Efficiency of Recovery of the Bioactive Principles of Plants by Comparison between Solid–Liquid Extraction in Mixture and Single-Vegetable Matrices via Maceration and RSLDE
Abstract
:1. Introduction
2. Results and Discussion
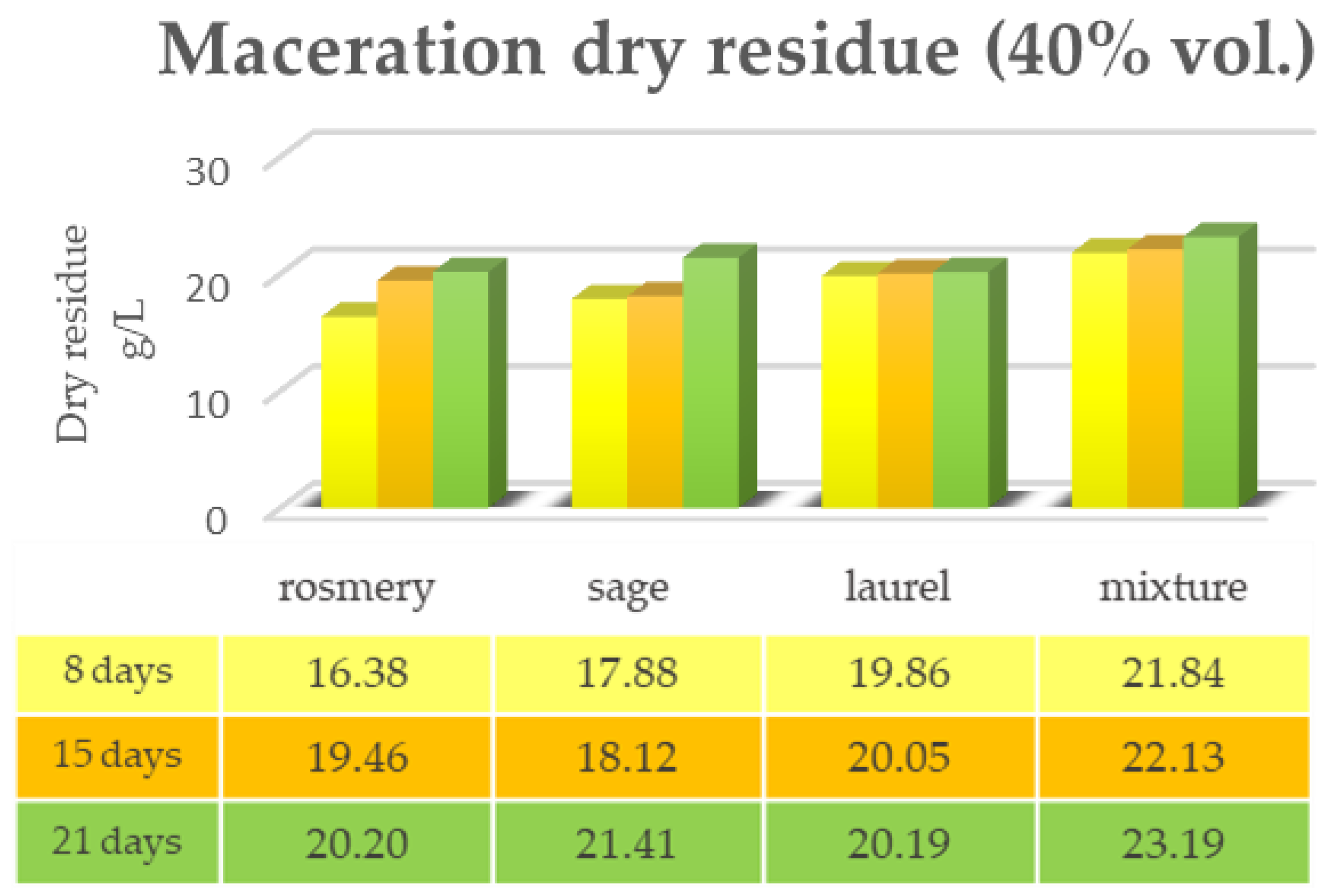
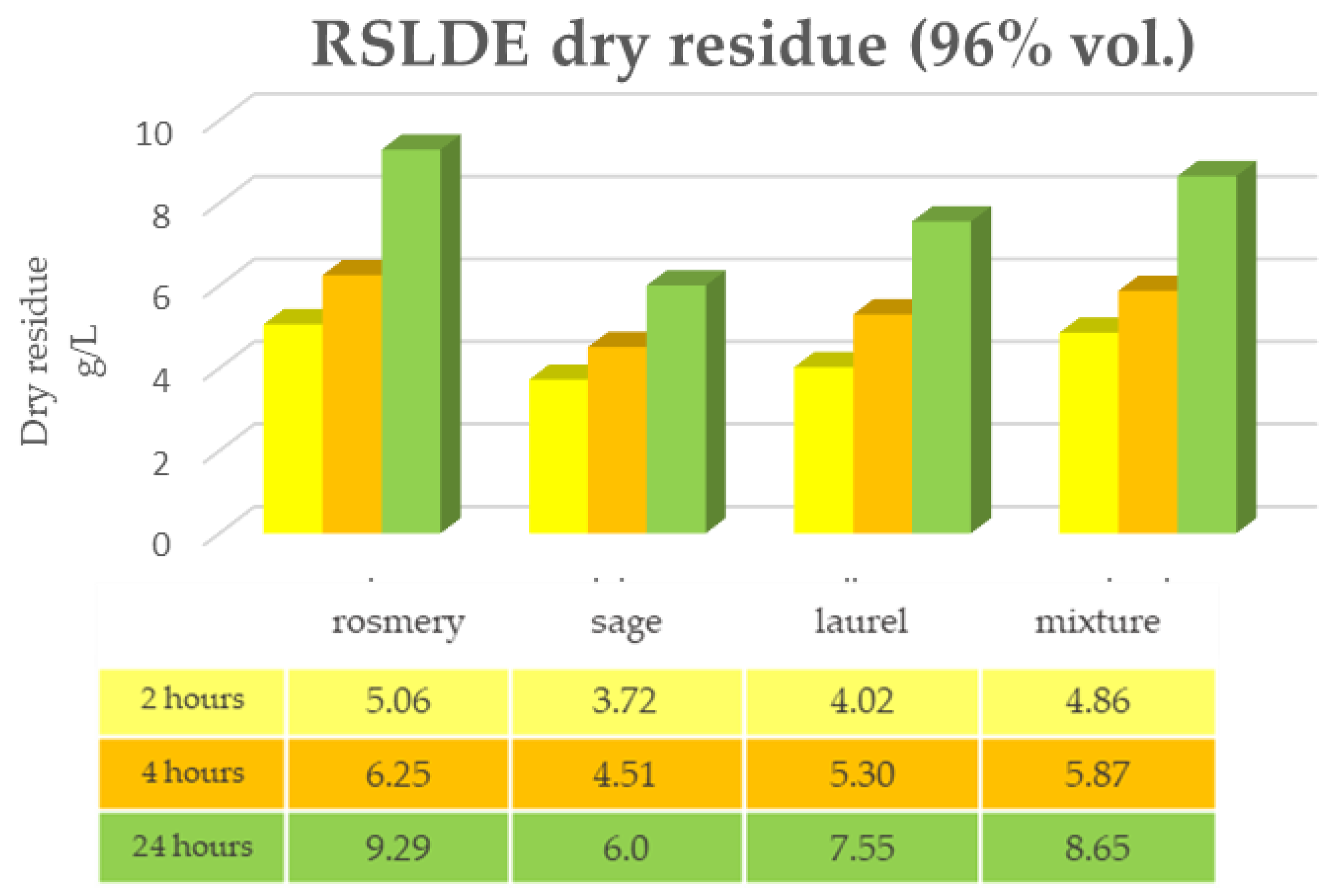
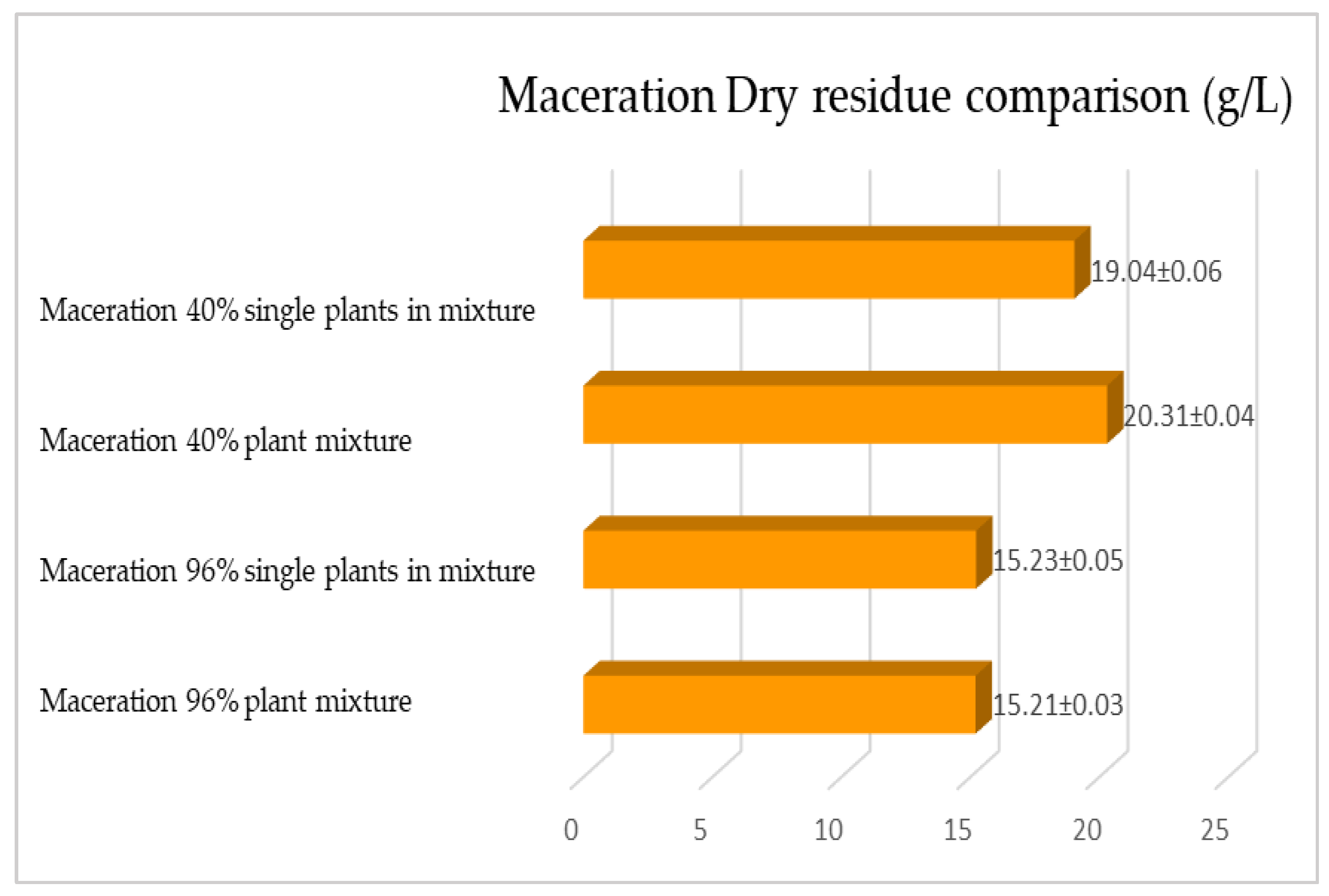
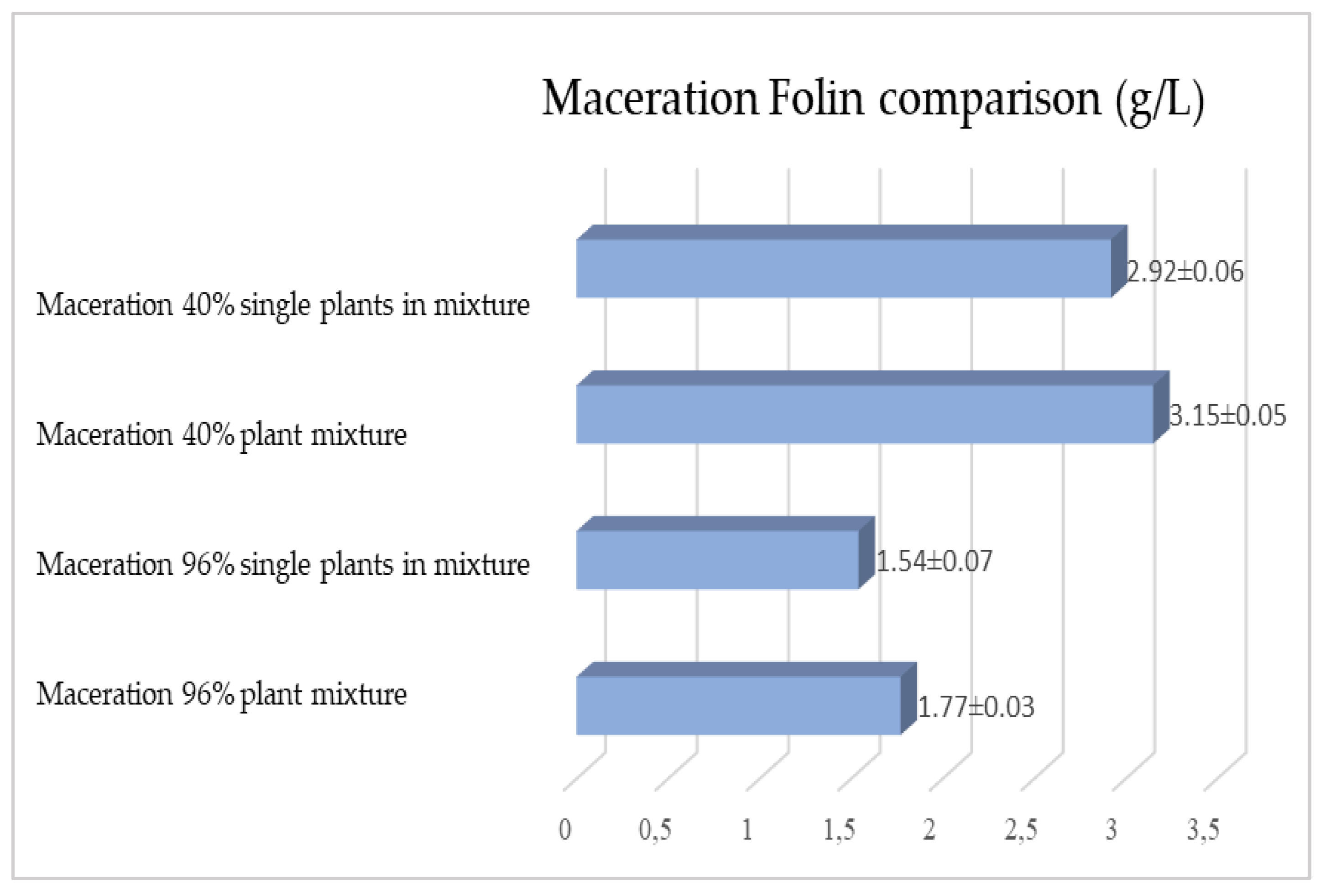
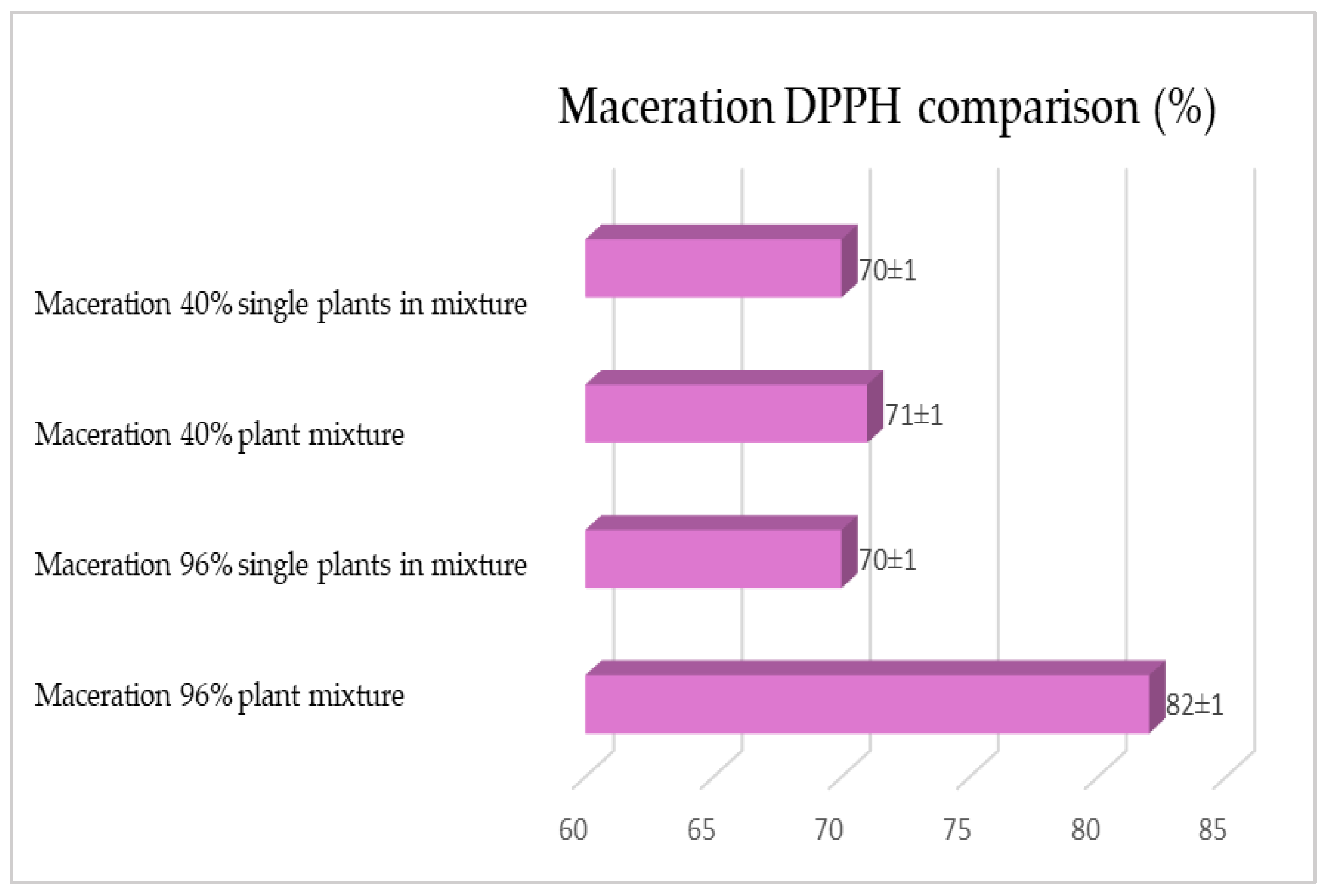
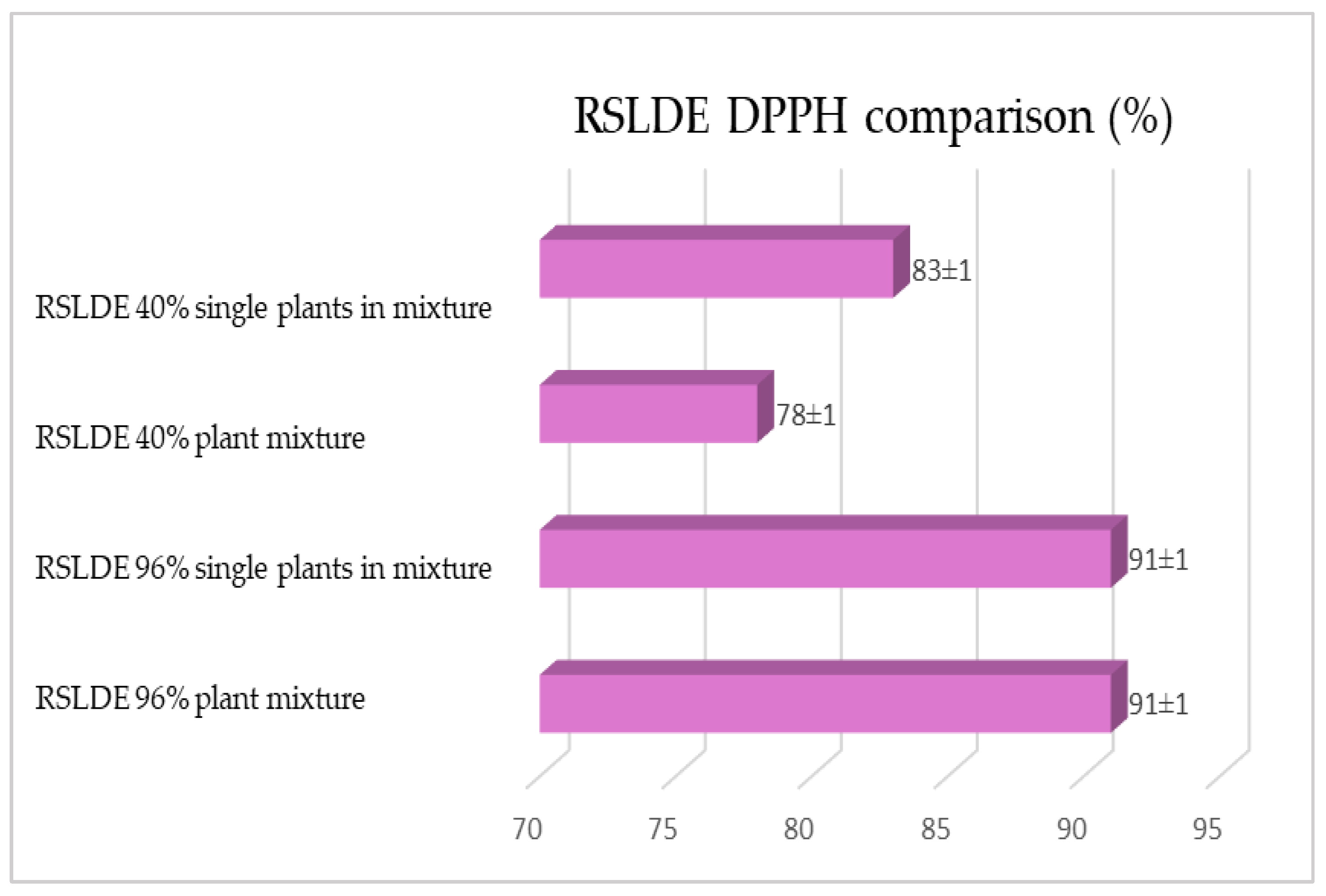
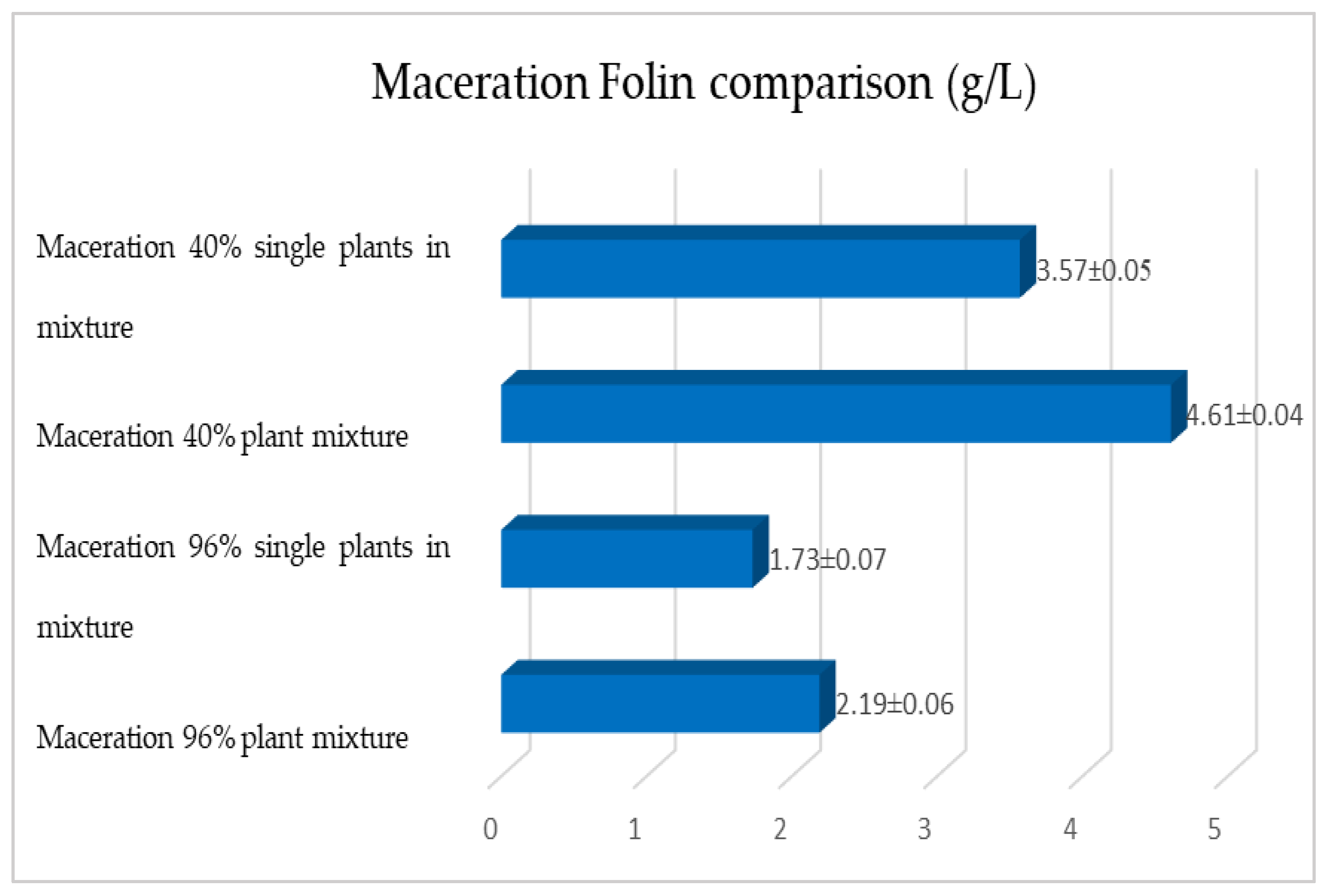
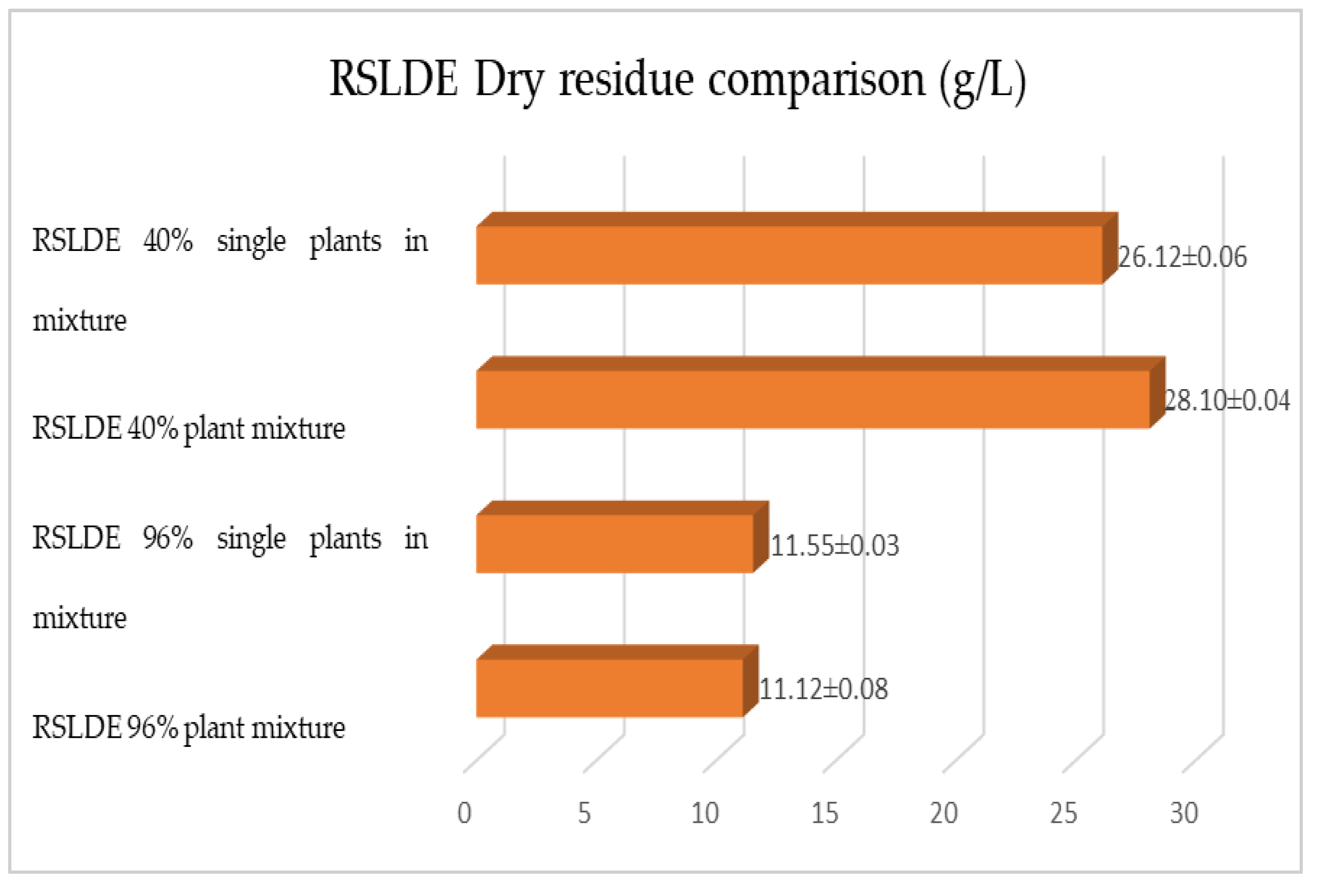
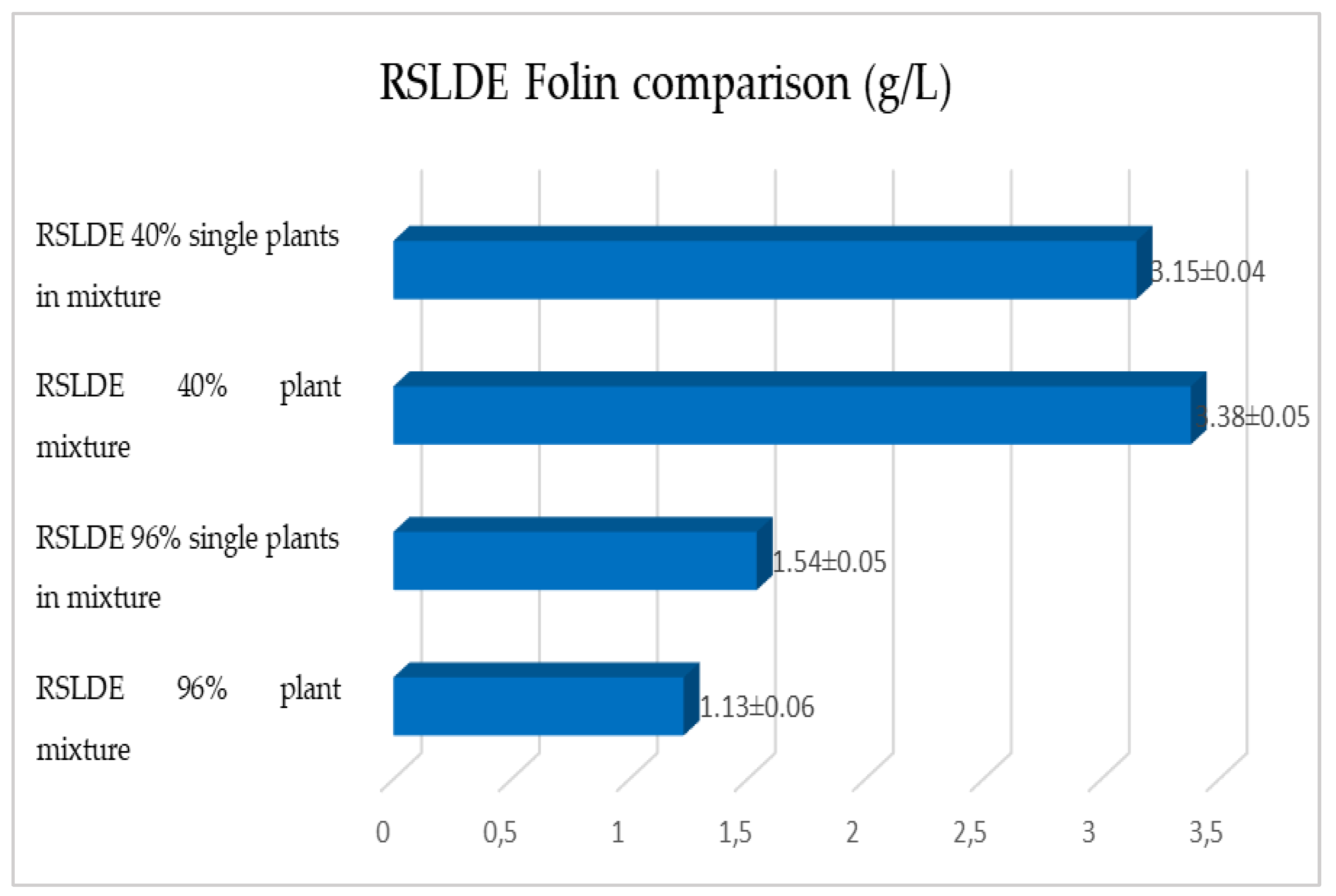
2.1. Maceration vs. RSLDE
2.2. Leaf Extraction
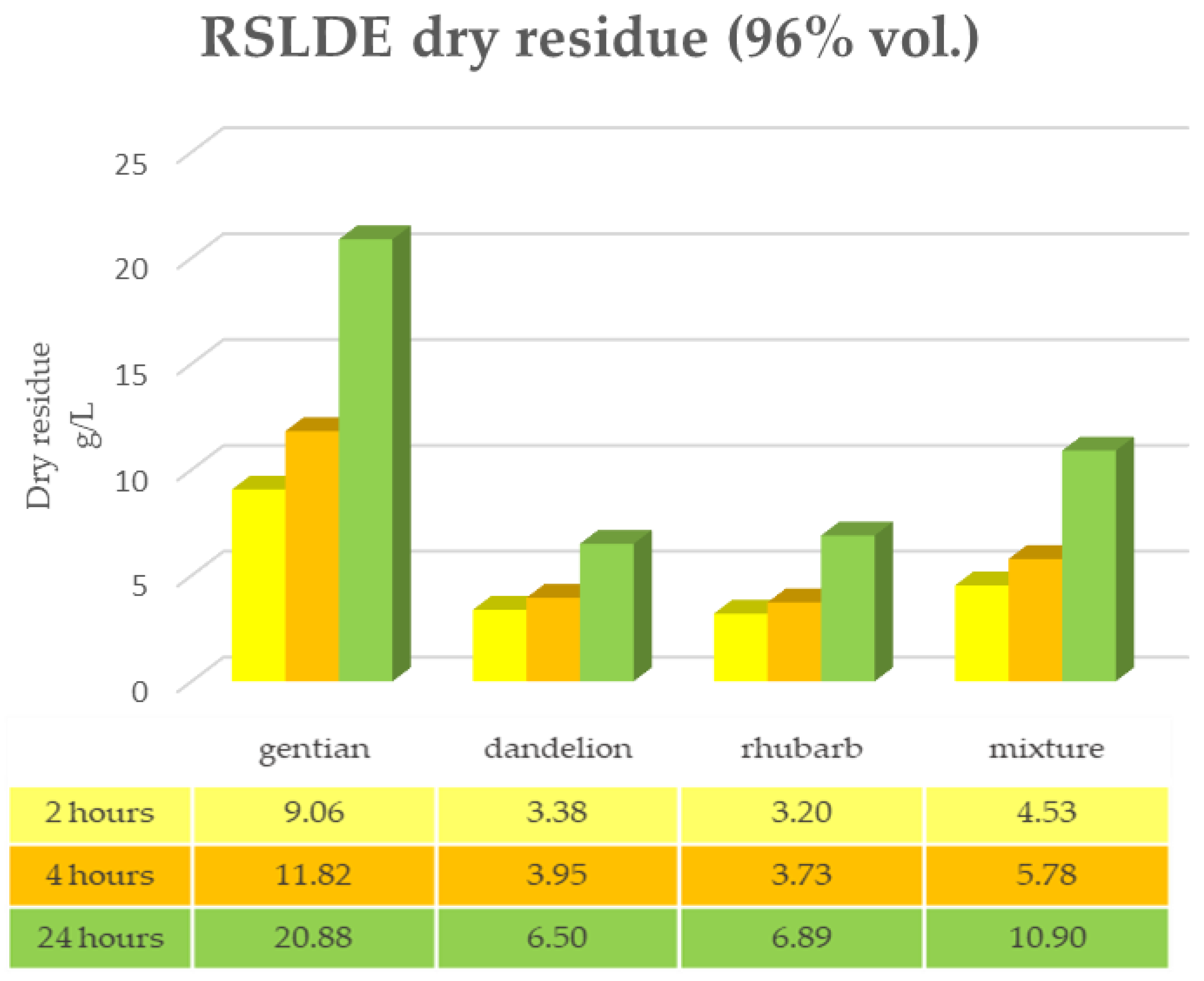
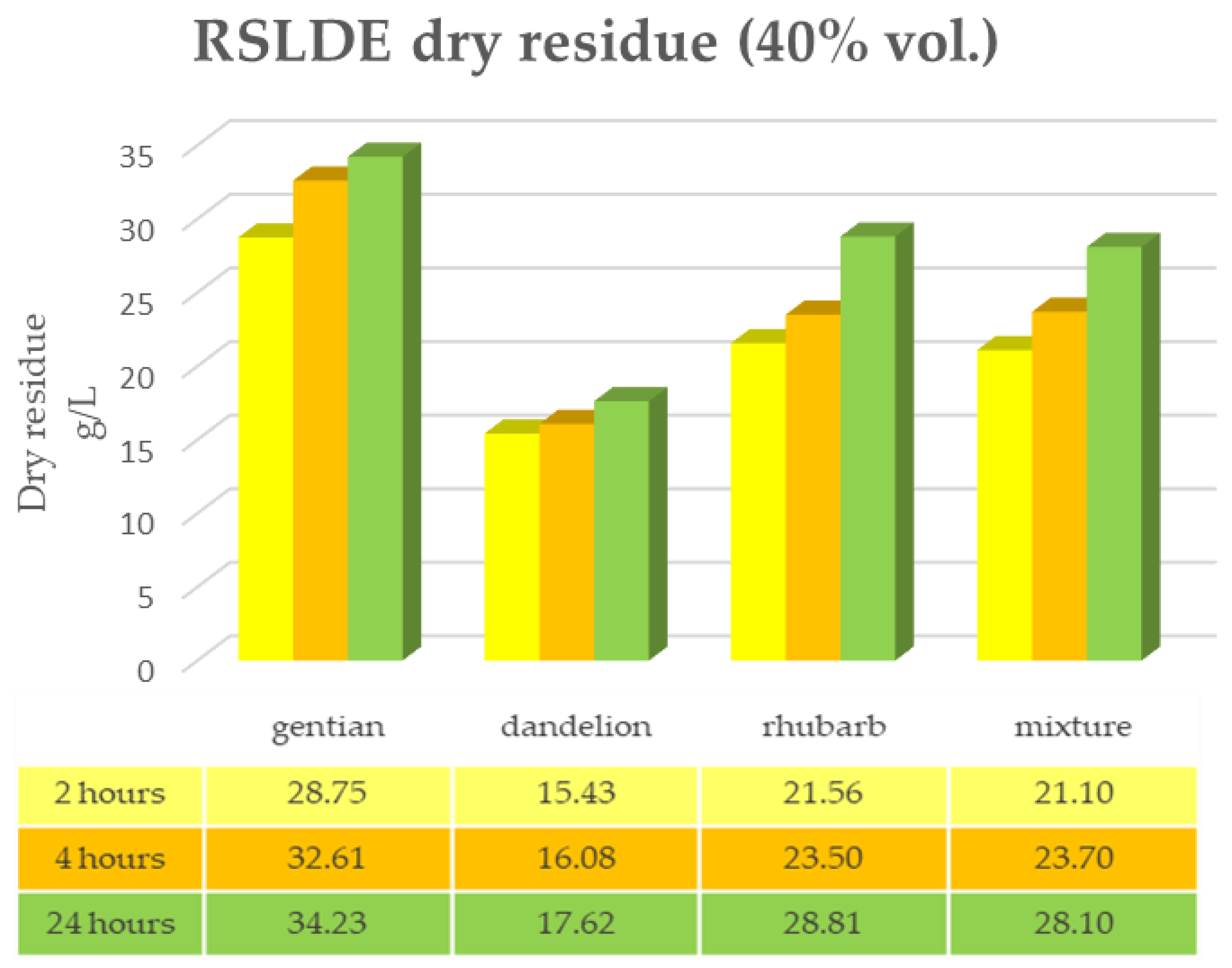
2.3. Root Extraction
3. Materials and Methods
3.1. Plant Matrices
3.2. Analysis of Total Phenols by Folin–Ciocalteu Reagent
3.3. DPPH Assay
3.4. FRAP Assay
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide research trends on medicinal plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Hussein, R.A.; El-anssary, A.A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. Herb. Med. 2019, 1, 3. [Google Scholar]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant Effects and Mechanisms of Medicinal Plants and Their Bioactive Compounds for the Prevention and Treatment of Type 2 Diabetes: An Updated Review. Oxidative Med. Cell. Longev. 2020. [Google Scholar] [CrossRef] [Green Version]
- Sekhon-Loodu, S.; Rupasinghe, H.P.V. Evaluation of Antioxidant, Antidiabetic and Antiobesity Potential of Selected Traditional Medicinal Plants. Front. Nutr. 2019, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Guleria, S.; Razdan, V.K.; Babu, V. Synergistic antioxidant and antimicrobial activities of essential oils of some selected medicinal plants in combination and with synthetic compounds. Ind. Crops Prod. 2020, 154, 112569. [Google Scholar] [CrossRef]
- Ghuman, S.; Ncube, B.; Finnie, J.; McGaw, L.; Njoya, E.M.; Coopoosamy, R.; Van Staden, J. Antioxidant, anti-inflammatory and wound healing properties of medicinal plant extracts used to treat wounds and dermatological disorders. S. Afr. J. Bot. 2019, 126, 232–240. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Islam, S.U.; Ahmed, M.B.; Ahsan, H.; Lee, Y.S. Recent molecular mechanisms and beneficial effects of phytochemicals and plant-based whole foods in reducing LDL-C and preventing cardiovascular disease. Antioxidants 2021, 10, 784. [Google Scholar] [CrossRef]
- Mickymaray, S. Efficacy and Mechanism of Traditional Medicinal Plants and Bioactive Compounds against Clinically Important Pathogens. Antibiotics 2019, 8, 257. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [Green Version]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of Polyphenols from Aromatic and Medicinal Plants: An Overview of the Methods and the Effect of Extraction Parameters. Polyphen. Plants 2019, 243–259. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Ge, S.; Lin, S. Review of Distribution, Extraction Methods, and Health Benefits of Bound Phenolics in Food Plants. J. Agric. Food Chem. 2020, 68, 3330–3343. [Google Scholar] [CrossRef] [PubMed]
- Kamil Hussain, M.; Saquib, M.; Faheem Khan, M. Techniques for extraction, isolation, and standardization of bioactive compounds from medicinal plants. In Natural Bio-Active Compounds: Volume 2: Chemistry, Pharmacology and Health Care Practices; Springer: Berlin/Heidelberg, Germany, 2019; pp. 179–200. [Google Scholar]
- Stéphane, F.F.Y.; Jules, B.K.J.; Batiha, G.E.-S.; Ali, I.; Bruno, L.N. Extraction of Bioactive Compounds from Medicinal Plants and Herbs. Nat. Med. Plants 2021. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Usman, I.; Hussain, M.; Imran, A.; Afzaal, M.; Saeed, F.; Javed, M.; Afzal, A.; Ashfaqc, I.; Al Jbawi, E.; Saewan, S. Traditional and innovative approaches for the extraction of bioactive compounds. Int. J. Food Prop. 2022, 25, 1215–1233. [Google Scholar] [CrossRef]
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Priego-Capote, F. Solid–liquid extraction techniques. In Analytical Sample Preparation with Nano-and Other High-Performance Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 111–130. [Google Scholar]
- Naviglio, D.; Scarano, P.; Ciaravolo, M.; Gallo, M. Rapid Solid-Liquid Dynamic Extraction (RSLDE): A Powerful and Greener Alternative to the Latest Solid-Liquid Extraction Techniques. Foods 2019, 8, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walia, A.; Gupta, A.K.; Sharma, V. Role of bioactive compounds in human health. Acta Sci. Med. Sci. 2019, 3, 25–33. [Google Scholar]
- Sharifi-Rad, J.; Rodrigues, C.F.; Sharopov, F.; Docea, A.O.; Can Karaca, A.; Sharifi-Rad, M.; Kahveci Karıncaoglu, D.; Gülseren, G.; Özçelik, B.; Demircan, E.; et al. Diet, Lifestyle and Cardiovascular Diseases: Linking Pathophysiology to Cardioprotective Effects of Natural Bioactive Compounds. Int. J. Environ. Res. Public Health 2020, 17, 2326. [Google Scholar] [CrossRef] [Green Version]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Comparison of microwave, ultrasonic and conventional techniques for extraction of bioactive compounds from olive leaves (Olea europaea L.). Innov. Food Sci. Emerg. Technol. 2019, 58, 102234. [Google Scholar] [CrossRef]
- Gori, A.; Boucherle, B.; Rey, A.; Rome, M.; Fuzzati, N.; Peuchmaur, M. Development of an innovative maceration technique to optimize extraction and phase partition of natural products. Fitoterapia 2021, 148, 104798. [Google Scholar] [CrossRef] [PubMed]
- Soquetta, M.B.; Terra, L.D.M.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Farooq, S.; Shah, M.A.; Siddiqui, M.W.; Dar, B.N.; Mir, S.A.; Ali, A. Recent trends in extraction techniques of anthocyanins from plant materials. J. Food Meas. Charact. 2020, 14, 3508–3519. [Google Scholar] [CrossRef]
- Yılmaz, F.M.; Görgüç, A.; Uygun, Ö.; Bircan, C. Steviol glycosides and polyphenols extraction from Stevia rebaudiana Bertoni leaves using maceration, microwave-, and ultrasound-assisted techniques. Sep. Sci. Technol. 2021, 56, 936–948. [Google Scholar] [CrossRef]
- Naviglio, D.; Nebbioso, V.; Savastano, A.; Montesano, D.; Trucillo, P.; Gallo, M. High Efficiency and New Potential of RSLDE: A Green Technique for the Extraction of Bioactive Molecules from Not Completely Exhausted Plant Biomass and Organic Industrial Processing Waste. Appl. Sci. 2022, 12, 11726. [Google Scholar] [CrossRef]
- Palmieri, S.; Pellegrini, M.; Ricci, A.; Compagnone, D.; Lo Sterzo, C. Chemical Composition and Antioxidant Activity of Thyme, Hemp and Coriander Extracts: A Comparison Study of Maceration, Soxhlet, UAE and RSLDE Techniques. Foods 2020, 9, 1221. [Google Scholar] [CrossRef]
- Gallo, M.; Conte, E.; Naviglio, D. Analysis and Comparison of the Antioxidant Component of Portulaca Oleracea Leaves Obtained by Different Solid-Liquid Extraction Techniques. Antioxidants 2017, 6, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, M.; Vitulano, M.; Andolfi, A.; DellaGreca, M.; Conte, E.; Ciaravolo, M.; Naviglio, D. Rapid Solid-Liquid Dynamic Extraction (RSLDE): A New Rapid and Greener Method for Extracting Two Steviol Glycosides (Stevioside and Rebaudioside A) from Stevia Leaves. Plant Foods Hum. Nutr. 2017, 72, 141–148. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Ciaravolo, M.; Cirino, P.; Toscano, A.; Salvatore, F.; Gallo, M.; Naviglio, D.; Andolfi, A. Fatty Acids from Paracentrotus lividus Sea Urchin Shells Obtained via Rapid Solid Liquid Dynamic Extraction (RSLDE). Separations 2019, 6, 50. [Google Scholar] [CrossRef] [Green Version]
- Gallo, M.; Formato, A.; Ciaravolo, M.; Formato, G.; Naviglio, D. Study of the Kinetics of Extraction Process for The Production of Hemp Inflorescences Extracts by Means of Conventional Maceration (CM) and Rapid Solid-Liquid Dynamic Extraction (RSLDE). Separations 2020, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Salvatore, M.M.; De Gregorio, V.; Gallo, M.; Corsaro, M.M.; Casillo, A.; Vecchione, R.; Andolfi, A.; Naviglio, D.; Netti, P.A. Evaluation of Two Extraction Methods for the Analysis of Hydrophilic Low Molecular Weight Compounds from Ganoderma lucidum Spores and Antiproliferative Activity on Human Cell Lines. Appl. Sci. 2020, 10, 4033. [Google Scholar] [CrossRef]
- Jain, C.; Khatana, S.; Vijayvergia, R. Bioactivity of secondary metabolites of various plants: A review. Int. J. Pharm. Sci. Res. 2019, 10, 494–504. [Google Scholar]
- De Marco, A.; Luongo, G.; Di Marino, C.; De Tommaso, G.; Di Fabio, G.; Zarrelli, A. Silymarin from Silybum marianum by Naviglio’s extractor: A new and very efficient approach. Nat. Prod. Res. 2021, 35, 2621–2627. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Szerlauth, A.; Muráth, S.; Viski, S.; Szilagyi, I. Radical scavenging activity of plant extracts from improved processing. Heliyon 2019, 5, e02763. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naviglio, D.; Trifuoggi, M.; Varchetta, F.; Nebbioso, V.; Perrone, A.; Avolio, L.; De Martino, E.; Montesano, D.; Gallo, M. Efficiency of Recovery of the Bioactive Principles of Plants by Comparison between Solid–Liquid Extraction in Mixture and Single-Vegetable Matrices via Maceration and RSLDE. Plants 2023, 12, 2900. https://doi.org/10.3390/plants12162900
Naviglio D, Trifuoggi M, Varchetta F, Nebbioso V, Perrone A, Avolio L, De Martino E, Montesano D, Gallo M. Efficiency of Recovery of the Bioactive Principles of Plants by Comparison between Solid–Liquid Extraction in Mixture and Single-Vegetable Matrices via Maceration and RSLDE. Plants. 2023; 12(16):2900. https://doi.org/10.3390/plants12162900
Chicago/Turabian StyleNaviglio, Daniele, Marco Trifuoggi, Francesca Varchetta, Viviana Nebbioso, Angela Perrone, Laura Avolio, Eleonora De Martino, Domenico Montesano, and Monica Gallo. 2023. "Efficiency of Recovery of the Bioactive Principles of Plants by Comparison between Solid–Liquid Extraction in Mixture and Single-Vegetable Matrices via Maceration and RSLDE" Plants 12, no. 16: 2900. https://doi.org/10.3390/plants12162900
APA StyleNaviglio, D., Trifuoggi, M., Varchetta, F., Nebbioso, V., Perrone, A., Avolio, L., De Martino, E., Montesano, D., & Gallo, M. (2023). Efficiency of Recovery of the Bioactive Principles of Plants by Comparison between Solid–Liquid Extraction in Mixture and Single-Vegetable Matrices via Maceration and RSLDE. Plants, 12(16), 2900. https://doi.org/10.3390/plants12162900










