Comparatively Evolution and Expression Analysis of GRF Transcription Factor Genes in Seven Plant Species
Abstract
:1. Introduction
2. Results
2.1. Identification of GRF Gene Members in Seven Plants
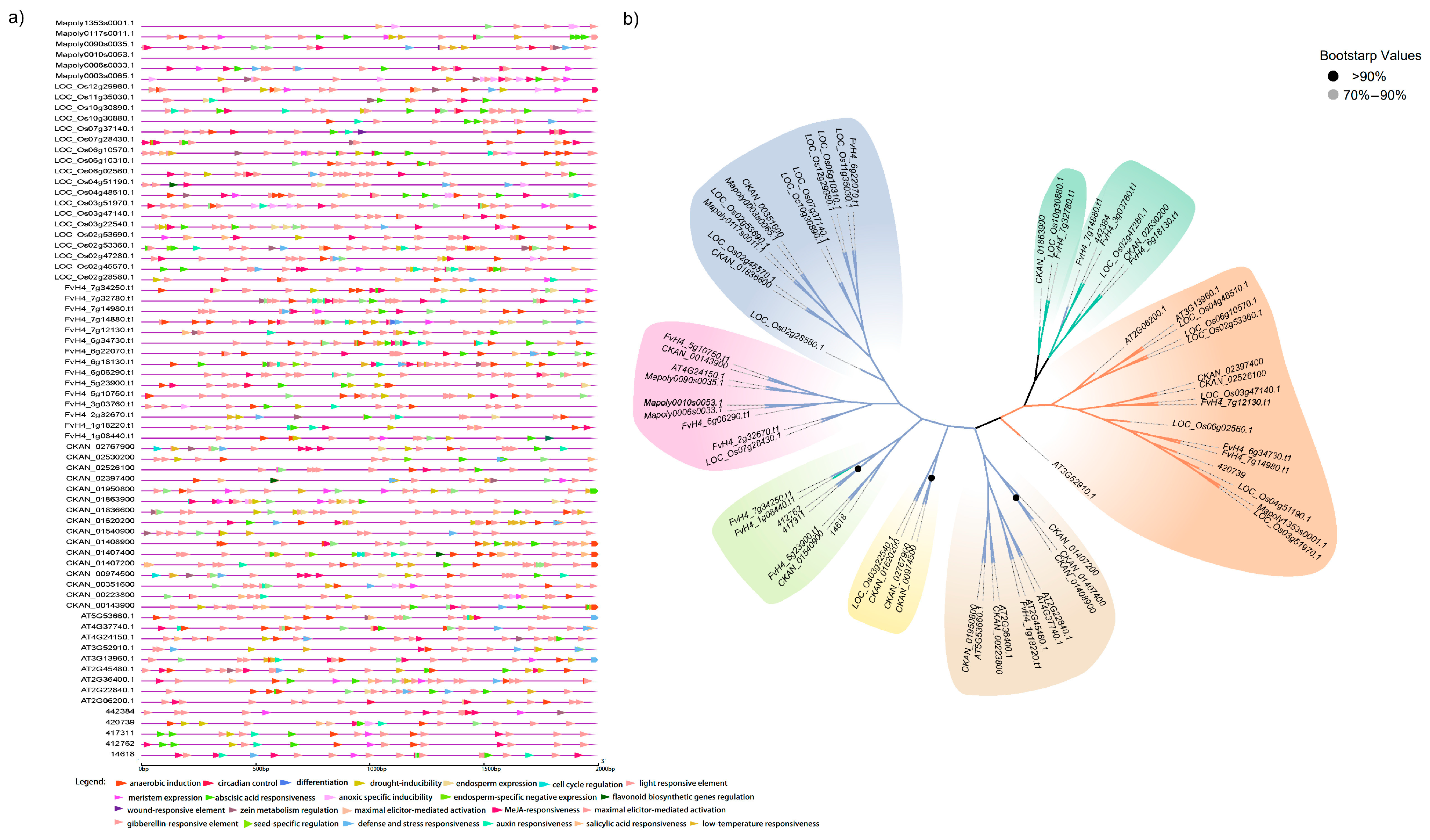
2.2. Cis-Acting Analysis in GRF Promoters
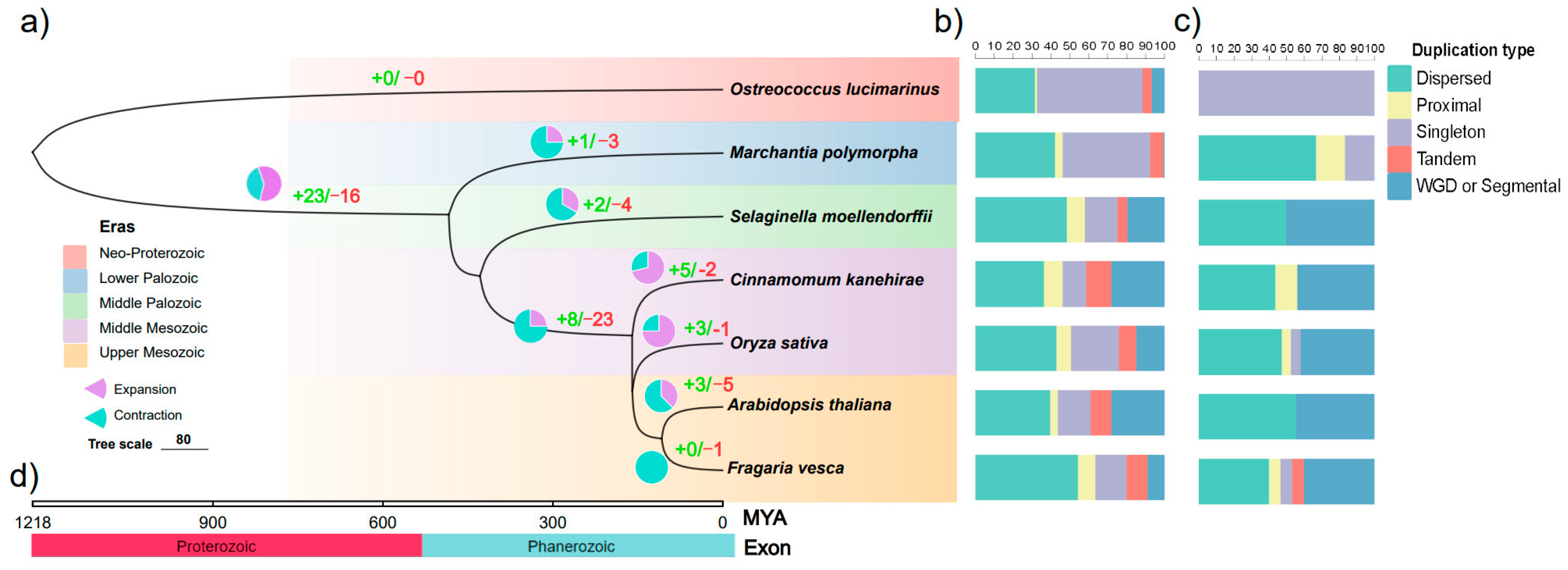
2.3. Duplication and Loss of GRF Gene Family in Seven Typical Species
2.4. The Evolution of Syntenic GRF Genes among Inter- and Intra- Representative Plants Was Accompanied by Genome-Wide Polyploidy Events
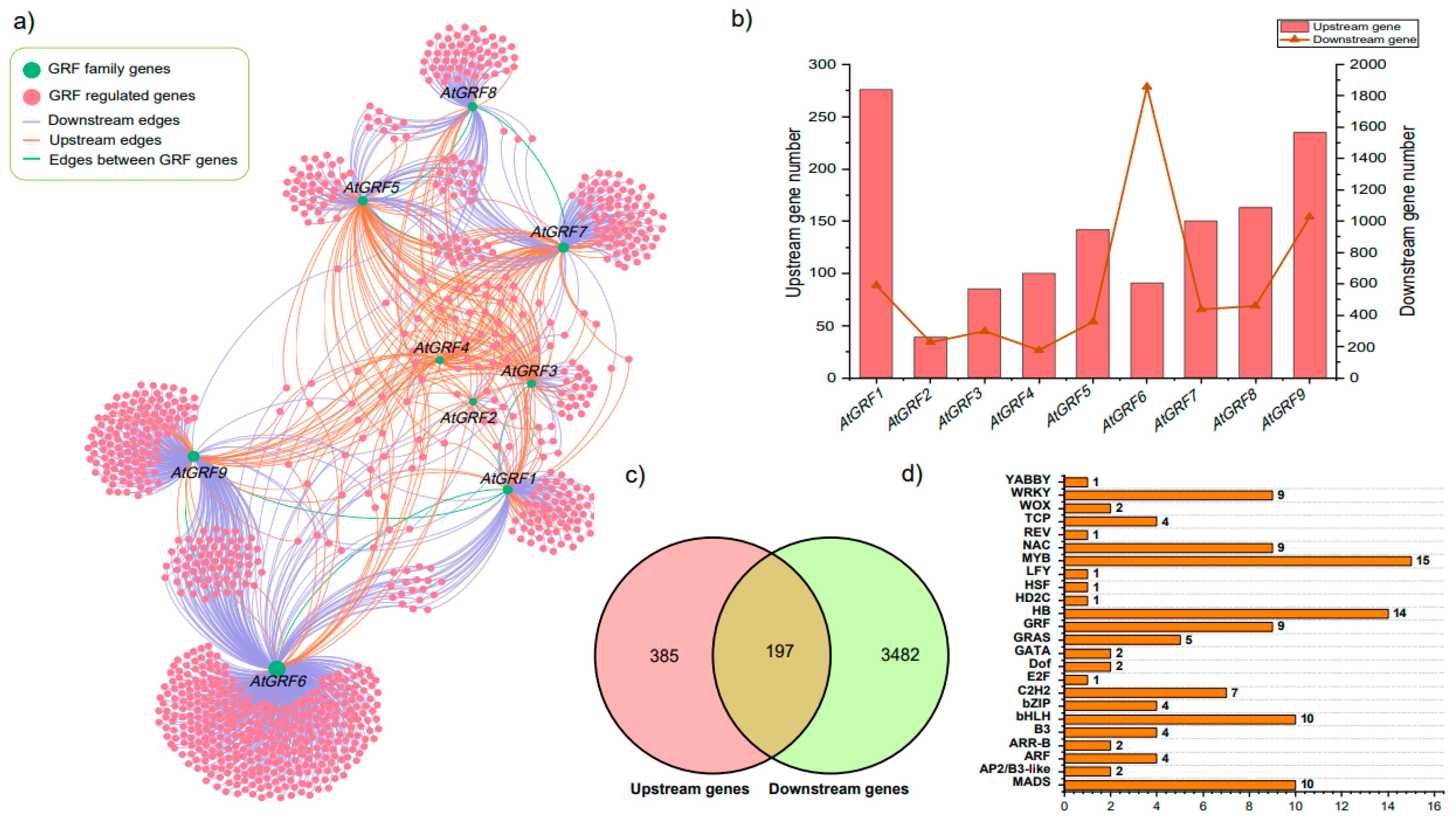
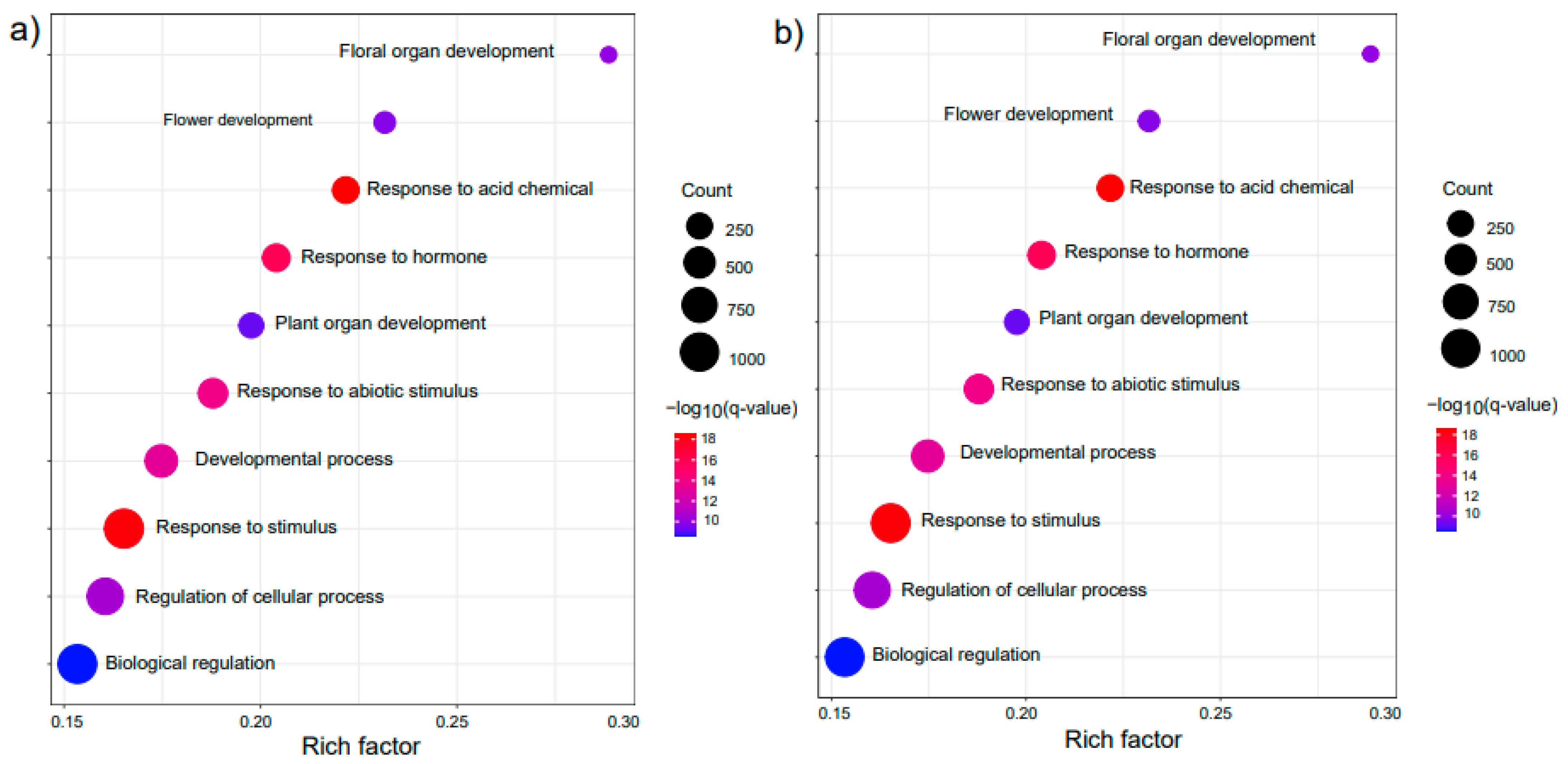
2.5. GRFs May Participate in External Stimuli and Floral Development
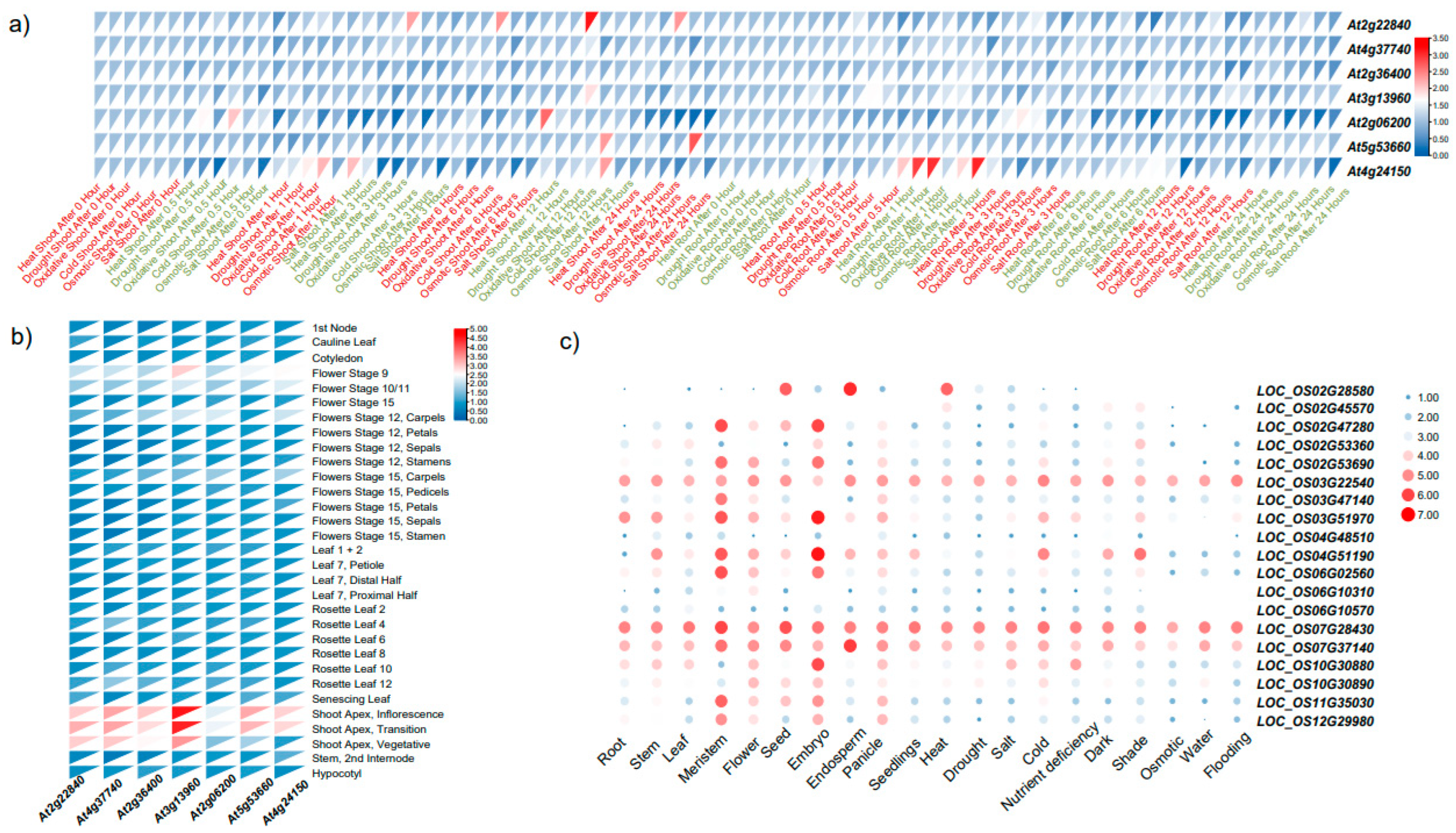
2.6. Exploring the Expression Patterns of GRF Gene Family under Diverse Conditions
2.7. Exploring the Expression Patterns of GRFs Target Genes under Diverse Conditions
2.8. Phylogeny, Expression Level and Interactive Network Analysis of GRF and GIF Gene Family in Arabidopsis
3. Discussion
4. Materials and Methods
4.1. GRF Gene Family Identification and Physicochemical Properties
4.2. Phylogenetic Tree Construction and Conserved Motif Comparison
4.3. Identification and Visualization of Cis-Acting Elements in the Promoters of GRF Genes
4.4. Duplication and Loss Detection for GRF Genes
4.5. Syntenic GRF Genes Recognition
4.6. Interaction Network Construction of GRF Gene Family
4.7. Functional Enrichment Analysis of Main Target Genes
4.8. Expression Patterns of GRF and GRF-Regulated Genes in Different Tissues and under Diverse Conditions
4.9. GRF-GIF Interaction Network Construction
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Downey, J.P.; Gregurek, D.; Keskinkilic, E.; Padilla, R. 10th International Symposium on High-Temperature Metallurgical Processing, 1st ed.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Cao, Z.; Tang, H.; Cai, Y.; Zeng, B.; Zhao, J.; Tang, X.; Lu, M.; Wang, H.; Zhu, X.; Wu, X.; et al. Natural variation of HTH5 from wild rice, Oryza rufipogon Griff., is involved in conferring high-temperature tolerance at the heading stage. Plant Biotechnol. J. 2022, 20, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castroverde, C.D.M.; Dina, D. Temperature regulation of plant hormone signaling during stress and development. J. Exp. Bot. 2021, 72, 7436–7458. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Zhang, R. High Temperature Effects on Electron and Proton Circuits of Photosynthesis. J. Integr. Plant Biol. 2010, 52, 712–722. [Google Scholar] [CrossRef] [PubMed]
- He, N.-Y.; Chen, L.-S.; Sun, A.-Z.; Zhao, Y.; Yin, S.-N.; Guo, F.-Q. A nitric oxide burst at the shoot apex triggers a heat-responsive pathway in Arabidopsis. Nat. Plants 2022, 8, 434–450. [Google Scholar] [CrossRef]
- Qin, L.; Chen, H.; Wu, Q.; Wang, X. Identification and exploration of the GRF and GIF families in maize and foxtail millet. Physiol. Mol. Biol. Plants 2022, 28, 1717–1735. [Google Scholar]
- Du, W.; Yang, J.; Li, Q.; Su, Q.; Yi, D.; Pang, Y. Genome-Wide Identification and Characterization of Growth Regulatory Factor Family Genes in Medicago. Int. J. Mol. Sci. 2022, 23, 6905. [Google Scholar] [CrossRef]
- Li, Z.; Xie, Q.; Yan, J.; Chen, J.; Chen, Q. Genome-Wide Identification and Characterization of the Abiot-ic-Stress-Responsive GRF Gene Family in Diploid Woodland Strawberry (Fragaria vesca). Plants 2021, 10, 1916. [Google Scholar] [CrossRef]
- van der Knaap, E.; Kim, J.H.; Kende, H. A Novel Gibberellin-Induced Gene from Rice and Its Potential Regulatory Role in Stem Growth. Plant Physiol. 2000, 122, 695–704. [Google Scholar] [CrossRef] [Green Version]
- Fonini, L.S.; Lazzarotto, F.; Barros, P.M.; Cabreira-Cagliari, C.; Martins, M.A.B.; Saibo, N.J.; Turchetto-Zolet, A.C.; Margis-Pinheiro, M. Molecular evolution and diversification of the GRF transcription factor family. Genet. Mol. Biol. 2020, 43, 20200080. [Google Scholar] [CrossRef]
- Kim, J.H.; Kende, H. A transcriptional coactivator, AtGIF1, is involved in regulating leaf growth and morphology in Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 13374–13379. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Tsukaya, H. Regulation of plant growth and development by the GROWTH-REGULATING FACTOR and GRF-INTERACTING FACTOR duo. J. Exp. Bot. 2015, 66, 6093–6107. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Li, X.; Hou, Y.; Li, Y.; Hu, Y. Functional conservation and divergence in plant-specific GRF gene family revealed by sequences and expression analysis. Open Life Sci. 2022, 17, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Omidbakhshfard, M.A.; Proost, S.; Fujikura, U.; Mueller-Roeber, B. Growth-Regulating Factors (GRFs): A Small Transcription Factor Family with Important Functions in Plant Biology. Mol. Plant 2015, 8, 998–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debernardi, J.M.; Tricoli, D.M.; Ercoli, M.F.; Hayta, S.; Ronald, P.; Palatnik, J.F.; Dubcovsky, J. A GRF–GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat. Biotechnol. 2020, 38, 1274–1279. [Google Scholar] [CrossRef]
- Lantzouni, O.; Alkofer, A.; Falter-Braun, P.; Schwechheimer, C. GROWTH-REGULATING FACTORS Interact with DELLAs and Regulate Growth in Cold Stress. Plant Cell 2020, 32, 1018–1034. [Google Scholar] [CrossRef]
- Wu, W.; Li, J.; Wang, Q.; Lv, K.; Du, K.; Zhang, W.; Li, Q.; Kang, X.; Wei, H. Growth-regulating factor 5 (GRF5)-mediated gene regulatory network promotes leaf growth and expansion in poplar. New Phytol. 2021, 230, 612–628. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, W.; Wang, Y.; He, Q.; Shu, F.; Liu, H.; Wang, J.; Wang, J.; Yuan, L.; Deng, H. OsGRF4 controls grain shape, panicle length and seed shattering in rice. J. Integr. Plant Biol. 2016, 58, 836–847. [Google Scholar] [CrossRef] [Green Version]
- Piya, S.; Liu, J.; Burch-Smith, T.; Baum, T.J.; Hewezi, T. A role for Arabidopsis growth-regulating factors 1 and 3 in growth–stress antagonism. J. Exp. Bot. 2020, 71, 1402–1417. [Google Scholar] [CrossRef]
- Horiguchi, G.; Kim, G.-T.; Tsukaya, H. The transcription factor AtGRF5 and the transcription coactivator AN3 regulate cell proliferation in leaf primordia of Arabidopsis thaliana. Plant J. 2005, 43, 68–78. [Google Scholar] [CrossRef]
- Liu, J.; Hua, W.; Yang, H.-L.; Zhan, G.-M.; Li, R.-J.; Deng, L.-B.; Wang, X.-F.; Liu, G.-H.; Wang, H.-Z. The BnGRF2 gene (GRF2-like gene from Brassica napus) enhances seed oil production through regulating cell number and plant photosynthesis. J. Exp. Bot. 2012, 63, 3727–3740. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Mizoi, J.; Kidokoro, S.; Maruyama, K.; Nakajima, J.; Nakashima, K.; Mitsuda, N.; Takiguchi, Y.; Ohme-Takagi, M.; Kondou, Y.; et al. Arabidopsis growth-regulating factor7 functions as a transcriptional repressor of abscisic acid- and osmotic stress-responsive genes, including DREB2A. Plant Cell 2012, 24, 3393–3405. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.-Q.; Jian, H.-J.; Yang, B.; Lu, K.; Zhang, A.-X.; Liu, P.; Li, J.-N. Genome-wide analysis and expression profiling of the GRF gene family in oilseed rape (Brassica napus L.). Gene 2017, 620, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Chen, C.; Jiang, L.; Zhang, J.; Ren, Q. Genome-wide identification, classification and expression analysis of the JmjC domain-containing histone demethylase gene family in maize. BMC Genom. 2019, 20, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Kabir, N.; Wei, Z.; Sun, Z.; Wang, J.; Qi, J.; Liu, M.; Liu, J.; Zhou, K. Genome-wide identification and expression profile of GhGRF gene family in Gossypium hirsutum L. PeerJ 2022, 10, e13372. [Google Scholar] [CrossRef]
- Shang, S.; Wu, C.; Huang, C.; Tie, W.; Yan, Y.; Ding, Z.; Xia, Z.; Wang, W.; Peng, M.; Tian, L.; et al. Genome-Wide Analysis of the GRF Family Reveals Their Involvement in Abiotic Stress Response in Cassava. Genes 2018, 9, 110. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Choi, D.; Kende, H. The AtGRF family of putative transcription factors is involved in leaf and cotyledon growth in Arabidopsis. Plant J. 2003, 36, 94–104. [Google Scholar] [CrossRef]
- Choi, D.; Kim, J.H.; Kende, H. Whole Genome Analysis of the OsGRF Gene Family Encoding Plant-Specific Putative Transcription Activators in Rice (Oryza sativa L.). Plant Cell Physiol. 2004, 45, 897–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Chen, S.; Shi, L.; Gong, D.; Zhang, S.; Zhao, Q.; Zhan, D.; Vasseur, L.; Wang, Y.; Yu, J.; et al. Haplotype-resolved genome assembly provides insights into evolutionary history of the tea plant Camellia sinensis. Nat. Genet. 2021, 53, 1250–1259. [Google Scholar] [CrossRef]
- Chan, A.P.; Crabtree, J.; Zhao, Q.; Lorenzi, H.; Orvis, J.; Puiu, D.; Melake-Berhan, A.; Jones, K.M.; Redman, J.; Chen, G.; et al. Draft genome sequence of the oilseed species Ricinus communis. Nat. Biotechnol. 2010, 28, 951–956. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Tucker, J.R.; Bekele, W.A.; You, F.M.; Fu, Y.-B.; Khanal, R.; Yao, Z.; Singh, J.; Boyle, B.; Beattie, A.D.; et al. Genome Assembly of the Canadian two-row Malting Barley cultivar AAC Synergy. G3 Genes Genomes Genet. 2021, 11, jkab031. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Wang, H.; Sun, J.; Ma, L.; Miao, F.; Zhang, Z.; Cheng, Y.; Huang, J.; Yang, G.; et al. A High-Quality Genome Assembly of Sorghum dochna. Front. Genet. 2022, 13, 844385. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, S.; Lan, L.; Xie, M.; Cheng, S.; Gan, X.; Huang, G.; Du, G.; Yu, K.; Ni, X.; et al. De novo genome assembly of a foxtail millet cultivar Huagu11 uncovered the genetic difference to the cultivar Yugu1, and the genetic mechanism of imazethapyr tolerance. BMC Plant Biol. 2021, 21, 271. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ma, X.; Zhang, J.; Zhou, Y.; Liu, M.; Huang, L.; Sun, S.; Zhang, X.; Gao, X.; Zhan, W.; et al. Chromosome conformation capture resolved near complete genome assembly of broomcorn millet. Nat. Commun. 2019, 10, 464. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Li, X.; Liu, H.; Wang, X.; Li, W.; Chen, M.-S.; Niu, L.-J. Chromatin Architectures Are Associated with Response to Dark Treatment in the Oil Crop Sesamum indicum, Based on a High-Quality Genome Assembly. Plant Cell Physiol. 2020, 61, 978–987. [Google Scholar] [CrossRef]
- Sankoff, D. Gene and genome duplication. Curr. Opin. Genet. Dev. 2001, 11, 681–684. [Google Scholar] [CrossRef]
- Hayes, S.; Schachtschabel, J.; Mishkind, M.; Munnik, T.; Arisz, S.A. Hot topic: Thermosensing in plants. Plant Cell Environ. 2021, 44, 2018–2033. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Qiao, L.; Chen, J.; Rong, Y.; Zhao, Y.; Cui, X.; Xu, J.; Hou, X.; Dong, C. Arabidopsis REM16 acts as a B3 domain transcription factor to promote flowering time via directly binding to the promoters of SOC1 and FT. Plant J. 2020, 103, 1386–1398. [Google Scholar] [CrossRef]
- Gan, E.S.; Xu, Y.; Wong, J.Y.; Goh, J.G.; Sun, B.; Wee, W.Y.; Huang, J.; Ito, T. Jumonji demethylases moderate pre-cocious flowering at elevated temperature via regulation of FLC in Arabidopsis. Nat. Commun. 2014, 5, 5098. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Hu, H.; Ren, H.; Yang, Z.; Qiu, Q.; Qi, W.; Liu, X.; Chen, X.; Cui, X.; Li, S.; et al. The Arabidopsis H3K27me3 demethylase JUMONJI 13 is a temperature and photoperiod dependent flowering repressor. Nat. Commun. 2019, 10, 1303. [Google Scholar] [CrossRef] [Green Version]
- Molina, I.; Ohlrogge, J.B.; Pollard, M. Deposition and localization of lipid polyester in developing seeds of Brassica napus and Arabidopsis thaliana. Plant J. 2008, 53, 437–449. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Jin, J.; Xie, X.; Zhang, H.; Chen, Q.; Luo, Z.; Yang, J. Genome-wide identification and analysis of the growth-regulating factor family in tobacco ( Nicotiana tabacum). Gene 2018, 639, 117–127. [Google Scholar] [CrossRef]
- Gao, B.; Chen, M.; Li, X.; Zhang, J. Ancient duplications and grass-specific transposition influenced the evolution of LEAFY transcription factor genes. Commun. Biol. 2019, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Qiao, X.; Li, Q.; Yin, H.; Qi, K.; Li, L.; Wang, R.; Zhang, S.; Paterson, A.H. Gene duplication and evolution in recurring polyploidization–diploidization cycles in plants. Genome Biol. 2019, 20, 38. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Guo, S.; Xu, Y.; Li, C.; Zhang, Z.; Zhang, D.; Xu, S.; Zhang, C.; Chong, K. OsmiR396d-Regulated OsGRFs Function in Floral Organogenesis in Rice through Binding to Their Targets OsJMJ706 and OsCR4. Plant Physiol. 2014, 165, 160–174. [Google Scholar] [CrossRef] [Green Version]
- Shao, W.; Chen, W.; Zhu, X.; Zhou, X.; Jin, Y.; Zhan, C.; Liu, G.; Liu, X.; Ma, D.; Qiao, Y. Genome-Wide Identification and Characterization of Wheat 14-3-3 Genes Unravels the Role of TaGRF6-A in Salt Stress Tolerance by Binding MYB Tran-scription Factor. Int. J. Mol. Sci. 2021, 22, 1904. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, X.; Chang, S.; Chu, Z.; Wang, H.; Han, S.; Wang, Y. Calcium-dependent protein kinase 21 phos-phorylates 14-3-3 proteins in response to ABA signaling and salt stress in rice. Biochem. Biophys. Res. Commun. 2017, 493, 1450–1456. [Google Scholar] [CrossRef]
- Xia, J.; Wang, D.; Peng, Y.; Wang, W.; Wang, Q.; Xu, Y.; Li, T.; Zhang, K.; Li, J.; Xu, X. Genome-Wide Analysis of the YABBY Transcription Factor Family in Rapeseed (Brassica napus L.). Genes 2021, 12, 981. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.; Zhang, W.; Fu, Y.; Huang, H. ASYMMETRIC LEAVES1, an Arabidopsis gene that is involved in the control of cell differentiation in leaves. Planta 2002, 214, 694–702. [Google Scholar] [CrossRef]
- Jin, S.; Kim, S.Y.; Susila, H.; Nasim, Z.; Youn, G.; Ahn, J.H. FLOWERING LOCUS M isoforms differentially affect the subcellular localization and stability of SHORT VEGETATIVE PHASE to regulate temperature-responsive flowering in Arabidopsis. Mol. Plant 2022, 15, 1696–1709. [Google Scholar] [CrossRef]
- Tian, T.; Yu, R.; Suo, Y.; Cheng, L.; Li, G.; Yao, D.; Song, Y.; Wang, H.; Li, X.; Gao, G. A Genome-Wide Analysis of StTGA Genes Reveals the Critical Role in Enhanced Bacterial Wilt Tolerance in Potato During Ralstonia solanacearum Infection. Front. Genet. 2022, 13, 894844. [Google Scholar] [CrossRef]
- Chen, J.; Nolan, T.M.; Ye, H.; Zhang, M.; Tong, H.; Xin, P.; Chu, J.; Chu, C.; Li, Z.; Yin, Y. Arabidopsis WRKY46, WRKY54, and WRKY70 Transcription Factors Are Involved in Brassinosteroid-Regulated Plant Growth and Drought Responses. Plant Cell 2017, 29, 1425–1439. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Liu, S.; Zhang, C.; He, J.; Ma, D.; Wang, X.; Dong, T.; Guo, F.; Cai, J.; Long, T.; et al. The unique sweet potato NAC transcription factor IbNAC3 modulates combined salt and drought stresses. Plant Physiol. 2023, 191, 747–771. [Google Scholar] [CrossRef]
- Luo, G.; Palmgren, M. GRF-GIF Chimeras Boost Plant Regeneration. Trends Plant Sci. 2021, 26, 201–204. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Anderson, J.B.; Chitsaz, F.; Derbyshire, M.K.; DeWeese-Scott, C.; Fong, J.H.; Geer, L.Y.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; et al. CDD: Specific functional annotation with the Conserved Domain Database. Nucleic Acids Res. 2009, 37, D205–D210. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37 (Suppl. S2), w202–w208. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, 256–259. [Google Scholar] [CrossRef] [Green Version]
- Mikhaylova, Y.V.; Puzanskiy, R.K.; Shishova, M.F. Evolution of 14-3-3 Proteins in Angiosperm Plants: Recurring Gene Duplication and Loss. Plants 2021, 10, 2724. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [Green Version]
- De Clercq, I.; Van de Velde, J.; Luo, X.; Liu, L.; Storme, V.; Van Bel, M.; Pottie, R.; Vaneechoutte, D.; Van Breusegem, F.; Vandepoele, K. Integrative inference of transcriptional networks in Arabidopsis yields novel ROS signalling regu-lators. Nat. Plants 2021, 7, 500–513. [Google Scholar] [CrossRef]
- Jacomy, M.; Venturini, T.; Heymann, S.; Bastian, M. ForceAtlas2, a Continuous Graph Layout Algorithm for Handy Network Visualization Designed for the Gephi Software. PLoS ONE 2014, 9, e98679. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2023, 51, D418–D427. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Amundson, K.K.; Borton, M.A.; Daly, R.A.; Hoyt, D.W.; Wong, A.; Eder, E.; Moore, J.; Wunch, K.; Wrighton, K.C.; Wilkins, M.J. Microbial colonization and persistence in deep fractured shales is guided by metabolic exchanges and viral predation. Microbiome 2022, 10, 5. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Wen, S.; Wu, Y.; Shang, L.; Wu, L.; Lyu, D.; Yu, H.; Wang, J.; Jian, H. Comparatively Evolution and Expression Analysis of GRF Transcription Factor Genes in Seven Plant Species. Plants 2023, 12, 2790. https://doi.org/10.3390/plants12152790
Cheng Z, Wen S, Wu Y, Shang L, Wu L, Lyu D, Yu H, Wang J, Jian H. Comparatively Evolution and Expression Analysis of GRF Transcription Factor Genes in Seven Plant Species. Plants. 2023; 12(15):2790. https://doi.org/10.3390/plants12152790
Chicago/Turabian StyleCheng, Zhihan, Shiqi Wen, Yuke Wu, Lina Shang, Lin Wu, Dianqiu Lyu, Hongtao Yu, Jichun Wang, and Hongju Jian. 2023. "Comparatively Evolution and Expression Analysis of GRF Transcription Factor Genes in Seven Plant Species" Plants 12, no. 15: 2790. https://doi.org/10.3390/plants12152790
APA StyleCheng, Z., Wen, S., Wu, Y., Shang, L., Wu, L., Lyu, D., Yu, H., Wang, J., & Jian, H. (2023). Comparatively Evolution and Expression Analysis of GRF Transcription Factor Genes in Seven Plant Species. Plants, 12(15), 2790. https://doi.org/10.3390/plants12152790





