Identification and Expression Analysis of the Alfin-like Gene Family in Tomato and the Role of SlAL3 in Salt and Drought Stresses
Abstract
:1. Introduction
2. Results
2.1. Identification and Characteristics of ALs in Tomato
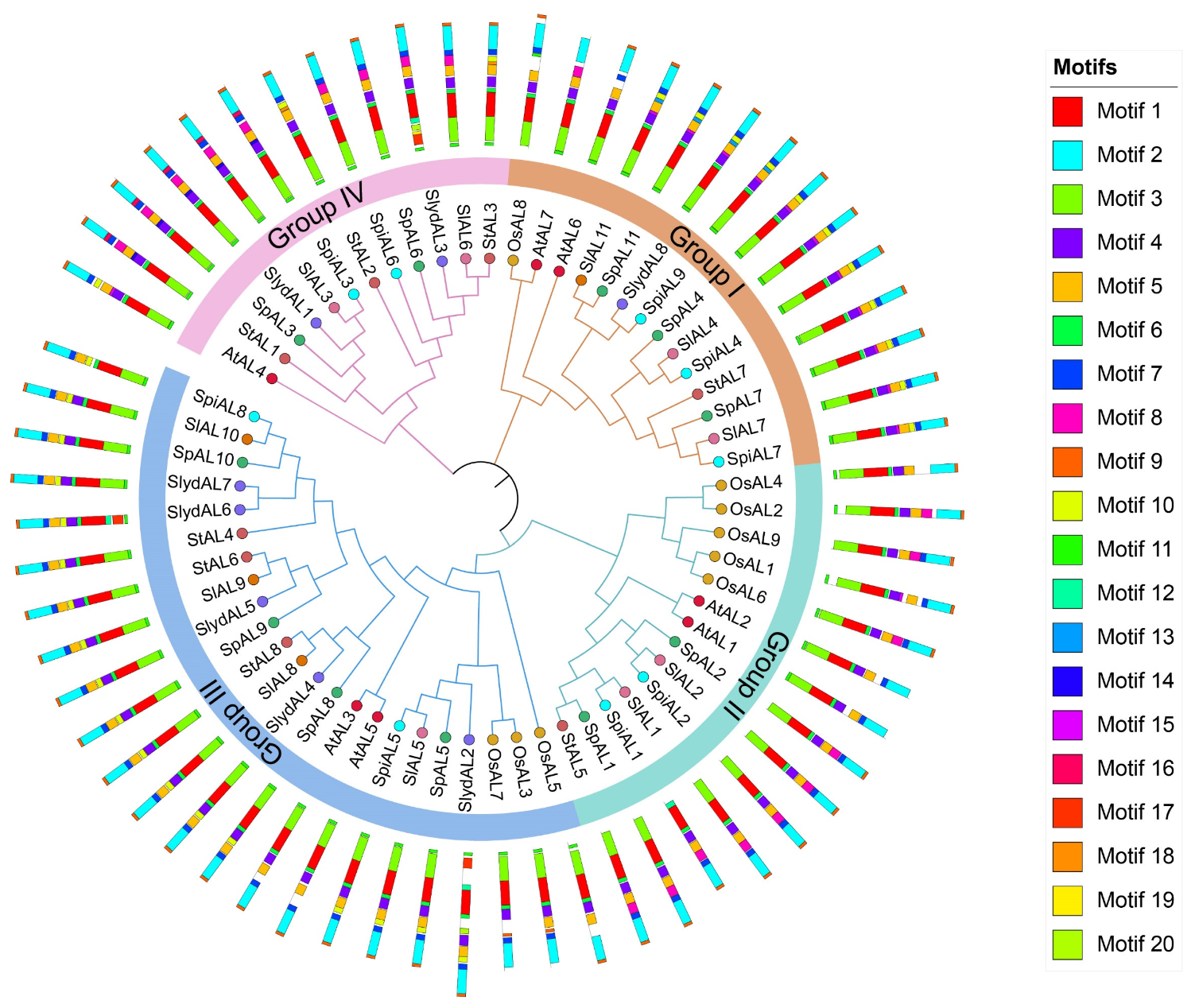
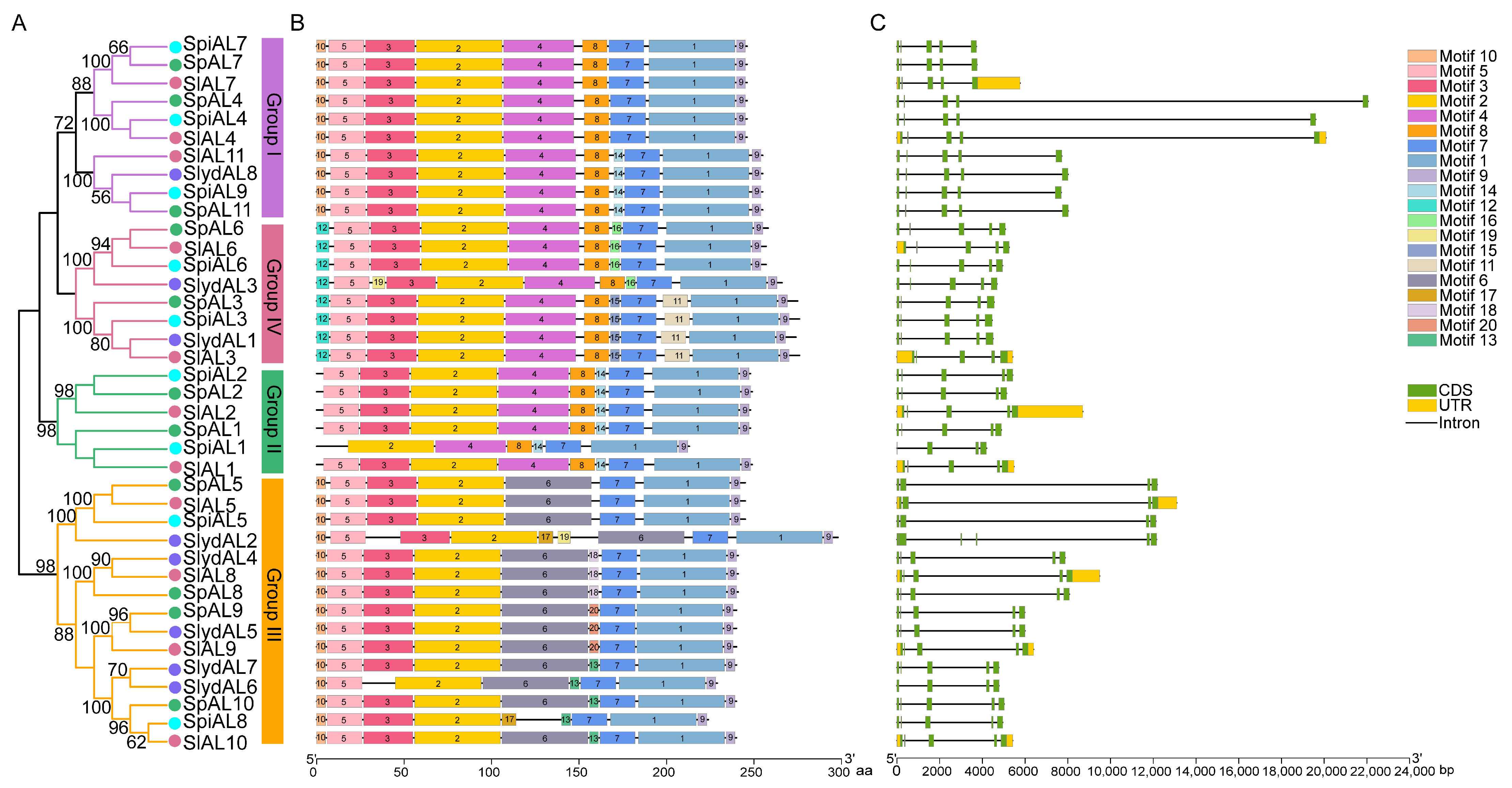
2.2. Phylogenetic Analyses, Gene Structure, and Conserved Motif Distributions of ALs in Tomato
2.3. Gene Structure and Conserved Motif Analysis of Tomato ALs
2.4. Cis-Acting Element Analysis of Tomato ALs
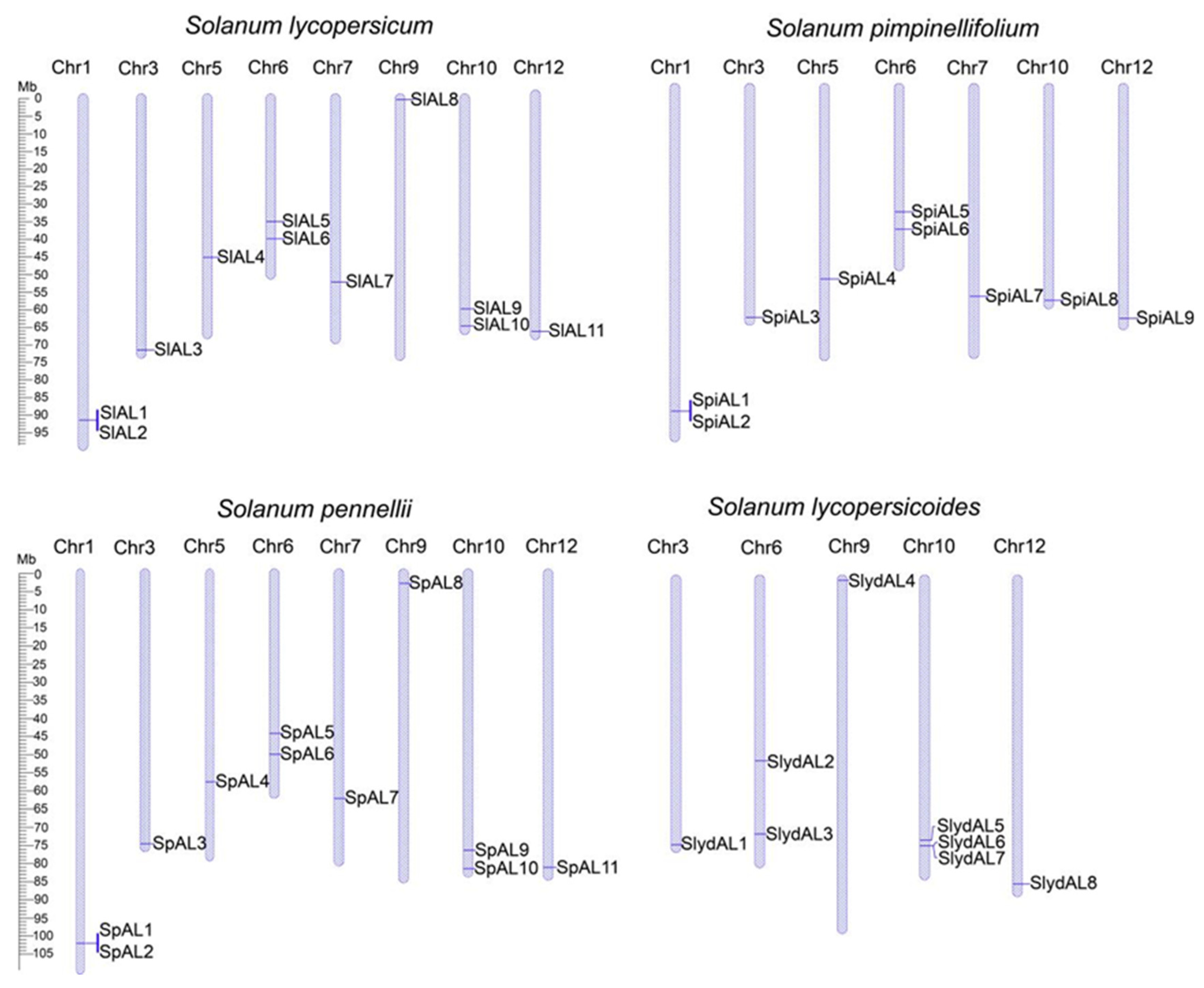
2.5. Chromosomal Location and Gene Duplication of AL Genes in Tomatoes
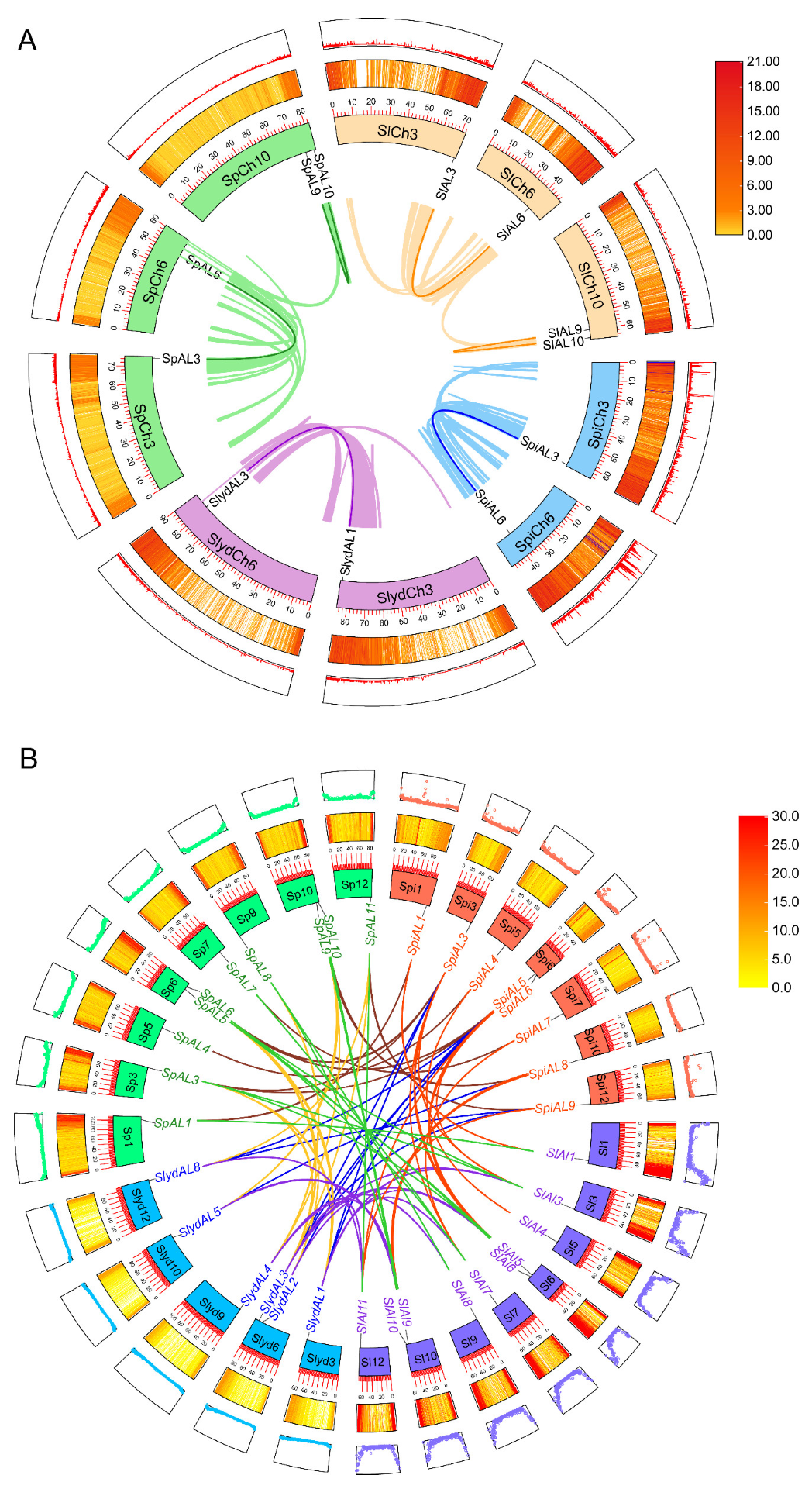
2.6. Collinearity and Evolutionary Analysis of AL Genes in Tomato
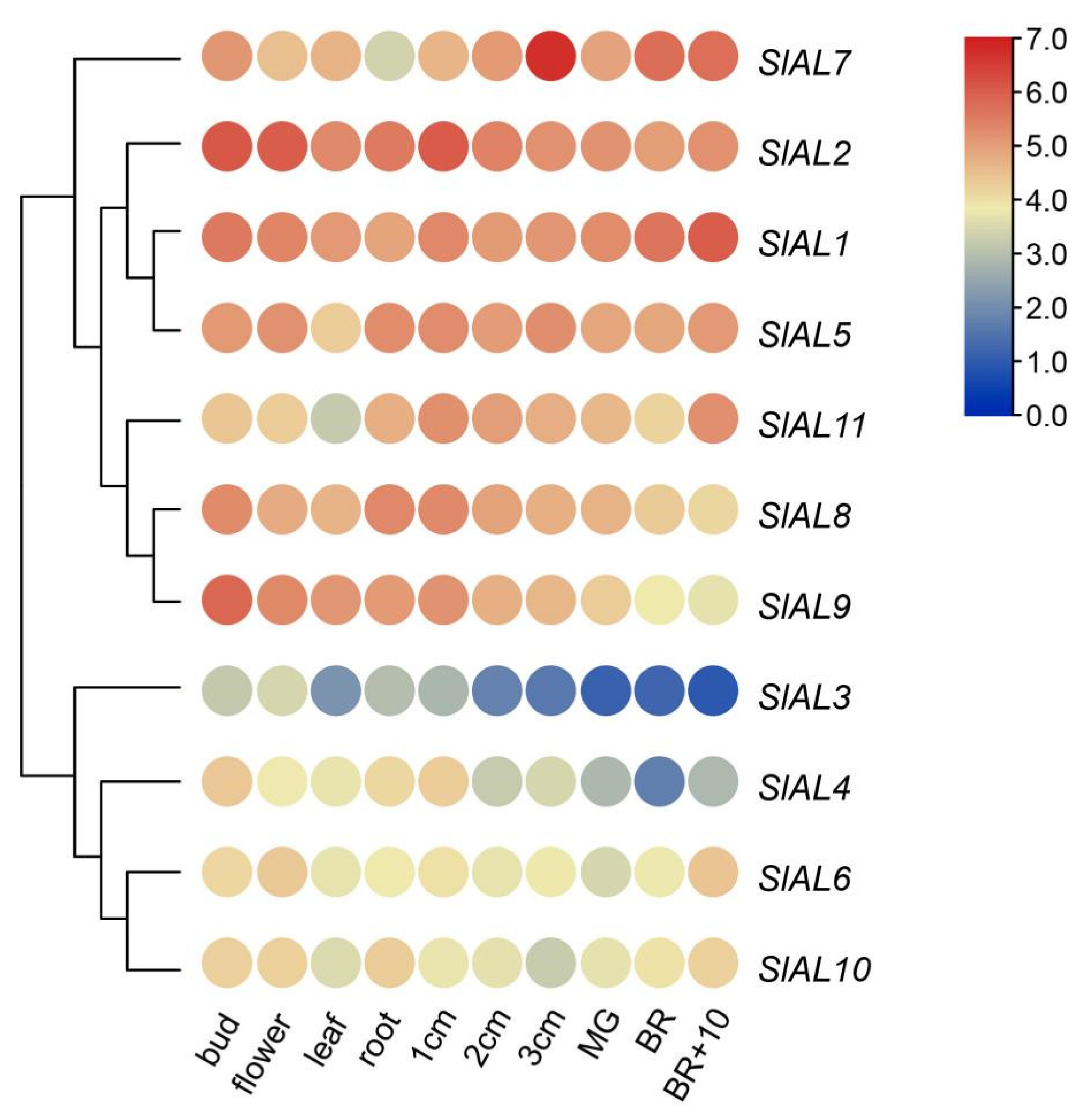
2.7. Expression Patterns of AL Genes in Tomato
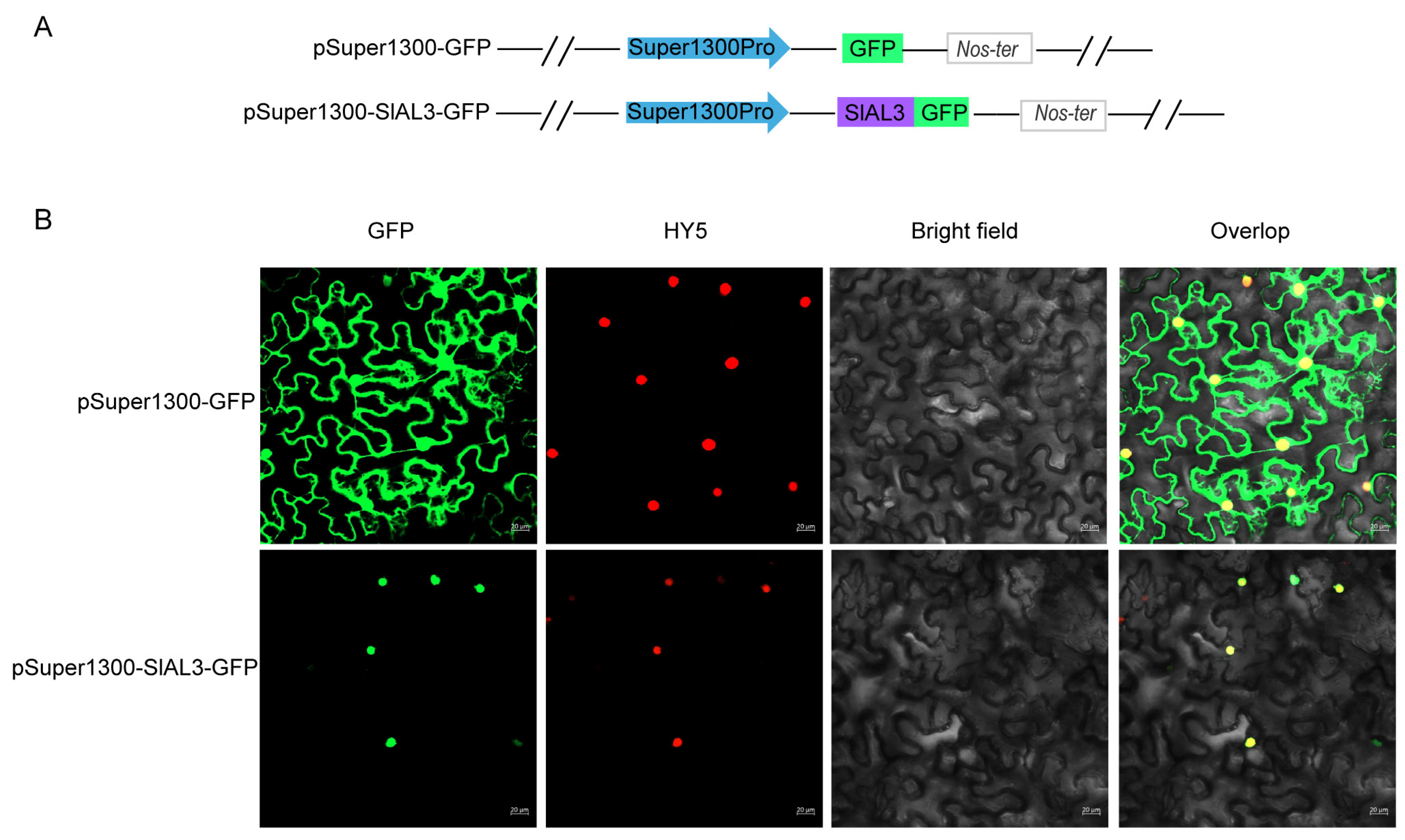
2.8. Subcellular Localization Analysis
3. Discussion
4. Materials and Methods
4.1. Retrieval and Identification of Putative AL Proteins in Four Tomato Species
4.2. Gene Structure, Chromosomal Mapping and Cis-Acting Element Analysis of Tomato ALs
4.3. Phylogeny and Conserved Motif Composition
4.4. Calculation of Ka/Ks Ratoos and Subcellular Localization Analysis
4.5. Gene Duplication and Synteny Analysis of the AL Gene Family
4.6. Plant Treatments
4.7. qRT-PCR Analysis
4.8. Expression Patterns of SlALs
4.9. Subcellular Localization Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Hu, M.; Xu, W.; Wang, Y.; Huang, K.; Zhang, C.; Wen, J. Understanding the Molecular Mechanism of Anther Development under Abiotic Stresses. Plant Mol. Biol. 2021, 150, 1–10. [Google Scholar] [CrossRef] [PubMed]
- De Nadal, E.; Ammerer, G.; Posas, F. Controlling Gene Expression in Response to Stress. Nat. Rev. Genet. 2011, 12, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Hrmova, M.; Hussain, S.S. Plant Transcription Factors Involved in Drought and Associated Stresses. Int. J. Mol. Sci. 2021, 22, 5662. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional Regulatory Networks in Cellular Responses and Tolerance to Dehydration and Cold Stresses. Annu. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to Drought and Salt Stress in Plants: Unraveling the Signaling Networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef] [Green Version]
- Chandrika, N.N.P.; Sundaravelpandian, K.; Yu, S.M.; Schmidt, W. ALFIN-LIKE 6 Is Involved in Root Hair Elongation during Phosphate Deficiency in Arabidopsis. New Phytol. 2013, 198, 709–720. [Google Scholar] [CrossRef]
- Winicov, I. Alfin1 Transcription Factor Overexpression Enhances Plant Root Growth under Normal and Saline Conditions and Improves Salt Tolerance in Alfalfa. Planta 2000, 210, 416–422. [Google Scholar] [CrossRef]
- Molitor, A.M.; Bu, Z.; Yu, Y.; Shen, W.H. Arabidopsis AL PHD-PRC1 Complexes Promote Seed Germination through H3K4me3-to-H3K27me3 Chromatin State Switch in Repression of Seed Developmental Genes. PLoS Genet. 2014, 10, e1004091. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Gao, J.; Yang, F.; Kua, C.S.; Liu, J.; Cannon, C.H. Molecular Evolutionary Analysis of the Alfin-Like Protein Family in Arabidopsis lyrata, Arabidopsis thaliana, and Thellungiella halophila. PLoS ONE 2013, 8, e66838. [Google Scholar] [CrossRef] [Green Version]
- Bastola, D.R.; Pethe, V.V.; Winicov, I. Alfin1, a Novel Zinc-Finger Protein in Alfalfa Roots That Binds to Promoter Elements in the Salt-Inducible MsPRP2 Gene. Plant Mol. Biol. 1998, 38, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Lee, D.; Chung, W.I.; Kwon, C.S. Arabidopsis ING and Alfin1-like Protein Families Localize to the Nucleus and Bind to H3K4me3/2 via Plant Homeodomain Fingers. Plant J. 2009, 58, 511–524. [Google Scholar] [CrossRef]
- Krochko, J.E.; Pramanik, S.K.; Bewley, J.D. Contrasting Storage Protein Synthesis and Messenger RNA Accumulation during Development of Zygotic and Somatic Embryos of Alfalfa (Medicago sativa L.). Plant Physiol. 1992, 99, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Kayum, M.A.; Park, J.I.; Ahmed, N.U.; Jung, H.J.; Saha, G.; Kang, J.G.; Nou, I.S. Characterization and Stress-Induced Expression Analysis of Alfin-like Transcription Factors in Brassica rapa. Mol. Genet. Genom. 2015, 290, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Kayum, M.A.; Park, J.I.; Ahmed, N.U.; Saha, G.; Chung, M.Y.; Kang, J.G.; Nou, I.S. Alfin-like Transcription Factor Family: Characterization and Expression Profiling against Stresses in Brassica oleracea. Acta Physiol. Plant. 2016, 38, 127. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, J.; Zheng, Q.; Jiang, Y.; Zhang, M.; Zhu, S. Genome-Wide Identification and Comparative Analysis of Alfin-like Transcription Factors in Maize. Genes Genom. 2017, 39, 261–275. [Google Scholar] [CrossRef]
- Giovannoni, J. Molecular Biology of Fruit Maturation and Ripening. Annu. Rev. Plant Biol. 2001, 52, 725–749. [Google Scholar] [CrossRef]
- Bolger, A.; Scossa, F.; Bolger, M.E.; Lanz, C.; Maumus, F.; Tohge, T.; Quesneville, H.; Alseekh, S.; Sørensen, I.; Lichtenstein, G.; et al. The Genome of the Stress-Tolerant Wild Tomato Species Solanum pennellii. Nat. Genet. 2014, 46, 1034–1038. [Google Scholar] [CrossRef]
- Sato, S.; Tabata, S.; Hirakawa, H.; Asamizu, E.; Shirasawa, K.; Isobe, S.; Kaneko, T.; Nakamura, Y.; Shibata, D.; Aoki, K.; et al. The Tomato Genome Sequence Provides Insights into Fleshy Fruit Evolution. Nature 2012, 485, 635. [Google Scholar]
- Takei, H.; Shirasawa, K.; Kuwabara, K.; Toyoda, A.; Matsuzawa, Y.; Iioka, S.; Ariizumi, T. De Novo Genome Assembly of Two Tomato Ancestors, Solanum pimpinellifolium and Solanum lycopersicum Var. Cerasiforme, by Long-Read Sequencing. DNA Res. 2021, 28, dsaa029. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, Y.Q.; Tao, J.J.; Chen, H.W.; Li, Q.T.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; Chen, S.Y. The Alfin-like Homeodomain Finger Protein AL5 Suppresses Multiple Negative Factors to Confer Abiotic Stress Tolerance in Arabidopsis. Plant J. 2015, 81, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Koralewski, T.E.; Krutovsky, K.V. Evolution of Exon-Intron Structure and Alternative Splicing. PLoS ONE 2011, 6, e18055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, M.; Conery, J.S. The Evolutionary Fate and Consequences of Duplicate Genes. Science 2000, 290, 1151–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, M.A.; Haubold, B.; Mitchell-Olds, T. Comparative Evolutionary Analysis of Chalcone Synthase and Alcohol Dehydrogenase Loci in Arabidopsis, Arabis, and Related Genera (Brassicaceae). Mol. Biol. Evol. 2000, 17, 1483–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Ling, L.; Jiang, Z.; Tan, W.; Liu, Z.; Wu, L.; Zhao, Y.; Xia, S.; Ma, J.; Wang, G.; et al. Genome-wide Identification and Expression Analysis of the 14-3-3 Gene Family in Soybean (Glycine max). PeerJ 2019, 7, e7950. [Google Scholar] [CrossRef] [Green Version]
- Jaillon, O.; Aury, J.M.; Noel, B.; Policriti, A.; Clepet, C.; Casagrande, A.; Choisne, N.; Aubourg, S.; Vitulo, N.; Jubin, C.; et al. The Grapevine Genome Sequence Suggests Ancestral Hexaploidization in Major Angiosperm Phyla. Nature 2007, 449, 463–467. [Google Scholar]
- Winicov, I.; Bastola, D.R. Transgenic Overexpression of the Transcription Factor Alfin1 Enhances Expression of the Endogenous MsPRP2 Gene in Alfalfa and Improves Salinity Tolerance of the Plants. Plant Physiol. 1999, 120, 473–480. [Google Scholar] [CrossRef] [Green Version]
- Schindler, U.; Beckmann, H.; Cashmore, A.R. HAT3. 1, a Novel Arabidopsis Homeodomain Protein Containing a Conserved Cysteine-rich Region. Plant J. 1993, 4, 137–150. [Google Scholar] [CrossRef]
- Tao, J.; Wei, W.; Pan, W.; Lu, L.; Li, Q.; Ma, J.; Zhang, W.; Ma, B.; Chen, S.; Zhang, J. An Alfin-like Gene from Atriplex Hortensis Enhances Salt and Drought Tolerance and Abscisic Acid Response in Transgenic Arabidopsis. Sci. Rep. 2018, 8, 2707. [Google Scholar] [CrossRef] [Green Version]
- Qu, L.J.; Zhu, Y.X. Transcription Factor Families in Arabidopsis: Major Progress and Outstanding Issues for Future Research. Curr. Opin. Plant Biol. 2006, 9, 544–549. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, T.; Tian, C.; Sun, S.; Li, J.; Chen, M. Transcription Factors in Rice: A Genome-Wide Comparative Analysis between Monocots and Eudicots. Plant Mol. Biol. 2005, 59, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Davis, C.I.; Mekhedov, S.L.; Hartl, D.L.; Koonin, E.V.; Kondrashov, F.A. Selection for Short Introns in Highly Expressed Genes. Nat. Genet. 2002, 31, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Le Hir, H.; Nott, A.; Moore, M.J. How Introns Influence and Enhance Eukaryotic Gene Expression. Trends Biochem. Sci. 2003, 28, 215–220. [Google Scholar] [CrossRef]
- Xie, D.W.; Wang, X.N.; Fu, L.S.; Sun, J.; Zheng, W.; Li, Z.F. Identification of the Trehalose-6-Phosphate Synthase Gene Family in Winter Wheat and Expression Analysis under Conditions of Freezing Stress. J. Genet. 2015, 94, 55–65. [Google Scholar] [CrossRef]
- Qin, F.; Sakuma, Y.; Tran, L.S.P.; Maruyama, K.; Kidokoro, S.; Fujita, Y.; Fujita, M.; Umezawa, T.; Sawano, Y.; Miyazono, K.I.; et al. Arabidopsis DREB2A-Interacting Proteins Function as Ring E3 Ligases and Negatively Regulate Plant Drought Stress-Responsive Gene Expression. Plant Cell 2008, 20, 1693–1707. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Organization of Cis-Acting Regulatory Elements in Osmotic- and Cold-Stress-Responsive Promoters. Trends Plant Sci. 2005, 10, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Winicov, I. CDNA Encoding Putative Zinc Finger Motifs from Salt-Tolerant Alfalfa (Medicago Sativa L.) Cells. Plant Physiol. 1993, 102, 681. [Google Scholar] [CrossRef] [Green Version]
- Wani, S.H.; Kumar, V.; Khare, T.; Guddimalli, R.; Parveda, M.; Solymosi, K.; Suprasanna, P.; Kavi Kishor, P.B. Engineering Salinity Tolerance in Plants: Progress and Prospects. Planta 2020, 251, 76. [Google Scholar] [CrossRef]
- Kong, H.; Landherr, L.L.; Frohlich, M.W.; Leebens-Mack, J.; Ma, H.; DePamphilis, C.W. Patterns of Gene Duplication in the Plant SKP1 Gene Family in Angiosperms: Evidence for Multiple Mechanisms of Rapid Gene Birth. Plant J. 2007, 50, 873–885. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A Software for Comprehensive Analysis of DNA Polymorphism Data. Bioinform. 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A Toolkit for Detection and Evolutionary Analysis of Gene Synteny and Collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An Information Aesthetic for Comparative Genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fei, Z.; Joung, J.G.; Tang, X.; Zheng, Y.; Huang, M.; Lee, J.M.; McQuinn, R.; Tieman, D.M.; Alba, R.; Klee, H.J.; et al. Tomato Functional Genomics Database: A Comprehensive Resource and Analysis Package for Tomato Functional Genomics. Nucleic Acids Res. 2011, 39, D1156–D1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, R.; Wang, J.; Guo, B.; Yang, T.; Hu, J.; Wang, B.; Yu, Q. Identification and Expression Analysis of the Alfin-like Gene Family in Tomato and the Role of SlAL3 in Salt and Drought Stresses. Plants 2023, 12, 2829. https://doi.org/10.3390/plants12152829
Jin R, Wang J, Guo B, Yang T, Hu J, Wang B, Yu Q. Identification and Expression Analysis of the Alfin-like Gene Family in Tomato and the Role of SlAL3 in Salt and Drought Stresses. Plants. 2023; 12(15):2829. https://doi.org/10.3390/plants12152829
Chicago/Turabian StyleJin, Ruixin, Juan Wang, Bin Guo, Tao Yang, Jiahui Hu, Baike Wang, and Qinghui Yu. 2023. "Identification and Expression Analysis of the Alfin-like Gene Family in Tomato and the Role of SlAL3 in Salt and Drought Stresses" Plants 12, no. 15: 2829. https://doi.org/10.3390/plants12152829




