GA20ox Family Genes Mediate Gibberellin and Auxin Crosstalk in Moso bamboo (Phyllostachys edulis)
Abstract
1. Introduction
2. Results
2.1. Analysis of the Chromosomal Distribution and Evolutionary Patterns of the GA20ox Gene Family
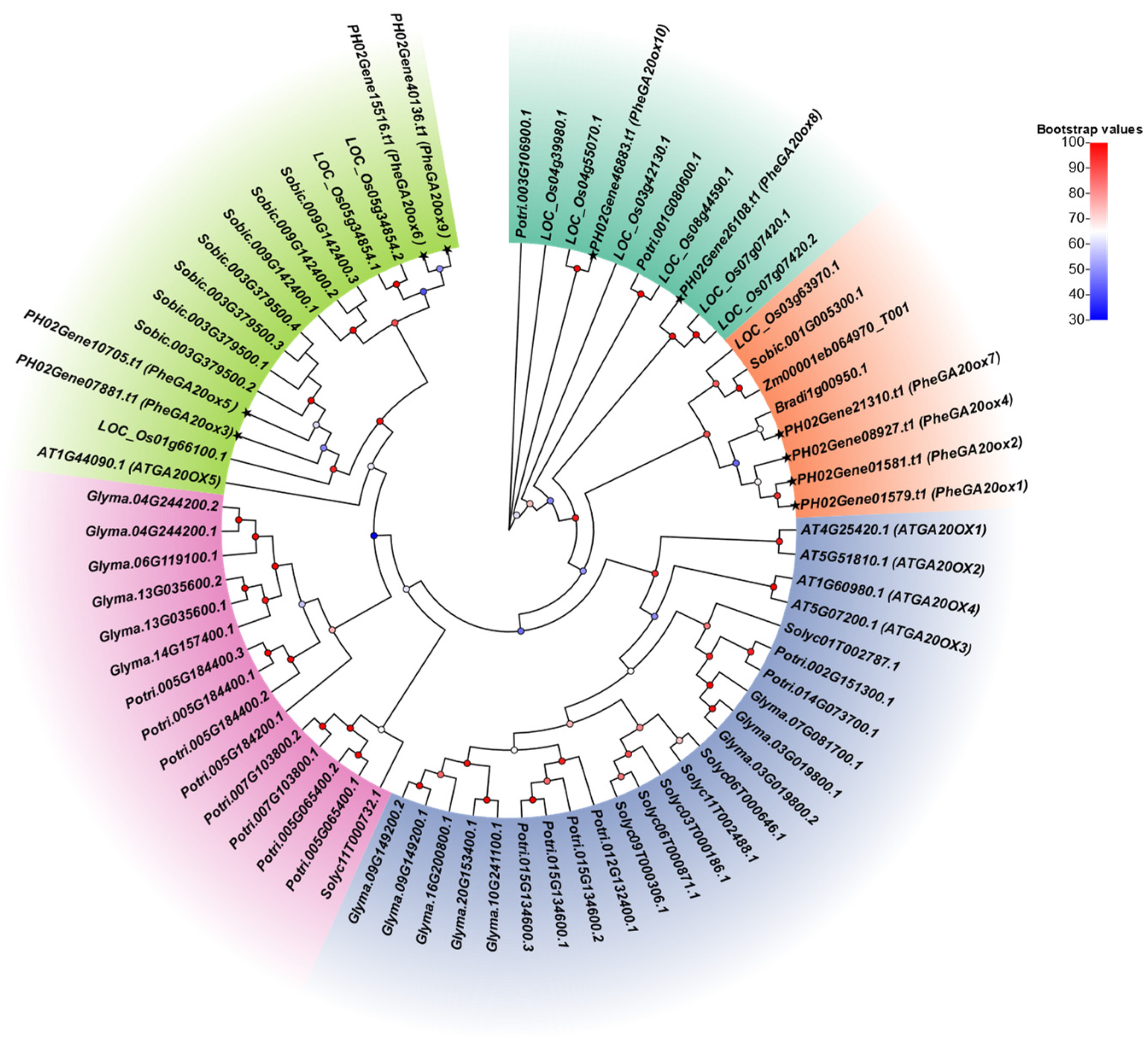
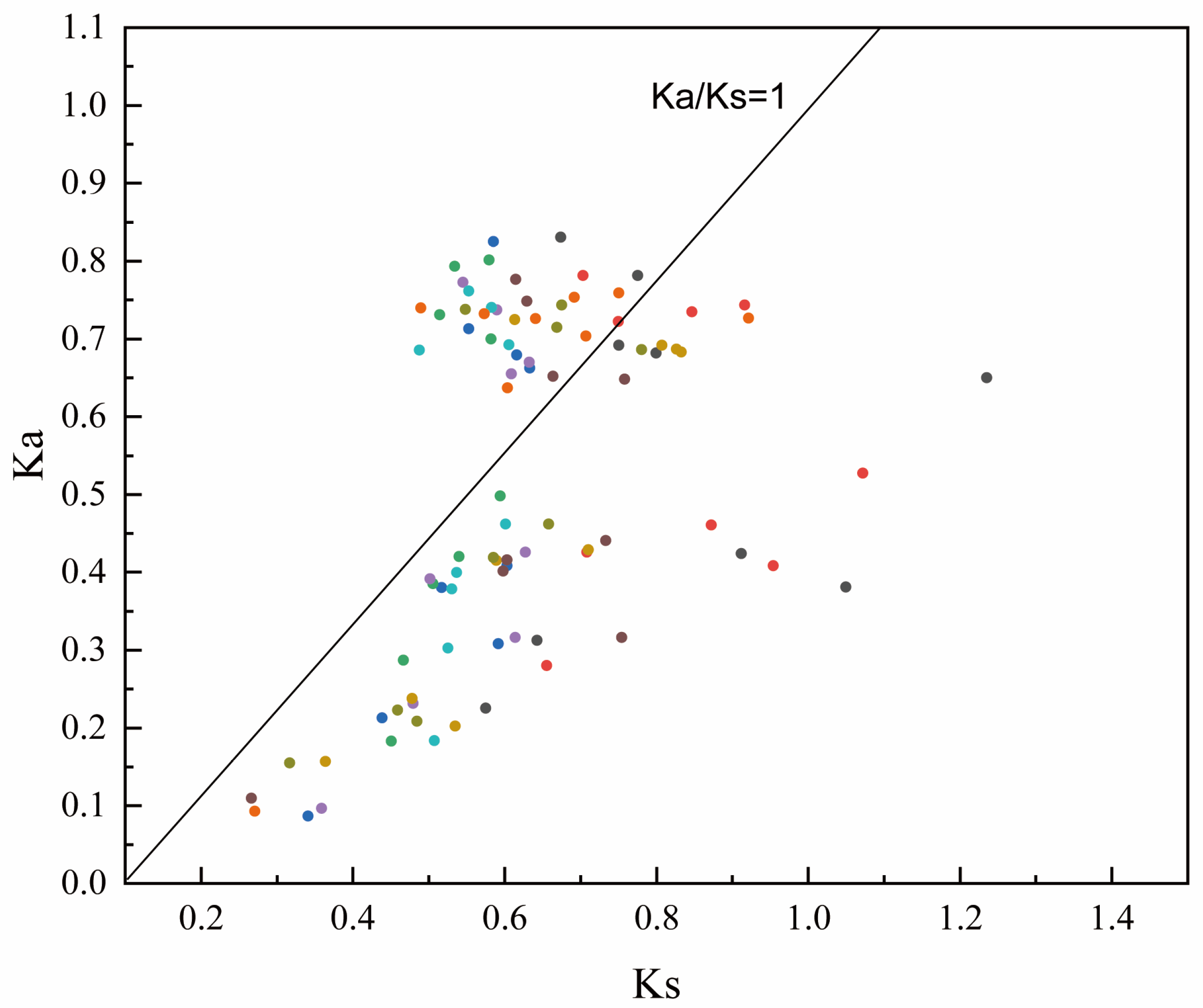
2.2. Phylogenetic Tree Construction and Selection Pressure Analysis of the GA20ox Gene Family
2.3. Analysis of the Expression Pattern of the GA20ox Gene Family
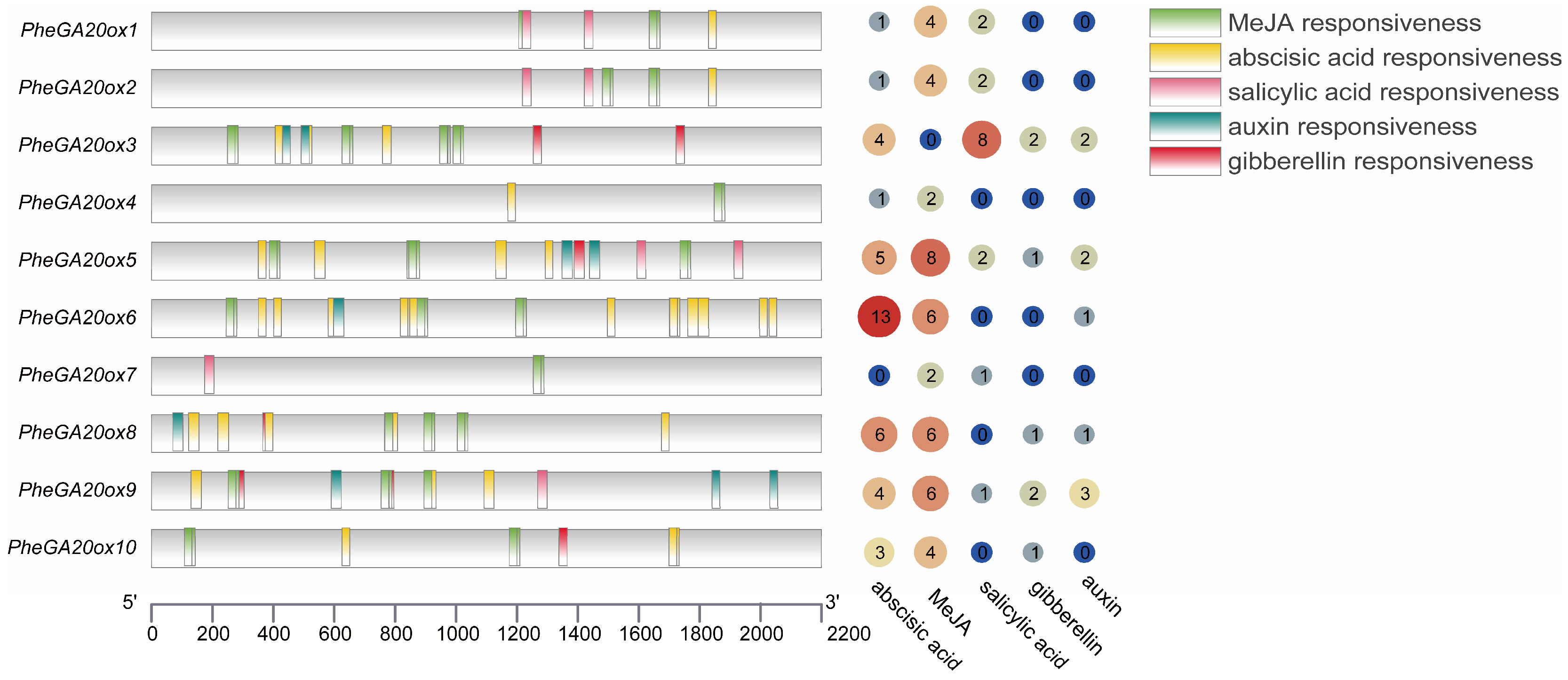
2.4. Analysis of Phytohormone Response Elements in the Promoter Region of GA20ox Family Genes
2.5. Analysis of Upstream Regulators of PheGA20ox3 and PheGA20ox6
2.6. Analysis of the PheARF47-Regulated PheGA20ox Promoter Region
3. Discussion
4. Materials and Methods
4.1. Gene Family Identification and Physicochemical Property Analysis
4.2. Analysis of Evolutionary Patterns
4.3. Phylogenetic Tree Construction
4.4. Transcriptome Data Acquisition
4.5. Promoter Analysis
4.6. Yeast One-Hybrid Assay
4.7. Dual Luciferase Reporter Assay
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ritonga, F.N.; Zhou, D.; Zhang, Y.; Song, R.; Li, C.; Li, J.; Gao, J. The Roles of Gibberellins in Regulating Leaf Development. Plants 2023, 12, 1243. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xia, W.; Li, H.; Zeng, H.; Wei, B.; Han, S.; Yin, C. Salinity inhibits rice seed germination by reducing α-amylase activity via decreased bioactive gibberellin content. Front. Plant Sci. 2018, 9, 275. [Google Scholar] [CrossRef] [PubMed]
- Durbak, A.; Yao, H.; McSteen, P. Hormone signaling in plant development. Curr. Opin. Plant Biol. 2012, 15, 92–96. [Google Scholar] [CrossRef]
- Vanhaelewyn, L.; Viczián, A.; Prinsen, E.; Bernula, P.; Serrano, A.M.; Arana, M.V.; Ballaré, C.L.; Nagy, F.; Van Der Straeten, D.; Vandenbussche, F. Differential UVR8 Signal across the Stem Controls UV-B—Induced Inflorescence Phototropism. Plant Cell 2019, 31, 2070–2088. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, N.; Sun, T.P.; Gubler, F. Gibberellin Signaling: Biosynthesis, Catabolism, and Response Pathways. Plant Cell 2002, 14, S61–S80. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Lisa, G.; Omar, R.S.; Domenico, M.; Kwame, A.A.; Marco, M.; Lorenzo, C.; Urska, V.; Claudio, M. Gibberellin metabolism in Vitis vinifera L. during bloom and fruit-set: Functional characterization and evolution of grapevine gibberellin oxidases. J. Exp. Bot. 2013, 64, 4403–4419. [Google Scholar]
- López-Cristoffanini, C.; Serrat, X.; Jáuregui, O.; Nogués, S.; López-Carbonell, M. Phytohormone Profiling Method for Rice: Effects of GA20ox Mutation on the gibberellin Content of Japonica Rice Varieties. Front. Plant Sci. 2019, 10, 733. [Google Scholar] [CrossRef]
- Xu, Y.; Jia, Q.; Zhou, G.; Zhang, X.Q.; Angessa, T.; Broughton, S.; Yan, G.; Zhang, W.; Li, C. Characterization of the sdw1 semi-dwarf gene in barley. BMC Plant Biol. 2017, 17, 11. [Google Scholar] [CrossRef]
- Peng, J.; Richards, D.E.; Hartley, N.M.; Murphy, G.P.; Devos, K.M.; Flintham, J.E.; Beales, J.; Fish, L.J.; Worland, A.J.; Pelica, F. ‘Green revolution’genes encode mutant gibberellin response modulators. Nature 1999, 400, 256–261. [Google Scholar] [CrossRef]
- Appleford, N.E.; Evans, D.J.; Lenton, J.R.; Gaskin, P.; Croker, S.J.; Devos, K.M.; Phillips, A.L.; Hedden, P. Function and transcript analysis of gibberellin-biosynthetic enzymes in wheat. Planta 2006, 223, 568–582. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Ptošková, K.; Szecówka, M.; Jaworek, P.; Tarkowská, D.; Petřík, I.; Pavlović, I.; Novák, O.; Thomas, S.G.; Phillips, A.L.; Hedden, P. Changes in the concentrations and transcripts for gibberellins and other hormones in a growing leaf and roots of wheat seedlings in response to water restriction. BMC Plant Biol. 2022, 22, 284. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huang, L.; Zhang, Y.; Yan, Z.; Wang, N. Overexpression of PdeGATA3 results in a dwarf phenotype in poplar by promoting the expression of PdeSTM and altering the content of gibberellins. Tree Physiol. 2022, 42, 2614–2626. [Google Scholar] [CrossRef]
- Yang, T.; Davies, P.J.; Reid, J.B. Genetic dissection of the relative roles of auxin and gibberellin in the regulation of stem elongation in intact light-grown peas. Plant Physiol. 1996, 110, 1029–1034. [Google Scholar] [CrossRef]
- Salem, J.; Hassanein, A.; El-Wakil, D.A.; Loutfy, N. Interaction between Growth Regulators Controls In Vitro Shoot Multiplication in Paulownia and Selection of NaCl-Tolerant Variants. Plants 2022, 11, 498. [Google Scholar] [CrossRef]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in fruit development and maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, M.; Alabadí, D.; Pérez-Gómez, J.; García-Cárcel, L.; Phillips, A.L.; Hedden, P.; Blázquez, M.A. Transcriptional regulation of gibberellin metabolism genes by auxin signaling in Arabidopsis. Plant Physiol. 2006, 142, 553–563. [Google Scholar] [CrossRef]
- Gou, J.; Strauss, S.H.; Tsai, C.J.; Fang, K.; Chen, Y.; Jiang, X.; Busov, V.B. Gibberellins regulate lateral root formation in Populus through interactions with auxin and other hormones. Plant Cell 2010, 22, 623–639. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.J.; Zhang, J.Z. Aux/IAA Gene Family in Plants: Molecular Structure, Regulation, and Function. Int. J. Mol. Sci. 2018, 19, 259. [Google Scholar] [CrossRef]
- Liu, X.; Xu, T.; Dong, X.; Liu, Y.; Liu, Z.; Shi, Z.; Wang, Y.; Qi, M.; Li, T. The role of gibberellins and auxin on the tomato cell layers in pericarp via the expression of ARFs regulated by miRNAs in fruit set. Acta Physiol. Plant. 2016, 38, 77. [Google Scholar] [CrossRef]
- Li, Y.; Han, S.; Qi, Y. Advances in structure and function of auxin response factor in plants. J. Integr. Plant Biol. 2023, 65, 617–632. [Google Scholar] [CrossRef] [PubMed]
- Lobovikov, M.; Paudel, S.; Ball, L.; Piazza, M.; Guardia, M.; Wu, J.; Ren, H. World Bamboo Resources: A Thematic Study Prepared in the Framework of the Global Forest Resources Assessment 2005; FAO: Rome, Italy, 2007. [Google Scholar]
- Ma, N.; Lai, G.; Zhang, P.; Zhang, H. The Genus Phyllostachys in China; Zhejiang Science and Technology Publishing House: Hangzhou, China, 2014. [Google Scholar]
- Wang, T.; Liu, L.; Wang, X.; Liang, L.; Yue, J.; Li, L. Comparative Analyses of Anatomical Structure, Phytohormone Levels, and Gene Expression Profiles Reveal Potential Dwarfing Mechanisms in Shengyin Bamboo (Phyllostachys edulis f. tubaeformis). Int. J. Mol. Sci. 2018, 19, 1697. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Cai, M.; Mu, C.; Zheng, H.; Cheng, Z.; Xie, Y.; Gao, J. Integrative analysis of exogenous auxin mediated plant height regulation in Moso bamboo (Phyllostachys edulis). Ind. Crops Prod. 2023, 200, 116852. [Google Scholar] [CrossRef]
- Chen, M.; Guo, L.; Ramakrishnan, M.; Fei, Z.; Vinod, K.K.; Ding, Y.; Jiao, C.; Gao, Z.; Zha, R.; Wang, C.; et al. Rapid growth of Moso bamboo (Phyllostachys edulis): Cellular roadmaps, transcriptome dynamics, and environmental factors. Plant Cell 2022, 34, 3577–3610. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, Y.; Fu, Y.; Zhou, M.; Tang, D. Genome-wide identification and expression analysis of gibberellin biosynthesis, metabolism and signaling family genes in Phyllostachys edulis. Chin. J. Biotechnol. 2019, 35, 647–666. [Google Scholar]
- Yang, Y.; Wang, S.; Xu, H.; Sun, H.; Zhao, H.; Chen, D.; Gao, Z. Genome-wide identification of the enzyme genes involved in gibberellin biosynthesis and their expression analyses in Moso bamboo. Genom. Appl. Biol. 2018, 37, 3966–3977. [Google Scholar]
- Bai, Y.; Dou, Y.; Xie, Y.; Zheng, H.; Gao, J. Phylogeny, transcriptional profile, and auxin-induced phosphorylation modification characteristics of conserved PIN proteins in Moso bamboo (Phyllostachys edulis). Int. J. Biol. Macromol. 2023, 234, 123671. [Google Scholar] [CrossRef]
- Hedden, P.; Phillips, A.L. Gibberellin metabolism: New insights revealed by the genes. Trends Plant Sci. 2000, 5, 523–530. [Google Scholar] [CrossRef]
- Han, F.; Zhu, B. Evolutionary analysis of three gibberellin oxidase genesin rice, Arabidopsis, and soybean. Gene 2011, 473, 23–35. [Google Scholar] [CrossRef]
- Zhang, C.; Nie, X.; Kong, W.; Deng, X.; Sun, T.; Liu, X.; Li, Y. Genome-Wide Identification and Evolution Analysis of the Gibberellin Oxidase Gene Family in Six Gramineae Crops. Genes 2022, 13, 863. [Google Scholar] [CrossRef]
- Landry, C.R.; Oh, J.; Hartl, D.L.; Cavalieri, D. Genome-wide scan reveals that genetic variation for transcriptional plasticity in yeast is biased towards multi-copy and dispensable genes. Gene 2006, 366, 343–351. [Google Scholar] [CrossRef]
- Barker, R.; Fernandez Garcia, M.N.; Powers, S.J.; Vaughan, S.; Bennett, M.J.; Phillips, A.L.; Thomas, S.G.; Hedden, P. Mapping sites of gibberellin biosynthesis in the Arabidopsis root tip. New Phytol. 2021, 229, 1521–1534. [Google Scholar] [CrossRef] [PubMed]
- Ming, W.J.; Fa, C.R.; Xing, H.; Hang, Q.L.; Rui, L.Y. Studies on the Gene of Key Component GA20-oxidase for Gibberellin Biosynthesis in Plant. Biotechnol. Bull. 2016, 32, 1–12. [Google Scholar]
- Boden, S.A.; Weiss, D.; Ross, J.J.; Davies, N.W.; Trevaskis, B.; Chandler, P.M.; Swain, S.M. EARLY FLOWERING3 Regulates Flowering in Spring Barley by Mediating Gibberellin Production and FLOWERING LOCUS T Expression. Plant Cell 2014, 26, 1557–1569. [Google Scholar] [CrossRef]
- Liao, X.; Li, M.; Liu, B.; Yan, M.; Yu, X.; Zi, H.; Liu, R.; Yamamuro, C. Interlinked regulatory loops of ABA catabolism and biosynthesis coordinate fruit growth and ripening in woodland strawberry. Proc. Natl. Acad. Sci. USA 2018, 115, E11542–E11550. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Chen, Y.; Cai, G.; Cai, R.; Hu, Z.; Wang, H. Tree Visualization By One Table (tvBOT): A web application for visualizing, modifying and annotating phylogenetic trees. Nucleic Acids Res. 2023, 51, W587–W592. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cheng, Z.; Ma, Y.; Bai, Q.; Li, X.; Cao, Z.; Wu, Z.; Gao, J. The association of hormone signalling genes, transcription and changes in shoot anatomy during Moso bamboo growth. Plant Biotechnol. J. 2018, 16, 72–85. [Google Scholar] [CrossRef]
- Wang, W.; Gu, L.; Ye, S.; Zhang, H.; Cai, C.; Xiang, M.; Gao, Y.; Wang, Q.; Lin, C.; Zhu, Q. Genome-wide analysis and transcriptomic profiling of the auxin biosynthesis, transport and signaling family genes in Moso bamboo (Phyllostachys heterocycla). BMC Genom. 2017, 18, 870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.; Zhu, Q.; Gao, Y.; Wang, H.; Zhao, L.; Wang, Y.; Xi, F.; Wang, W.; Yang, Y. Transcriptome characterization of Moso bamboo (Phyllostachys edulis) seedlings in response to exogenous gibberellin applications. BMC Plant Biol. 2018, 18, 125. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Xie, Y.; Cai, M.; Jiang, J.; Wu, C.; Zheng, H.; Gao, J. GA20ox Family Genes Mediate Gibberellin and Auxin Crosstalk in Moso bamboo (Phyllostachys edulis). Plants 2023, 12, 2842. https://doi.org/10.3390/plants12152842
Bai Y, Xie Y, Cai M, Jiang J, Wu C, Zheng H, Gao J. GA20ox Family Genes Mediate Gibberellin and Auxin Crosstalk in Moso bamboo (Phyllostachys edulis). Plants. 2023; 12(15):2842. https://doi.org/10.3390/plants12152842
Chicago/Turabian StyleBai, Yucong, Yali Xie, Miaomiao Cai, Jutang Jiang, Chongyang Wu, Huifang Zheng, and Jian Gao. 2023. "GA20ox Family Genes Mediate Gibberellin and Auxin Crosstalk in Moso bamboo (Phyllostachys edulis)" Plants 12, no. 15: 2842. https://doi.org/10.3390/plants12152842
APA StyleBai, Y., Xie, Y., Cai, M., Jiang, J., Wu, C., Zheng, H., & Gao, J. (2023). GA20ox Family Genes Mediate Gibberellin and Auxin Crosstalk in Moso bamboo (Phyllostachys edulis). Plants, 12(15), 2842. https://doi.org/10.3390/plants12152842



