMed]
- Viapiana, A.; Wesolowski, M. The Phenolic Contents and Antioxidant Activities of Infusions of Sambucus nigra L. Plant Foods Hum. Nutr. 2017, 72, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Starchenko, G.; Hrytsyk, A.; Raal, A.; Koshovyi, O. Phytochemical profile and pharmacological activities of water and hydroethanolic dry extracts of Calluna vulgaris (L.) Hull. herb. Plants 2020, 9, 751. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A comparative review on the extraction, antioxidant content and antioxidant potential of different parts of walnut (Juglans regia L.) fruit and tree. Molecules 2019, 24, 2133. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, F.; Moreira, T.; Pinto, D.; Pimentel, F.B.; Costa, A.S.G.; Nunes, M.A.; Gonçalves Albuquerque, T.; Costaa, H.S.; Palmeira-de-Oliveira, A.; Oliveira, A.I.; et al. S The phytochemical and bioactivity profiles of wild Calluna vulgaris L. flowers. Food Res. Int. 2018, 111, 724–731. [Google Scholar] [CrossRef] [Green Version]
- Dezmirean, D.S.; Paşca, C.; Moise, A.R.; Bobiş, O. Plant sources responsible for the chemical composition and main bioactive properties of poplar-type propolis. Plants 2021, 10, 22. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Ortan, A.; Fierascu, I.C.; Fierascu, I. In vitro and in vivo evaluation of antioxidant properties of wild-growing plants. A short review. Curr. Opin. Food Sci. 2018, 24, 1–8. [Google Scholar] [CrossRef]
- Debbache, N.; Atmani, D.; Atmani, D. Chemical analysis and biological activities of Populus nigra, flower buds extracts as source of propolis in Algeria. Ind. Crops Prod. 2014, 53, 85–92. [Google Scholar] [CrossRef]
- Sundararajan, R.; Haja, N.A.; Venkatesan, K.; Mukherjee, K.; Saha, B.P.; Bandyopadhyay, A.; Mukherjee, P.K. Cytisus scoparius Link-A natural antioxidant. BMC Complement. Altern. Med. 2006, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2021, 73, 285–290. Available online: http://www.elsevier.com/locate/foodchem (accessed on 15 June 2023). [CrossRef]
- Jayaprakasha, G.K.; Selvi, T.; Sakariah, K.K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res. Int. 2003, 36, 117–122. Available online: http://www.elsevier.com/locate/foodres (accessed on 15 June 2023). [CrossRef]
- Geetha, S.; Ram, M.S.; Singh, V.; Ilavazhagan, G.; Sawhney, R.C. Anti-oxidant and immunomodulatory properties of seabuckthorn (Hippophae rhamnoides)—An in vitro study. J. Ethnopharmacol. 2022, 79, 373–378. Available online: http://www.elsevier.com/locate/jethpharm (accessed on 15 June 2023). [CrossRef]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds-recent developments. Trends Food Sci. Technol. 2012, 12, 401–413. [Google Scholar]
- Zhao, J.; Qiu, J.; Zhou, Y.-W.; Hu, X.-J.; Yang, A.-F. Quality disclosure in the presence of strategic consumers. J. Retail. Consum. Serv. 2020, 55, 1–6. [Google Scholar]
- Pereira-Netto, A.B. Tropical Fruits as Natural, Exceptionally Rich, Sources of Bioactive Compounds. Int. J. Fruit Sci. 2018, 18, 231–242. [Google Scholar] [CrossRef]
- Dudonné, S.; Poupard, P.; Coutiére, P.; Woillez, M.; Richard, T.; Mérillon, J.M.; Vitrac, X. Phenolic composition and antioxidant properties of poplar bud (Populus nigra) extract: Individual antioxidant contribution of phenolics and transcriptional effect on skin aging. J. Agric. Food Chem. 2011, 59, 4527–4536. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). LWT-Food Sci. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Valentão, P.; Andrade, P.B.; Ferreira, I.C.F.R.; Ferreres, F.; Bento, A.; Seabra, R.; Estevinho, L. Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol. 2007, 45, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, E.; Greco, G.; Potenza, L.; Calcabrini, C.; Fimognari, C. Natural products to fight cancer: A focus on Juglans Regia. Toxins 2018, 10, 469. [Google Scholar] [CrossRef] [Green Version]
- Kalčíková, G.; Zagorc-Končan, J.; Gotvajn, A.Ž. Artemia salina acute immobilization test: A possible tool for aquatic ecotoxicity assessment. Water Sci. Technol. 2012, 66, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, O.V.; Grigorev, V.Y.; Razdolsky, A.N.; Grigoryeva, L.D.; Dearden, J.C. Effect of the structural factors of organic compounds on the acute toxicity toward Daphnia magna. SAR QSAR Environ. Res. 2020, 31, 615–641. [Google Scholar] [CrossRef] [PubMed]
- de Fátima, M.; Barroso, S. Efeitos Ecotoxicológicos de Pesticidas e Factores Abióticos em Daphnia magna; Environmental Contamination and Toxicology. Master’s Dissertation, Instituto de Ciências Biomédicas Abel Salazar, Universidade do Porto, Porto, Portugal, July 2009. [Google Scholar]
- Skowrońska, W.; Granica, S.; Czerwińska, M.E.; Osińska, E.; Bazylko, A. Sambucus nigra L. leaves inhibit TNF-α secretion by LPS-stimulated human neutrophils and strongly scavenge reactive oxygen species. J. Ethnopharmacol. 2022, 290, 115116. Available online: https://ssrn.com/abstract=3993339 (accessed on 15 June 2023).
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef]
- Goufo, P.; Singh, R.K.; Cortez, I. A reference list of phenolic compounds (Including stilbenes) in grapevine (Vitis vinifera L.) roots, woods, canes, stems, and leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef]
- Allegro, G.; Pastore, C.; Valentini, G.; Filippetti, I. The Evolution of Phenolic Compounds in Vitis vinifera L. Red Berries during Ripening: Analysis and Role on Wine Sensory—A Review. Agronomy 2021, 11, 999. [Google Scholar] [CrossRef]
- Amaral, J.S.; Seabra, R.M.; Andrade, P.B.; Valentão, P.; Pereira, J.A.; Rerreres, F. Phenolic profile in the quality control of walnut (Juglans regia L.) leaves. Food Chem. 2004, 88, 373–379. [Google Scholar] [CrossRef]
- Zheng, W.H.; Bai, H.Y.; Han, S.; Bao, F.; Zhang, K.X.; Sun, L.L.; Du, H.; Yang, Z.G. Analysis on the Constituents of Branches, Berries, and Leaves of Hippophae rhamnoides L. by UHPLC-ESI-QTOF-MS and Their Anti-Inflammatory Activities. Nat. Prod. Commun. 2019, 14, 1934578X19871404. [Google Scholar] [CrossRef] [Green Version]
- Pires, A.F.M. Caracterização Química e Avaliação das Propriedades Bioativas das Sumidades Floridas de Calluna vulgaris (L.) Hull; Pharmacy and Natural Products Chemistry. Master’s Dissertation, Instituto Politécnico de Bragança, Bragança, Portugal, 2018. [Google Scholar]
- di Lorenzo, C.; Frigerio, G.; Colombo, F.; de Sousa, L.P.; Altindişli, A.; Dell’Agli, M.; Restani, P. Phenolic profile and antioxidant activity of different raisin (Vitis vinifera L.) samples. BIO Web Conf. 2016, 7, 04006. [Google Scholar] [CrossRef]
- Ćavar Zeljković, S.; Šišková, J.; Komzáková, K.; De Diego, N.; Kaffková, K.; Tarkowski, P. Phenolic Compounds and Biological Activity of Selected Mentha Species. Plants 2021, 10, 550. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Ramalhosa, E.; Pires, P.; Verdial, J.; Valentão, P.; Andrade, P.; Bento, A.; Pereira, J.A. Vitis vinifera leaves towards bioactivity. Ind. Crops Prod. 2013, 43, 434–440. [Google Scholar] [CrossRef]
- Żurek, N.; Pawłowska, A.; Pycia, K.; Grabek-Lejko, D.; Kapusta, I.T. Phenolic Profile and Antioxidant, Antibacterial, and Antiproliferative Activity of Juglans regia L. Male Flowers. Molecules 2022, 27, 2762. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I.; Topal, F.; Çakmakçi, R.; Bilsel, M.; Gören, A.C.; Erdogan, U. Pomological Features, Nutritional Quality, Polyphenol Content Analysis, and Antioxidant Properties of Domesticated and 3 Wild Ecotype Forms of Raspberries (Rubus idaeus L.). J. Food Sci. 2011, 76, C585–C593. [Google Scholar] [CrossRef]
- Rieger, G.; Müller, M.; Guttenberger, H.; Bucar, F. Influence of altitudinal variation on the content of phenolic compounds in wild populations of Calluna vulgaris, Sambucus nigra, and Vaccinium myrtillus. J. Agric. Food Chem. 2008, 56, 9080–9086. [Google Scholar] [CrossRef] [PubMed]
- Suvanto, J.; Tahtinen, P.; Valkamaa, S.; Engstrom, M.T.; Karonen, M.; Salminen, J.-P. Variability in Foliar Ellagitannins of Hippophae rhamnoides L. and Identification of a New Ellagitannin, Hippophaenin C. J. Agric. Food Chem. 2018, 66, 613–620. [Google Scholar] [CrossRef]
- Pavlović, A.V.; Papetti, A.; Zagorac, D.Č.D.; Gašić, U.M.; Mišić, D.M.; Tešić, Ž.L.; Natić, M.M. Phenolics composition of leaf extracts of raspberry and blackberry cultivars grown in Serbia. Ind. Crops Prod. 2016, 87, 304–314. [Google Scholar] [CrossRef]
- Salejda, A.; Tril, U.; Krasnowska, G. The effect of Sea Buckthorn (Hippophae rhamnoides L.) berries on some quality characteristics of cooked pork sausages. Int. J. Nutr. Food Eng. 2014, 8, 604–607. [Google Scholar]
- Negi, P.S.; Chauhan, A.S.; Sadia, G.A.; Rohinishree, Y.S.; Ramteke, R.S. Antioxidant and antibacterial activities of various seabuckthorn (Hippophae rhamnoides L.) seed extracts. Food Chem. 2005, 92, 119–124. [Google Scholar] [CrossRef]
- Bennacer, A.; Sahir-Halouane, F.; Aitslimane-Aitkaki, S.; Oukali, Z.; Oliveira, I.V.; Rahmouni, N.; Aissaoui, M. Structural characterization of phytochemical content, antibacterial and antifungal activities of Juglans regia L. leaves cultivated in Algeria. Biocatal. Agric. Biotechnol. 2022, 40, 102304. [Google Scholar] [CrossRef]
- Othman, S.; Pinela, J.; Barros, L. Valorisation of quince (Cydonia oblonga Mill.) peel as a source of nutrients and bioactive polyphenols. Master’s Dissertation, Biotechnological Engineering, Instituto Politécnico de Bragança, Bragança, Portugal, 2021. [Google Scholar]
- Baydar, N.G.; Özkan, G.; Saǧdiç, O. Total phenolic contents and antibacterial activities of grape (Vitis vinifera L.) extracts. Food Control 2004, 15, 335–339. [Google Scholar] [CrossRef]
- Noumi, E.; Snoussi, M.; Hajlaoui, H.; Valentin, E.; Bakhrouf, A. Antifungal properties of Salvadora persica and Juglans regia L. extracts against oral Candida strains. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 81–88. [Google Scholar] [CrossRef]
- Colombo, F.; Di Lorenzo, C.; Regazzoni, L.; Fumagalli, M.; Sangiovanni, E.; Peres de Sousa, L.; Bavaresco, L.; Tomasi, D.; Bosso, A.; Aldini, G.; et al. Phenolic profiles and anti-inflammatory activities of sixteen table grape (Vitis vinifera L.) varieties. Food Funct. 2019, 10, 1797–1807. [Google Scholar] [CrossRef]
- Tapia-Salazar, M.; Diaz-Sosa, V.R.; Cárdenas-Chávez, D.L. Toxicological effect and enzymatic disorder of non-studied emerging contaminants in Artemia salina model. Toxicol. Rep. 2022, 9, 210–218. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Mitić, Z.S.; Stojanović-Radić, Z.Z.; Jovanović, S.; Cvetković, V.J.; Nikolić, J.S.; Ickovski, J.D.; Mitrović, T.L.; Nikolić, B.M.; Zlatković, B.K.; Stojanović, G.S. Essential Oils of Three Balkan Abies Species: Chemical Profiles, Antimicrobial Activity and Toxicity toward Artemia salina and Drosophila melanogaster. Chem. Biodivers. 2022, 19, e202200235. [Google Scholar] [CrossRef] [PubMed]
- Yoo, G.; Lee, I.K.; Park, S.; Kim, N.; Park, J.H.; Kim, S.H. Optimization of Extraction Conditions for Phenolic Acids from the Leaves of Melissa officinalis L. Using Response Surface Methodology. Pharmacogn. Mag. 2018, 14, 155–161. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Duarte, L.C.; Lourenço, A.; Roseiro, L.B.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Distillery Residues from Cistus ladanifer (Rockrose) as Feedstock for the Production of Added-Value Phenolic Compounds and Hemicellulosic Oligosaccharides. Bioenerg. Res. 2019, 12, 347–358. [Google Scholar] [CrossRef]
- Pinela, J.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antioxidant activity, ascorbic acid, phenolic compounds and sugars of wild and commercial Tuberaria lignosa samples: Effects of drying and oral preparation methods. Food Chem. 2012, 135, 1028–1035. [Google Scholar] [CrossRef]
- Takebayashi, J.; Iwahashi, N.; Ishimi, Y.; Tai, A. Development of a simple 96-well plate method for evaluation of antioxidant activity based on the oxidative haemolysis inhibition assay (OxHLIA). Food Chem. 2012, 134, 606–610. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- Rotilie, C.A.; Fass, R.J.; Prior, R.B.; Perkins, R.L. Microdilution technique for antimicrobial susceptibility testing of anaerobic bacteria. Antimicrob. Agents Chemother. 1975, 7, 311–315. [Google Scholar] [CrossRef] [Green Version]
- Sorgeloos, P.; Lavens, P. Food and Agriculture Organization of the United Nations. Manual on the Production and Use of Live Food for Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 1996. [Google Scholar]
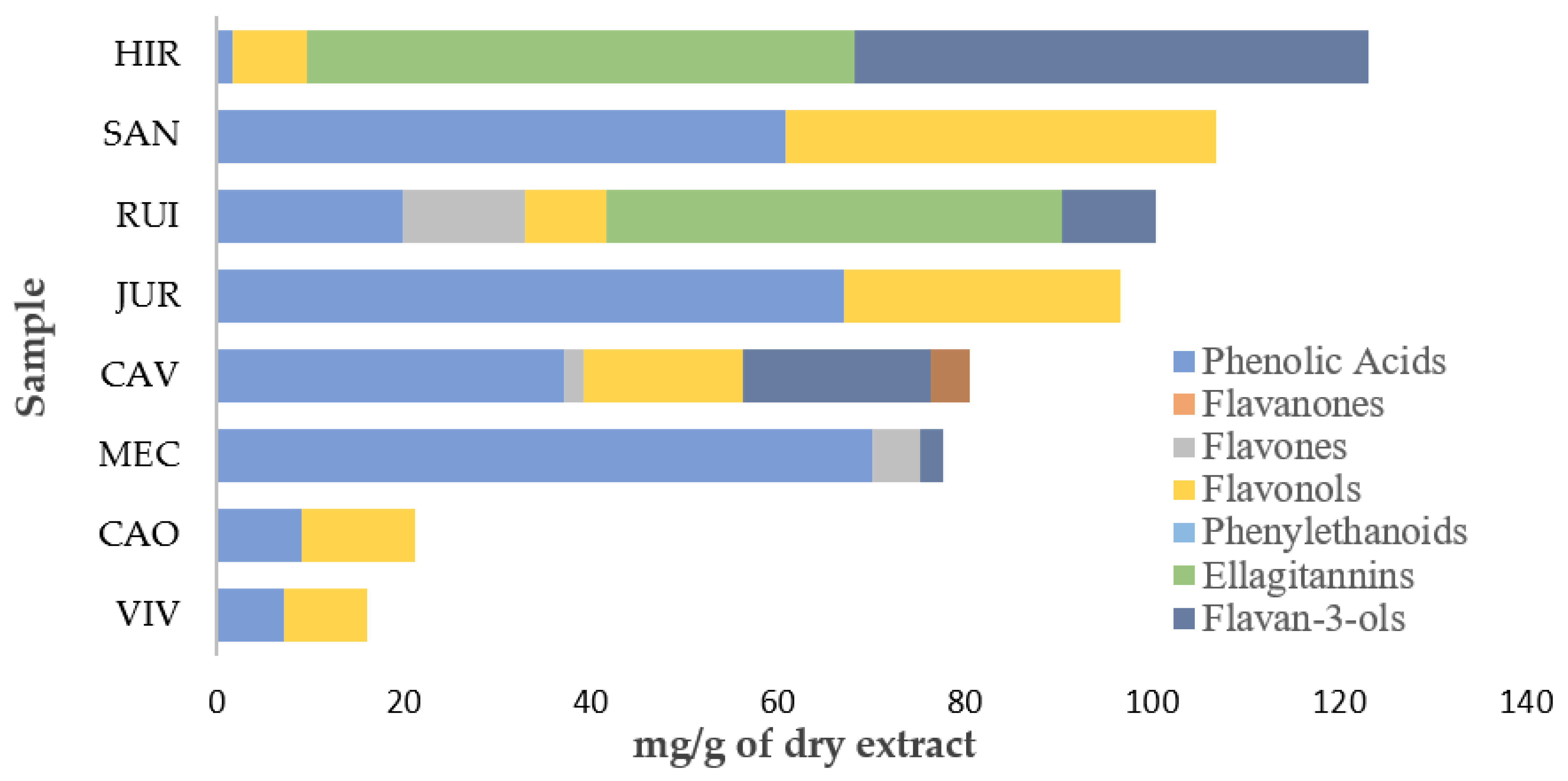
| Peak | Rt (min) | λmax (nm) | [M-H]− (m/z) | Tentative Identification | Content (mg/g) |
|---|---|---|---|---|---|
| Calendula officinalis L. | |||||
| 1 G | 6.31 | 322 | 353 | 5-O-Caffeoylquinic acid | 8.4 ± 0.5 |
| 2 A | 8.71 | 326 | 341 | Caffeic acid hexoside | 0.61 ± 0.01 |
| 3 F | 13.5 | 346 | 755 | Quercetin-3-O-rhamnosylrutinoside | 0.737 ± 0.001 |
| 4 F | 14.99 | 350 | 609 | Quercetin-3-O-rutinoside | 1.01 ± 0.02 |
| 5 J | 15.93 | 354 | 769 | Isorhamnetin-3-O-rhamnosylrutinoside | 0.65 ± 0.04 |
| 6 F | 16.69 | 353 | 595 | Quercetin-O-pentosylhexoside | 1.26 ± 0.02 |
| 7 F | 16.67 | 353 | 623 | Isorhamnetin-3-O-neohesperidoside | 1.31 ± 0.02 |
| 8 F | 19.17 | 346 | 549 | Quercetin-7-O-malonylhexoside | 0.99 ± 0.03 |
| 9 F | 19.84 | 343 | 593 | Kaempferol-O-rutinoside | 0.660 ± 0.002 |
| 10 F | 20.82 | 354 | 623 | Isorhamnetin-3-O-rutinoside | 3.5 ± 0.2 |
| 11 F | 22.02 | 343 | 477 | Isorhamnetin-3-O-glucoside | 0.86 ± 0.01 |
| 12 F | 24.64 | 353 | 519 | Isorhamnetin-3-O-(6″-acetyl)-glucoside | 1.06 ± 0.03 |
| Total Phenolic Acids | 9 ± 0.5 | ||||
| Total Flavonols | 12.1 ± 0.4 | ||||
| Total Phenolic Compounds | 21.0 ± 0.8 | ||||
| Calluna vulgaris (L.) Hull | |||||
| 1 G | 4.36 | 324 | 353 | 3-O-Caffeoylquinic acid | 4.70 ± 0.03 |
| 2 L | 4.94 | 279 | 575 | Proanthocyanidin dimer A type | 5.56 ± 0.08 |
| 3 L | 5.32 | 287 | 1151 | Procyanidin tetramer A type | 7.67 ± 0.04 |
| 4 G | 6.34 | 324 | 707 | Dimer of 3-O-Caffeoylquinic acid | 27.5 ± 0.5 |
| 5 G | 7.41 | 321 | 353 | 5-O-Caffeoylquinic acid | 4.90 ± 0.01 |
| 6 F | 8.64 | 340 | 465 | Dihydroquercetin-6-C-hesoxide | 1.72 ± 0.03 |
| 7 F | 9.77 | 341 | 465 | Dihydroquercetin-C-hesoxide | 0.83 ± 0.02 |
| 8 O | 13.08 | 285 | 435 | Taxifolin-O-pentoside | 4.026 ± 0.003 |
| 9 L | 14.35 | 283 | 1153 | Procyanidin dimer B-type | 6.8 ± 0.1 |
| 10 F | 14.73 | 350 | 595 | Quercetin-O-pentosyl-hexoside | 1.50 ± 0.02 |
| 11 F | 16.49 | 350 | 493 | Myricetin-3-O-glucuronide | 1.43 ± 0.03 |
| 12 F | 17.36 | 355 | 463 | Quercetin-3-O-glucoside | 6.11 ± 0.06 |
| 13 F | 19.26 | 350 | 609 | Quercetin-3-O-rutinoside | 1.18 ± 0.02 |
| 14 F | 20.14 | 352 | 433 | Quercetin-O-pentoside | 2.11 ± 0.02 |
| 15 F | 21.1 | 347 | 477 | Quercetin-3-O-glucuronide | 1.21 ± 0.02 |
| 16 F | 21.9 | 341 | 447 | Quercetin-O-rhamnoside | 1.00 ± 0.01 |
| 17 M | 23.15 | 346 | 621 | Luteolin acetyl pentosyl-hexoside | 2.1 ± 0.1 |
| Total Phenolic Acids | 37.1 ± 0.5 | ||||
| Total Flavan-3-ols | 20.1 ± 0.2 | ||||
| Total Flavones | 2.1 ± 0.1 | ||||
| Total Flavonols | 17.1 ± 0.2 | ||||
| Total Flavononols | 4.026 ± 0.003 | ||||
| Total Phenolic Compounds | 80 ± 1 | ||||
| Hippophae rhamnoides L. | |||||
| 1 I | 5.61 | 274 | 633 | Galloyl-HHDP-glucose | 56.3 ± 0.9 |
| 2 I | 6.31 | 276 | 935 | Galloyl-bis-HHDP-glucose isomer I | 0.61 ± 0.03 |
| 3 P | 7.73 | 275 | 577 | Procyanidin dimer | 51 ± 1 |
| 4 L | 9.12 | 274 | 865 | Procyanidin trimer | 1.10 ± 0.01 |
| 5 F | 10.62 | 350 | 831 | Isorhamnetin-O-hydroxyferuloylhexoside-O-hexoside | 1.32 ± 0.02 |
| 6 L | 11.48 | 274 | 1441 | B-type (epi)catechin pentamer | 0.98 ± 0.03 |
| 7 I | 11.89 | 275 | 935 | Galloyl-bis-HHDP-glucose | 0.184 ± 0.007 |
| 8 F | 12.56 | 352 | 463 | Quercetin-3-O-glucoside | 0.96 ± 0.03 |
| 9 L | 13.51 | 275 | 865 | Procyanidin trimer | 0.887 ± 0.001 |
| 10 L | 14.58 | 281 | 1153 | Procyanidin tetramer | 1.31 ± 0.05 |
| 11 I | 15.02 | 275 | 1567 | Saguiin H10 | 0.47 ± 0.02 |
| 12 F | 16.63 | 350 | 609 | Quercetin-3-O-rutinoside | 1.37 ± 0.03 |
| 13 H | 17.31 | 362 | 433 | Ellagic acid pentoside | 1.70 ± 0.03 |
| 14 F | 17.67 | 282 | 935 | Quercetin-O-glucosyl-glucoside | 1.60 ± 0.02 |
| 15 I | 18.29 | 350 | 961 | Galloyl-bis-HHDP-glucose isomer II | 1.12 ± 0.04 |
| 16 F | 20.82 | 353 | 623 | Isorhamnetin-3-O-rutinoside | 1.24 ± 0.03 |
| 17 F | 22.01 | 350 | 477 | Isorhamnetin-3-O-glucoside | 0.87 ± 0.02 |
| 18 F | 31.9 | 338 | 593 | Kaempferol-O-rutinoside | 0.581 ± 0.003 |
| Total Phenolic Acids | 1.70 ± 0.03 | ||||
| Total Flavonols | 7.9 ± 0.2 | ||||
| Total Ellagitannins | 59 ± 1 | ||||
| Total Flavan-3-ols | 55 ± 1 | ||||
| Total Phenolic Compounds | 123 ± 2 | ||||
| Juglans regia L. | |||||
| 1 G | 4.28 | 322 | 353 | 3-O-Caffeoylquinic acid | 25.0 ± 0.4 |
| 2 G | 5.57 | 312 | 337 | cis 4-p-Coumaroylquinic acid | 12.4 ± 0.4 |
| 3 G | 6.28 | 324 | 353 | 5-O-Caffeoylquinic acid | 11.8 ± 0.5 |
| 4 G | 7.32 | 311 | 337 | trans 4-p-Coumaroylquinic acid | 3.99 ± 0.03 |
| 5 G | 8.57 | 314 | 337 | cis 5-p-Coumaroylquinic acid | 3.89 ± 0.02 |
| 6 G | 10.54 | 325 | 337 | trans 5-p-Coumaroylquinic acid | 5.20 ± 0.03 |
| 7 F | 16.51 | 331 | 463 | Quercetin-3-O-glucoside | 0.92 ± 0.01 |
| 8 F | 17.31 | 354 | 463 | Quercetin-3-O-hexoside | 13.4 ± 0.1 |
| 9 F | 19.17 | 350 | 433 | Quercetin-O-pentoside | 1.82 ± 0.02 |
| 10 F | 20.01 | 351 | 433 | Quercetin-O-pentoside | 7.11 ± 0.08 |
| 11 F | 21.08 | 348 | 447 | Quercetin-3-O-rhamnoside | 3.5 ± 0.2 |
| 12 C | 22.85 | 344 | 417 | Luteolin-O-pentoside | 4.0 ± 0.1 |
| 13 A | 24.29 | 328 | 501 | Caffeic acid derivative | 0.68 ± 0.02 |
| 14 F | 25.69 | 332 | 431 | Kaempferol-O-deoxyhexosyl | 0.89 ± 0.01 |
| 15 F | 26.71 | 340 | 489 | Acetylquercetin-O-rhamnoside isomer I | 0.90 ± 0.01 |
| 16 F | 28.67 | 341 | 489 | Acetylquercetin-O-rhamnoside isomer II | 0.92 ± 0.03 |
| Total Phenolic Acids | 67 ± 1 | ||||
| Total Flavonols | 29.5 ± 0.5 | ||||
| Total Phenolic Compounds | 96 ± 2 | ||||
| Mentha cervina L. | |||||
| 1 D | 4.16 | 278 | 197 | Syringic acid | 0.89 ± 0.01 |
| 2 L | 6.51 | 318 | 305 | Gallocatechin | 2.42 ± 0.07 |
| 3 B | 8.23 | 322 | 313 | Salvianolic acid F | 1.48 ± 0.03 |
| 4 E | 8.79 | 319 | 593 | Apigenin 6,8-C-diglucoside | 5.18 ± 0.07 |
| 5 B | 13.35 | 322 | 537 | Lithospermic acid A isomer I | 2.61 ± 0.05 |
| 6 B | 14.52 | 320 | 539 | Yannaneic acid D isomer I | 4.32 ± 0.04 |
| 7 B | 16.25 | 321 | 539 | Yannaneic acid D isomer II | 4.7 ± 0.3 |
| 8 B | 17.41 | 321 | 717 | Salvianolic acid A | 6.0 ± 0.3 |
| 9 B | 19.98 | 321 | 719 | Sagerinic acid | 2.68 ± 0.04 |
| 10 B | 20.48 | 327 | 717 | Salvianolic acid L | 28.7 ± 0.3 |
| 11 B | 21.87 | 328 | 359 | cis-Rosmarinic acid | 5.37 ± 0.03 |
| 12 B | 22.87 | 331 | 359 | trans-Rosmarinic acid | 2.54 ± 0.02 |
| 13 B | 23.95 | 325 | 537 | Lithospermic acid A isomer II | 3.28 ± 0.04 |
| 14 B | 25.89 | 322 | 521 | Rosmarinic acid hexoside | 1.33 ± 0.03 |
| 15 B | 29.73 | 323 | 537 | Lithospermic acid A isomer III | 6.11 ± 0.08 |
| Total Phenolic Acids | 70 ± 1 | ||||
| Total Flavan-3-ols | 2.42 ± 0.07 | ||||
| Total Flavones | 5.18 ± 0.07 | ||||
| Total Phenolic Compounds | 78 ± 1 | ||||
| Rubus idaeus L. | |||||
| 1 G | 4.34 | 324 | 353 | 3-O-Caffeoylquinic acid | 7.8 ± 0.1 |
| 2 K | 5.15 | 323 | 417 | Dihydroxybenzoic acid-O-dipentoside | 6.9 ± 0.4 |
| 3 A | 5.66 | 321 | 341 | Caffeic acid hexoside | 3.4 ± 0.1 |
| 4 L | 8.28 | 280 | 577 | Procyanidin dimer | 10.13 ± 0.05 |
| 5 C | 9.62 | 322 | 401 | Apigenin-O-pentoside | 1.337 ± 0.001 |
| 6 H | 12.21 | 280 | 1401 | Lambertianin C | 11.7 ± 0.2 |
| 7 I | 12.82 | 280 | 935 | Galloyl-bis-HHDP-glucoside | 36.8 ± 0.4 |
| 8 H | 16.54 | 364 | 433 | Ellagic acid pentoside | 1.78 ± 0.02 |
| 9 F | 17.21 | 352 | 477 | Quercetin-O-glucuronide | 4.5 ± 0.2 |
| 10 F | 19.01 | 324 | 607 | Kaempferol glucuronyl-rhamnoside | 1.11 ± 0.03 |
| 11 F | 19.84 | 325 | 593 | Kaempferol-O-rutinoside | 0.97 ± 0.02 |
| 12 M | 20.74 | 342 | 461 | Luteolin-glucuronide | 11.65 ± 0.05 |
| 13 F | 22.47 | 330 | 461 | Kaempferol-O-glucoronide | 1.24 ± 0.03 |
| 14 F | 22.82 | 326 | 447 | Quercetin-3-O-rhamnoside | 0.95 ± 0.02 |
| Total Phenolic Acids | 19.9 ± 0.7 | ||||
| Total Flavonols | 8.8 ± 0.3 | ||||
| Total Flavones | 12.99 ± 0.05 | ||||
| Total Flavan-3ols | 10.13 ± 0.05 | ||||
| Total Ellagitannins | 48.6 ± 0.6 | ||||
| Total Phenolic Compounds | 100 ± 2 | ||||
| Sambucus nigra L. | |||||
| 1 G | 4.17 | 322 | 353 | 3-O-Caffeoylquinic acid | 5.01 ± 0.03 |
| 2 G | 6.18 | 336 | 353 | 5-O-Caffeoylquinic acid | 52.0 ± 0.3 |
| 3 A | 8.76 | 323 | 179 | Caffeic acid | 1.19 ± 0.03 |
| 4 N | 10.09 | 312 | 337 | p-Coumaroylquinic acid | 2.17 ± 0.03 |
| 5 Q | 12.23 | 318 | 367 | Feruloyl-quinic acid | 0.45 ± 0.01 |
| 6 F | 13.01 | 331 | 625 | Quercetin-diglucoside | 0.680 ± 0.003 |
| 7 F | 13.97 | 325 | 755 | Kaempferol-O-hexosyl-O-rutinoside | 0.554 ± 0.003 |
| 8 F | 15.69 | 324 | 639 | Isorhamnetin dihexoside | 0.72 ± 0.03 |
| 9 F | 16.53 | 356 | 609 | Quercetin-3-O-rutinoside | 24.9 ± 0.4 |
| 10 F | 17.74 | 353 | 609 | Quercetin-deoxyhexosylhexoside | 3.9 ± 0.2 |
| 11 F | 18.94 | 343 | 549 | Quercetin-7-O-malonylhexoside | 3.8 ± 0.2 |
| 12 F | 19.85 | 341 | 593 | Kaempferol-O-rutinoside | 2.00 ± 0.02 |
| 13 F | 20.88 | 354 | 623 | Isorhamnetin-3-O-rutinoside | 5.5 ± 0.3 |
| 14 F | 21.94 | 346 | 477 | Kaempferol-3-O-glucoside | 2.495 ± 0.008 |
| 15 F | 24.59 | 349 | 519 | Isorhamnetin-3-O-acetyl-glucoside | 1.08 ± 0.02 |
| Total Phenolic Acids | 60.8 ± 0.4 | ||||
| Total Flavonols | 46 ± 1 | ||||
| Total Phenolic Compounds | 106 ± 2 | ||||
| Vitis vinifera L. | |||||
| 1 A | 4.55 | 328 | 311 | Caftaric acid | 4.03 ± 0.02 |
| 2 A | 5.92 | 311 | 295 | Cis-Coutaric acid | 1.522 ± 0.009 |
| 3 A | 6.53 | 315 | 295 | Trans-Coutaric acid | 1.52 ± 0.01 |
| 4 F | 17.28 | 354 | 477 | Quercetin-glucoronide | 3.29 ± 0.02 |
| 5 A | 17.73 | 354 | 463 | Quercetin-3-O-glucoside | 3.72 ± 0.09 |
| 6 F | 19.91 | 346 | 593 | Kaempferol-O-rutinoside | 0.89 ± 0.02 |
| 7 F | 21.07 | 346 | 447 | Kaempferol-7-O-hexoside | 1.062 ± 0.006 |
| Total Phenolic Acids | 7.1 ± 0.1 | ||||
| Total Flavonols | 8.97 ± 0.05 | ||||
| Total Phenolic Compounds | 16.0 ± 0.2 | ||||
| CAO | CAV | HIR | JUR | MEC | RUI | SAN | VIV | |
|---|---|---|---|---|---|---|---|---|
| Antioxidant Activity—IC50 (µg/mL) | ||||||||
| TBARS | 129 ± 22 a | 6 ± 1 d | 1.2 ± 0.1 d | 25 ± 1 c | 50 ± 3 b | 6 ± 0.3 d | 123 ± 4 a | 9 ± 1 d |
| DPPH | 64 ± 3 a | 8.7 ± 0.3 f | 5.3 ± 0.4 g | 26.39 ± 0.04 c | 32 ± 1 b | 9.9 ± 0.3 f | 14.0 ± 0.3 d | 11.7 ± 0.2 e |
| OxHLIA (Δt = 60 min) | 266 ± 12 a | 23 ± 1 e | 43 ± 1 d | 27 ± 1 e | 112 ± 6 b | 88 ± 3 c | 28 ± 1 e | 22 ± 1 e |
| OxHLIA (Δt = 120 min) | 500 ± 14 a | 47 ± 1 e | 79 ± 1 d | 51 ± 1 e | 331 ± 14 b | 222 ± 4 c | 44 ± 1 e | 49 ± 1 e |
| CAO | CAV | HIR | JUR | MEC | RUI | SAN | VIV | |
|---|---|---|---|---|---|---|---|---|
| Antimicrobial Activity—MIC (mg/mL) | ||||||||
| Gram-negative Bacteria | ||||||||
| E. cloacae | >10 | 5 | 2.5 | 10 | >10 | 2.5 | 10 | 10 |
| E. coli | >10 | >10 | 10 | >10 | >10 | 10 | >10 | >10 |
| P. aeruginosa | >10 | 10 | 5 | 10 | >10 | 10 | >10 | 10 |
| S. enterica | 10 | 10 | 2.5 | 10 | 10 | 2.5 | 10 | 10 |
| Y. enterocolitica | 10 | 10 | 1.25 | 2.5 | 10 | 5 | 10 | 5 |
| Gram-positive Bacteria | ||||||||
| B. cereus | 2.5 | 10 | 1.25 | 5 | 10 | 5 | 5 | 10 |
| L. monocytogenes | 2.5 | 2.5 | 0.6 | 2.5 | 10 | 2.5 | 1.25 | 10 |
| S. aureus | 5 | 5 | 0.6 | 2.5 | 10 | 2.5 | 10 | 5 |
| Fungi | ||||||||
| A. brasiliensis | 10 | 10 | 10 | 1.25 | 5 | 5 | 10 | 10 |
| A. fumigatus | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Cytotoxicity—GI50 (µg/mL) | Acute Toxicity A. franciscana LC50 (mg/L) | |||||
|---|---|---|---|---|---|---|
| Tumoral | Non-Tumoral | |||||
| AGS | CaCo-2 | MCF-7 | VERO | PLP2 | ||
| CAO | 340 ± 17 a | 213 ± 20 bc | 241 ± 17 ab | 261 ± 27 b | 214 ± 7 d | 200 < LC50 < 400 |
| CAV | 124 ± 2 e | 282 ± 21 a | 274 ± 19 a | 257 ± 24 b | 261 ± 10 b | 200 < LC50 < 400 |
| HIR | 183 ± 11 d | 194 ± 11 bc | 209 ± 11 b | 219 ± 19 b | 178 ± 3 e | >400 |
| JUR | 231 ± 21 c | 219 ± 4 b | 253 ± 12 a | 69 ± 1 c | 193 ± 18 de | >400 |
| MEC | 277 ± 25 b | 297 ± 21 a | >400 | 228 ± 8 b | 238 ± 10 c | >400 |
| RUI | 134 ± 7 e | 174 ± 17 c | 208 ± 16 b | >400 | 175 ± 6 e | >400 |
| SAN | >400 | >400 | >400 | 314 ± 5 a | 381 ± 3 a | >400 |
| VIV | 313 ± 30 ab | >400 | >400 | >400 | >400 | >400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins de Deus, B.; Fernandes, C.; Molina, A.K.; Xavier, V.; Pires, T.C.S.P.; Mandim, F.; Heleno, S.A.; Finimundy, T.C.; Barros, L. Chemical Characterization, Bioactivity and Toxicity of European Flora Plant Extracts in Search for Potential Natural Origin Preservatives. Plants 2023, 12, 2784. https://doi.org/10.3390/plants12152784
Martins de Deus B, Fernandes C, Molina AK, Xavier V, Pires TCSP, Mandim F, Heleno SA, Finimundy TC, Barros L. Chemical Characterization, Bioactivity and Toxicity of European Flora Plant Extracts in Search for Potential Natural Origin Preservatives. Plants. 2023; 12(15):2784. https://doi.org/10.3390/plants12152784
Chicago/Turabian StyleMartins de Deus, Breno, Conceição Fernandes, Adriana K. Molina, Virginie Xavier, Tânia C. S. P. Pires, Filipa Mandim, Sandrina A. Heleno, Tiane C. Finimundy, and Lillian Barros. 2023. "Chemical Characterization, Bioactivity and Toxicity of European Flora Plant Extracts in Search for Potential Natural Origin Preservatives" Plants 12, no. 15: 2784. https://doi.org/10.3390/plants12152784
APA StyleMartins de Deus, B., Fernandes, C., Molina, A. K., Xavier, V., Pires, T. C. S. P., Mandim, F., Heleno, S. A., Finimundy, T. C., & Barros, L. (2023). Chemical Characterization, Bioactivity and Toxicity of European Flora Plant Extracts in Search for Potential Natural Origin Preservatives. Plants, 12(15), 2784. https://doi.org/10.3390/plants12152784












