Seasonal Hydration Status of Common Bryophyte Species in Azorean Native Vegetation
Abstract
1. Introduction
2. Results
2.1. Variations of Field Water Content in Bryophytes along an Elevation Gradient
2.2. Relationship between Climate Variables and Field Water Content
2.3. Field Water Content of the Species Shared between Different Elevations
2.4. Seasonal Variations in the Relative Water Content (RWC) of Bryophytes in Native Vegetation
3. Discussion
3.1. How Did Elevation Impact the Bryophyte Field Water Content?
3.2. Lowland—Climate and Environmental Factors
3.3. Lowland—Traits and Species/Taxa Comparisons
3.4. Mid- and Highland Sites—Climate and Environmental Factors
3.5. Mid- and Highlands—Traits and Species/Taxa Comparisons
3.6. Did Bryophytes Reach Their Maximum Water Retention Capacity?
4. Materials and Methods
4.1. Study Sites
4.1.1. General Information
4.1.2. Climate Data
4.2. Study Species
- (i)
- Species that were abundant and widespread enough on each studied site to withstand monthly collections for one year without significantly depleting the populations. At lower altitudes the selected bryophyte species are commonly found around the island, while the other species are typically present in mature native forests, where, even the endemic and conservation-concern species are capable of forming extensive and healthy patches [16,68,86];
- (ii)
- Species that were large and relatively easy to recognize in the field [86];
- (iii)
4.3. Sampling Procedure in the Field
4.4. Processing Samples in the Laboratory
4.5. Data Analysis
4.5.1. Water Content
4.5.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| FWC | 01_Jan | 02_Feb | 03_Mar | 04_Apr | 05_May | 06_Jun | 07_Jul | 08_Aug | 09_Sep | 10_Oct | 11_Nov | 12_Dec |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Farol da Serreta (FS) | ||||||||||||
| Frullania acicularis | 0.83 ± 0.1 | 2.51 ± 0.2 | 0.41 ± 0.0 | 2.19 ± 0.3 | 2.77 ± 0.4 | 0.42 ± 0.0 | 0.37 ± 0.0 | 0.47 ± 0.1 | 0.43 ± 0.1 | 2.19 ± 0.3 | 0.98 ± 0.1 | 3.22 ± 0.6 |
| FS—Livertorts | 0.83 ± 0.1 | 2.51 ± 0.2 | 0.41 ± 0.0 | 2.19 ± 0.3 | 2.77 ± 0.4 | 0.42 ± 0.0 | 0.37 ± 0.0 | 0.47 ± 0.1 | 0.43 ± 0.1 | 2.19 ± 0.3 | 0.98 ± 0.1 | 3.22 ± 0.6 |
| Campy. brevipilus | 1.44 ± 0.5 | 2.96 ± 0.5 | 0.36 ± 0.1 | 3.50 ± 0.8 | 2.55 ± 0.3 | 0.31 ± 0.0 | 0.32 ± 0.1 | 0.26 ± 0.0 | 0.75 ± 0.2 | 3.14 ± 0.4 | 1.77 ± 0.3 | 3.10 ± 0.4 |
| Trich. brachydontium | 0.75 ± 0.1 | 2.12 ± 0.3 | 0.47 ± 0.0 | 1.37 ± 0.4 | 1.51 ± 0.2 | 0.38 ± 0.0 | 0.42 ± 0.1 | 0.36 ± 0.0 | 0.62 ± 0.3 | 1.55 ± 0.3 | 1.18 ± 0.2 | 2.12 ± 0.4 |
| FS—Mosses | 1.10 ± 0.5 | 2.54 ± 0.6 | 0.42 ± 0.1 | 2.44 ± 1.3 | 2.03 ± 0.6 | 0.34 ± 0.0 | 0.37 ± 0.1 | 0.31 ± 0.1 | 0.69 ± 0.2 | 2.35 ± 0.9 | 1.47 ± 0.4 | 2.61 ± 0.6 |
| FS Total | 1.01 ± 0.4 | 2.53 ± 0.5 | 0.41 ± 0.1 | 2.35 ± 1.0 | 2.28 ± 0.6 | 0.37 ± 0.1 | 0.37 ± 0.1 | 0.36 ± 0.1 | 0.60 ± 0.2 | 2.29 ± 0.7 | 1.31 ± 0.4 | 2.81 ± 0.7 |
| Pico da Lagoínha (PL) | ||||||||||||
| Plagiochila bifaria | 2.35 ± 0.2 | 6.74 ± 0.4 | 2.16 ± 0.1 | 7.85 ± 1.1 | 10.0 ± 1.2 | 1.90 ± 0.1 | 3.12 ± 0.6 | 1.68 ± 0.0 | 1.80 ± 0.0 | 6.39 ± 1.3 | 2.26 ± 0.1 | 6.18 ± 0.9 |
| Scapania gracilis | 4.98 ± 0.4 | 5.80 ± 0.5 | 2.27 ± 0.4 | 6.47 ± 1.2 | 6.62 ± 1.0 | 1.19 ± 0.1 | 3.67 ± 0.4 | 1.75 ± 0.2 | 1.94 ± 0.5 | 6.27 ± 1.6 | 3.55 ± 0.5 | 5.91 ± 0.4 |
| Frullania acicularis | 0.96 ± 0.0 | 5.57 ± 0.9 | 0.67 ± 0.1 | 5.73 ± 0.5 | 5.81 ± 0.6 | 1.02 ± 0.1 | 3.78 ± 0.3 | 0.68 ± 0.0 | 0.62 ± 0.1 | 3.54 ± 0.3 | 1.00 ± 0.5 | 5.77 ± 1.0 |
| PL—Livertorts | 2.76 ± 1.7 | 6.04 ± 0.8 | 1.70 ± 0.8 | 6.68 ± 1.3 | 7.49 ± 2.1 | 1.37 ± 0.4 | 3.52 ± 0.5 | 1.37 ± 0.5 | 1.45 ± 0.7 | 5.40 ± 1.8 | 2.27 ± 1.1 | 5.96 ± 0.8 |
| Sphagnum subnitens | 15.3 ± 0.3 | 19.2 ± 1.3 | 15.6 ± 1.4 | 18.6 ± 0.9 | 18.1 ± 0.5 | 8.38 ± 0.5 | 11.1 ± 0.8 | 8.14 ± 0.4 | 12.7 ± 0.2 | 16.8 ± 0.5 | 16.1 ± 1.8 | 16.8 ± 1.6 |
| Isothecium prolixum | 1.76 ± 0.2 | 4.81 ± 0.5 | 0.90 ± 0.1 | 4.89 ± 0.4 | 5.49 ± 0.8 | 1.18 ± 0.0 | 2.25 ± 0.3 | 1.00 ± 0.0 | 0.81 ± 0.1 | 3.36 ± 0.5 | 1.51 ± 0.3 | 4.77 ± 0.7 |
| Myur. hochstetteri | 4.73 ± 1.3 | 9.56 ± 0.2 | 1.29 ± 0.1 | 11.9 ± 1.4 | 8.87 ± 0.9 | 0.95 ± 0.0 | 2.43 ± 0.7 | 0.87 ± 0.1 | 0.93 ± 0.3 | 6.43 ± 0.6 | 2.59 ± 0.3 | 8.94 ± 0.7 |
| Thui. tamariscinum | 4.11 ± 0.5 | 5.31 ± 0.4 | 1.86 ± 0.5 | 5.10 ± 0.6 | 4.27 ± 0.4 | 1.28 ± 0.0 | 2.87 ± 0.3 | 1.21 ± 0.1 | 1.82 ± 1.0 | 5.88 ± 0.7 | 4.95 ± 0.6 | 5.68 ± 0.4 |
| PL—Mosses | 6.49 ± 5.4 | 9.71 ± 5.9 | 4.92 ± 6.4 | 10.1 ± 5.9 | 9.19 ± 5.6 | 2.95 ± 3.2 | 4.65 ± 3.8 | 2.81 ± 3.2 | 4.08 ± 5.2 | 8.12 ± 5.3 | 6.29 ± 6.0 | 9.06 ± 5.0 |
| PL Total | 4.89 ± 4.6 | 8.13 ± 4.8 | 3.54 ± 5.1 | 8.64 ± 4.8 | 8.46 ± 4.5 | 2.27 ± 2.6 | 4.17 ± 2.9 | 2.19 ± 2.5 | 2.95 ± 4.1 | 6.95 ± 4.4 | 4.57 ± 5.0 | 7.73 ± 4.0 |
| Serra de Santa Bárbara (SB)) | ||||||||||||
| Bazzania azorica | 9.28 ± 0.5 | 10.3 ± 1.0 | 6.39 ± 0.2 | 8.46 ± 0.7 | 7.09 ± 1.3 | 4.32 ± 0.4 | 4.63 ± 0.4 | 2.91 ± 0.0 | 5.66 ± 0.1 | 10.3 ± 0.3 | 5.52 ± 0.2 | 9.40 ± 0.6 |
| Herbertus azoricus | 6.50 ± 1.7 | 6.33 ± 1.5 | 1.35 ± 0.1 | 4.60 ± 0.4 | 5.24 ± 0.5 | 3.65 ± 0.9 | 2.66 ± 0.4 | 1.30 ± 0.1 | 2.86 ± 0.6 | 5.32 ± 0.9 | 2.33 ± 0.2 | 4.81 ± 0.2 |
| Lepidozia cupressina | 10.7 ± 0.4 | 9.80 ± 0.4 | 7.15 ± 0.5 | 9.07 ± 0.2 | 5.86 ± 0.4 | 5.95 ± 1.0 | 7.27 ± 0.6 | 2.19 ± 0.2 | 6.58 ± 0.5 | 12.4 ± 1.1 | 6.49 ± 0.4 | 10.2 ± 0.9 |
| Plagiochila bifaria | 6.77 ± 1.6 | 6.17 ± 0.9 | 2.20 ± 0.7 | 5.24 ± 0.8 | 6.46 ± 1.0 | 5.80 ± 0.9 | 5.48 ± 1.3 | 2.42 ± 0.1 | 5.62 ± 0.7 | 2.71 ± 0.2 | 6.85 ± 0.7 | |
| Scapania gracilis | 8.14 ± 1.3 | 6.21 ± 0.4 | 5.38 ± 0.8 | 7.79 ± 1.2 | 8.34 ± 1.8 | 3.32 ± 0.8 | 7.05 ± 1.2 | 2.22 ± 0.0 | 4.00 ± 0.7 | 9.48 ± 1.8 | 3.58 ± 0.1 | 7.85 ± 1.1 |
| Frullania acicularis | 7.76 ± 1.4 | 5.72 ± 0.7 | 1.20 ± 0.1 | 5.15 ± 0.7 | 8.08 ± 0.7 | 6.26 ± 1.3 | 4.14 ± 0.5 | 1.14 ± 0.1 | 1.91 ± 0.1 | 4.74 ± 0.4 | 2.08 ± 0.3 | 6.95 ± 0.8 |
| SB—Livertorts | 8.19 ± 1.8 | 7.42 ± 2.1 | 3.95 ± 2.5 | 6.72 ± 1.9 | 6.85 ± 1.5 | 4.88 ± 1.4 | 5.20 ± 1.8 | 2.03 ± 0.6 | 4.20 ± 1.8 | 7.97 ± 3.1 | 3.79 ± 1.7 | 7.68 ± 1.9 |
| Sphagnum subnitens | 17.0 ± 1.1 | 18.9 ± 1.0 | 13.2 ± 0.4 | 18.3 ± 1.3 | 16.9 ± 1.3 | 10.7 ± 0.8 | 15.2 ± 0.6 | 10.5 ± 0.5 | 14.0 ± 1.2 | 16.7 ± 1.0 | 14.1 ± 0.2 | 18.4 ± 0.5 |
| Polytri. commune | 3.21 ± 0.2 | 2.88 ± 0.2 | 2.55 ± 0.2 | 3.08 ± 0.2 | 3.03 ± 0.2 | 3.52 ± 0.5 | 2.68 ± 0.1 | 2.44 ± 0.1 | 3.04 ± 0.4 | 2.87 ± 0.4 | 2.58 ± 0.3 | 3.10 ± 0.2 |
| Campylopus shawii | 6.89 ± 0.7 | 5.43 ± 0.3 | 5.22 ± 0.3 | 4.91 ± 0.6 | 4.95 ± 0.3 | 4.20 ± 0.6 | 5.03 ± 0.5 | 1.91 ± 0.3 | 4.50 ± 0.3 | 7.27 ± 1.1 | 4.81 ± 0.2 | 6.57 ± 0.4 |
| SB—Mosses | 9.05 ± 6.1 | 9.09 ± 7.3 | 6.98 ± 4.7 | 8.77 ± 7.1 | 8.30 ± 6.4 | 6.14 ± 3.4 | 7.63 ± 5.6 | 4.96 ± 4.1 | 7.18 ± 5.1 | 8.93 ± 6.0 | 7.15 ± 5.1 | 9.37 ± 6.8 |
| SB Total | 8.47 ± 3.8 | 7.98 ± 4.5 | 4.96 ± 3.6 | 7.40 ± 4.4 | 7.33 ± 3.9 | 5.30 ± 2.3 | 6.01 ± 3.7 | 3.01 ± 2.7 | 5.32 ± 3.7 | 8.29 ± 4.2 | 4.91 ± 3.6 | 8.24 ± 4.2 |
Appendix B
| RWC | 01_Jan | 02_Feb | 03_Mar | 04_Apr | 05_May | 06_Jun | 07_Jul | 08_Aug | 09_Sep | 10_Oct | 11_Nov | 12_Dec |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Farol da Serreta (FS) | ||||||||||||
| Frullania acicularis | 6.72 ± 0.7 | 20.27 ± 1.3 | 3.33 ± 0.4 | 17.72 ± 2.6 | 22.36 ± 3.2 | 3.39 ± 0.2 | 3.01 ± 0.3 | 3.79 ± 0.4 | 3.49 ± 0.7 | 17.66 ± 2.1 | 7.94 ± 1.0 | 25.98 ± 5.1 |
| FS—Livertorts | 6.72 ± 0.7 | 20.27 ± 1.3 | 3.33 ± 0.4 | 17.72 ± 2.6 | 22.36 ± 3.2 | 3.39 ± 0.2 | 3.01 ± 0.3 | 3.79 ± 0.4 | 3.49 ± 0.7 | 17.66 ± 2.1 | 7.94 ± 1.0 | 25.98 ± 5.1 |
| Campy. brevipilus | 9.12 ± 3.0 | 18.70 ± 3.2 | 2.25 ± 0.3 | 22.11 ± 5.0 | 16.11 ± 2.1 | 1.93 ± 0.2 | 2.02 ± 0.7 | 1.65 ± 0.2 | 4.77 ± 1.4 | 19.85 ± 2.8 | 11.17 ± 2.1 | 19.57 ± 2.3 |
| Trich. brachydontium | 4.81 ± 0.5 | 13.55 ± 2.1 | 3.03 ± 0.2 | 8.76 ± 2.6 | 9.64 ± 1.4 | 2.40 ± 0.2 | 2.67 ± 0.5 | 2.27 ± 0.3 | 3.97 ± 1.8 | 9.92 ± 1.8 | 7.53 ± 1.1 | 13.54 ± 2.5 |
| FS—Mosses | 6.96 ± 3.0 | 16.13 ± 3.7 | 2.64 ± 0.5 | 15.44 ± 8.0 | 12.88 ± 3.8 | 2.17 ± 0.3 | 2.35 ± 0.7 | 1.96 ± 0.4 | 4.37 ± 1.6 | 14.88 ± 5.7 | 9.35 ± 2.5 | 16.55 ± 3.9 |
| FS Total | 6.88 ± 2.5 | 17.51 ± 3.7 | 2.87 ± 0.6 | 16.20 ± 6.6 | 16.04 ± 5.8 | 2.57 ± 0.7 | 2.57 ± 0.7 | 2.57 ± 1.0 | 4.08 ± 1.4 | 15.81 ± 4.9 | 8.88 ± 2.2 | 19.70 ± 6.2 |
| Pico da Lagoínha (PL) | ||||||||||||
| Plagiochila bifaria | 21.44 ± 1.6 | 61.60 ± 4.0 | 19.73 ± 0.7 | 71.75 ± 9.9 | 90.86 ± 9.7 | 17.36 ± 1.0 | 28.53 ± 5.3 | 15.30 ± 0.2 | 16.41 ± 0.4 | 58.3 ± 12.0 | 20.69 ± 1.2 | 56.50 ± 8.3 |
| Scapania gracilis | 46.64 ± 3.7 | 54.25 ± 5.0 | 21.21 ± 3.7 | 60.6 ± 11.4 | 61.96 ± 9.7 | 11.14 ± 0.7 | 34.31 ± 3.6 | 16.36 ± 2.1 | 18.12 ± 4.7 | 58.7 ± 15.1 | 33.24 ± 4.6 | 55.31 ± 3.7 |
| Frullania acicularis | 7.76 ± 0.4 | 44.98 ± 7.4 | 5.43 ± 0.6 | 46.26 ± 3.7 | 46.96 ± 5.1 | 8.23 ± 0.9 | 30.52 ± 2.7 | 5.52 ± 0.2 | 5.03 ± 0.6 | 28.56 ± 2.6 | 8.11 ± 3.8 | 46.63 ± 8.1 |
| PL—Livertorts | 25.3 ± 16.8 | 53.61 ± 8.8 | 15.46 ± 7.6 | 59.5 ± 13.6 | 66.6 ± 20.4 | 12.24 ± 4.0 | 31.12 ± 4.5 | 12.39 ± 5.2 | 13.19 ± 6.5 | 48.5 ± 18.0 | 20.7 ± 11.1 | 52.81 ± 8.0 |
| Sphagnum subnitens | 29.63 ± 0.6 | 37.01 ± 2.5 | 30.18 ± 2.7 | 35.93 ± 1.8 | 35.05 ± 1.0 | 16.19 ± 1.0 | 21.36 ± 1.6 | 15.73 ± 0.9 | 24.62 ± 0.4 | 32.52 ± 1.0 | 31.11 ± 3.6 | 32.54 ± 3.1 |
| Isothecium prolixum | 31.17 ± 2.9 | 84.92 ± 9.1 | 15.89 ± 0.9 | 86.31 ± 6.8 | 92.59 ± 6.8 | 20.83 ± 0.7 | 39.71 ± 6.1 | 17.74 ± 0.4 | 14.37 ± 1.3 | 59.31 ± 9.3 | 26.61 ± 4.9 | 84.2 ± 12.1 |
| Myur. hochstetteri | 24.05 ± 6.7 | 48.59 ± 1.2 | 6.56 ± 0.6 | 60.32 ± 7.3 | 45.07 ± 4.4 | 4.81 ± 0.1 | 12.37 ± 3.6 | 4.41 ± 0.3 | 4.74 ± 1.5 | 32.64 ± 3.1 | 13.14 ± 1.3 | 45.44 ± 3.7 |
| Thui. tamariscinum | 30.57 ± 4.0 | 39.44 ± 3.2 | 13.82 ± 3.4 | 37.88 ± 4.7 | 31.74 ± 2.8 | 9.49 ± 0.3 | 21.29 ± 2.0 | 9.01 ± 0.7 | 13.54 ± 7.3 | 43.67 ± 5.2 | 36.77 ± 4.3 | 42.23 ± 2.6 |
| PL—Mosses | 28.85 ± 4.8 | 52.5 ± 20.2 | 16.62 ± 9.0 | 55.1 ± 21.6 | 51.1 ± 25.4 | 12.83 ± 6.3 | 23.7 ± 10.8 | 11.72 ± 5.5 | 14.32 ± 8.0 | 42.0 ± 12.4 | 26.91 ± 9.6 | 51.1 ± 21.1 |
| PL Total | 27.3 ± 11.5 | 53.0 ± 16.2 | 16.12 ± 8.4 | 57.0 ± 18.5 | 57.8 ± 24.3 | 12.58 ± 5.4 | 26.87 ± 9.3 | 12.01 ± 5.3 | 13.83 ± 7.3 | 44.8 ± 15.1 | 24.2 ± 10.6 | 51.8 ± 16.6 |
| Serra de Santa Bárbara (SB) | ||||||||||||
| Bazzania azorica | 99.95 ± 0.1 | 100.0 ± 0.0 | 73.35 ± 2.2 | 95.27 ± 5.4 | 79.9 ± 11.6 | 49.57 ± 4.3 | 53.11 ± 4.1 | 33.45 ± 0.5 | 64.96 ± 1.0 | 100.0 ± 0.0 | 63.35 ± 2.8 | 100.0 ± 0.0 |
| Herbertus azoricus | 89.9 ± 10.3 | 87.5 ± 12.0 | 20.41 ± 2.2 | 69.60 ± 5.6 | 79.40 ± 7.9 | 55.3 ± 14.3 | 40.33 ± 5.3 | 19.66 ± 1.2 | 43.37 ± 9.3 | 80.6 ± 14.1 | 35.30 ± 3.2 | 72.82 ± 3.4 |
| Lepidozia cupressina | 74.11 ± 2.5 | 68.18 ± 2.7 | 49.77 ± 3.6 | 63.07 ± 1.2 | 40.79 ± 2.9 | 41.38 ± 7.1 | 50.59 ± 4.0 | 15.23 ± 1.2 | 45.75 ± 3.6 | 86.10 ± 7.8 | 45.17 ± 2.6 | 70.91 ± 6.2 |
| Plagiochila bifaria | 61.9 ± 14.9 | 56.38 ± 8.1 | 20.10 ± 6.8 | 47.87 ± 7.5 | 59.03 ± 9.4 | 52.99 ± 7.9 | 50.1 ± 11.8 | 22.08 ± 1.2 | 51.37 ± 6.7 | 24.80 ± 1.5 | 62.59 ± 6.3 | |
| Scapania gracilis | 76.2 ± 12.4 | 58.15 ± 3.7 | 50.34 ± 7.6 | 72.9 ± 11.3 | 78.1 ± 17.0 | 31.07 ± 7.4 | 65.9 ± 11.6 | 20.78 ± 0.4 | 37.47 ± 6.3 | 87.6 ± 15.9 | 33.50 ± 1.1 | 73.5 ± 10.4 |
| Frullania acicularis | 62. 7 ± 11.0 | 46.24 ± 5.9 | 9.72 ± 0.6 | 41.59 ± 5.9 | 65.31 ± 5.3 | 50.6 ± 10.2 | 33.46 ± 4.2 | 9.18 ± 0.9 | 15.45 ± 1.1 | 38.31 ± 3.6 | 16.77 ± 2.7 | 56.17 ± 6.1 |
| SB—Livertorts | 77.5 ± 16.7 | 69.4 ± 20.0 | 37.3 ± 23.0 | 65.1 ± 18.9 | 67.1 ± 17.0 | 46.8 ± 11.8 | 48.9 ± 12.5 | 20.06 ± 7.5 | 41.4 ± 17.0 | 74.0 ± 23.8 | 36.5 ± 15.3 | 72.7 ± 15.0 |
| Sphagnum subnitens | 32.93 ± 2.1 | 36.60 ± 2.0 | 25.46 ± 0.8 | 35.38 ± 2.4 | 32.71 ± 2.5 | 20.66 ± 1.5 | 29.34 ± 1.1 | 20.35 ± 1.0 | 27.07 ± 2.4 | 32.20 ± 1.9 | 27.15 ± 0.4 | 35.60 ± 1.0 |
| Polytri. commune | 63.42 ± 4.1 | 57.01 ± 3.0 | 50.35 ± 4.5 | 60.86 ± 3.7 | 59.98 ± 3.2 | 69.62 ± 9.4 | 52.95 ± 1.9 | 48.27 ± 2.7 | 60.20 ± 8.6 | 56.71 ± 7.5 | 51.05 ± 5.0 | 61.38 ± 4.0 |
| Campylopus shawii | 44.64 ± 4.3 | 35.20 ± 2.2 | 33.80 ± 2.1 | 31.81 ± 4.2 | 32.05 ± 2.1 | 27.20 ± 3.8 | 32.59 ± 3.1 | 12.36 ± 1.7 | 29.17 ± 2.1 | 47.10 ± 7.1 | 31.18 ± 1.6 | 42.58 ± 2.8 |
| SB—Mosses | 47.0 ± 13.4 | 42.9 ± 10.6 | 36.5 ± 11.0 | 42.7 ± 13.8 | 41.6 ± 13.7 | 39.2 ± 23.1 | 38.3 ± 11.0 | 27.0 ± 16.0 | 38.8 ± 16.4 | 45.3 ± 11.8 | 36.5 ± 11.2 | 46.5 ± 11.6 |
| SB Total | 67.3 ± 21.3 | 60.6 ± 21.4 | 37.0 ± 19.7 | 57.6 ± 20.2 | 58.6 ± 20.0 | 44.3 ± 16.6 | 45.4 ± 12.9 | 22.4 ± 11.4 | 40.4 ± 16.6 | 64.5 ± 24.6 | 36.5 ± 13.9 | 63.9 ± 18.6 |
Appendix C
| Climate Variables | 01_Jan | 02_Feb | 03_Mar | 04_Apr | 05_May | 06_Jun | 07_Jul | 08_Aug | 09_Sep | 10_Oct | 11_Nov | 12_Dec |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Farol da Serreta (FS) | ||||||||||||
| Precipitation (mm) | 137.74 | 127.10 | 108.19 | 95.91 | 52.50 | 49.80 | 34.20 | 52.90 | 90.70 | 123.78 | 138.94 | 140.01 |
| Temperature—min (°C) | 11.58 | 11.16 | 11.72 | 12.24 | 13.67 | 15.84 | 17.53 | 19.03 | 18.98 | 16.25 | 1409.00 | 12.69 |
| Temperature—max (°C) | 16.30 | 16.00 | 16.66 | 17.37 | 19.10 | 21.49 | 23.97 | 25.40 | 24.23 | 21.48 | 18.93 | 17.21 |
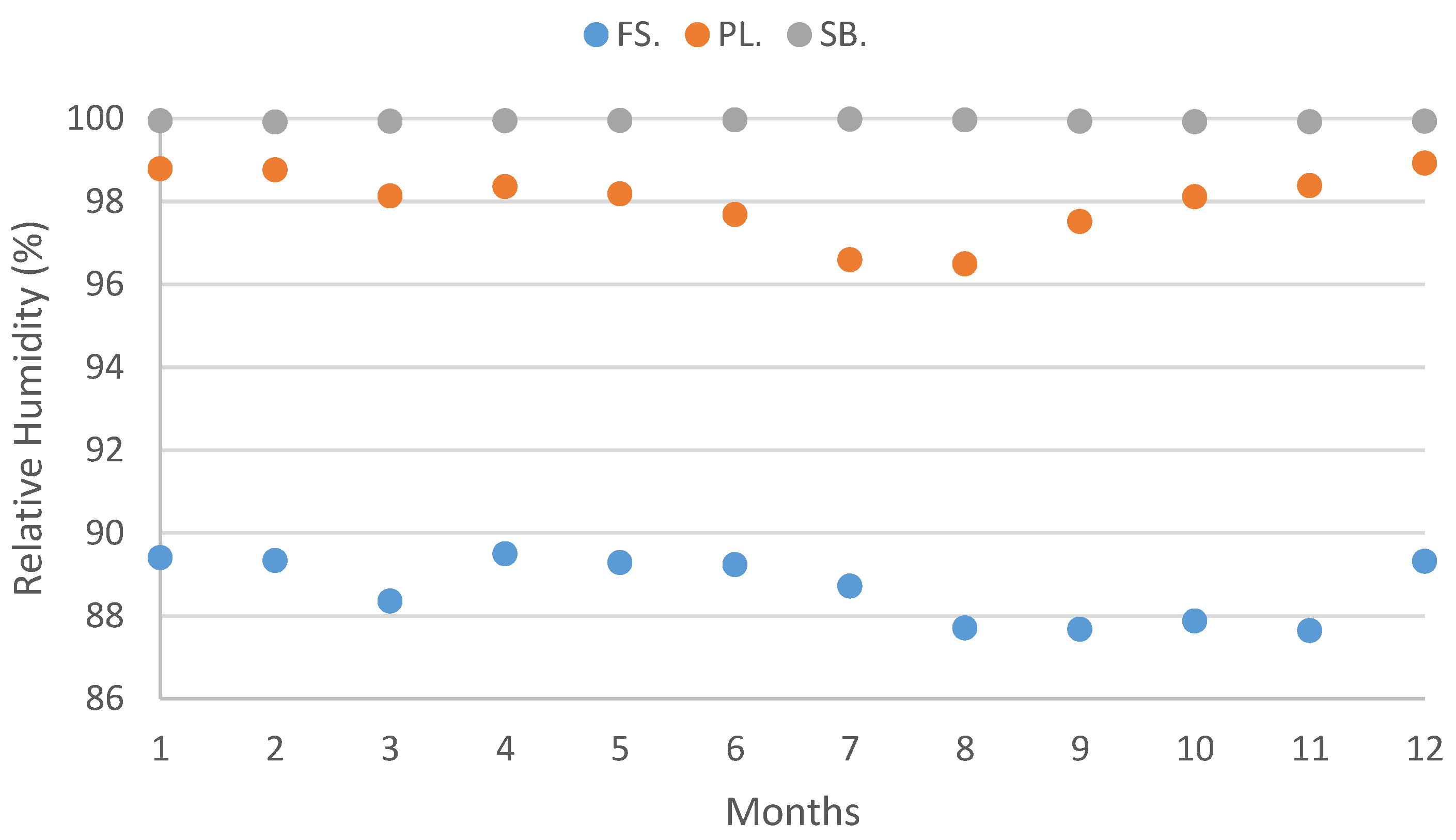
| Relative Humidity—min (%) | 83.22 | 83.65 | 82.22 | 83.79 | 83.59 | 84.46 | 83.08 | 82.10 | 81.62 | 81.34 | 80.64 | 83.16 |
| Relative Humidity—max (%) | 95.58 | 95.01 | 94.48 | 95.18 | 94.96 | 94.00 | 94.33 | 93.29 | 93.73 | 94.40 | 94.65 | 95.47 |
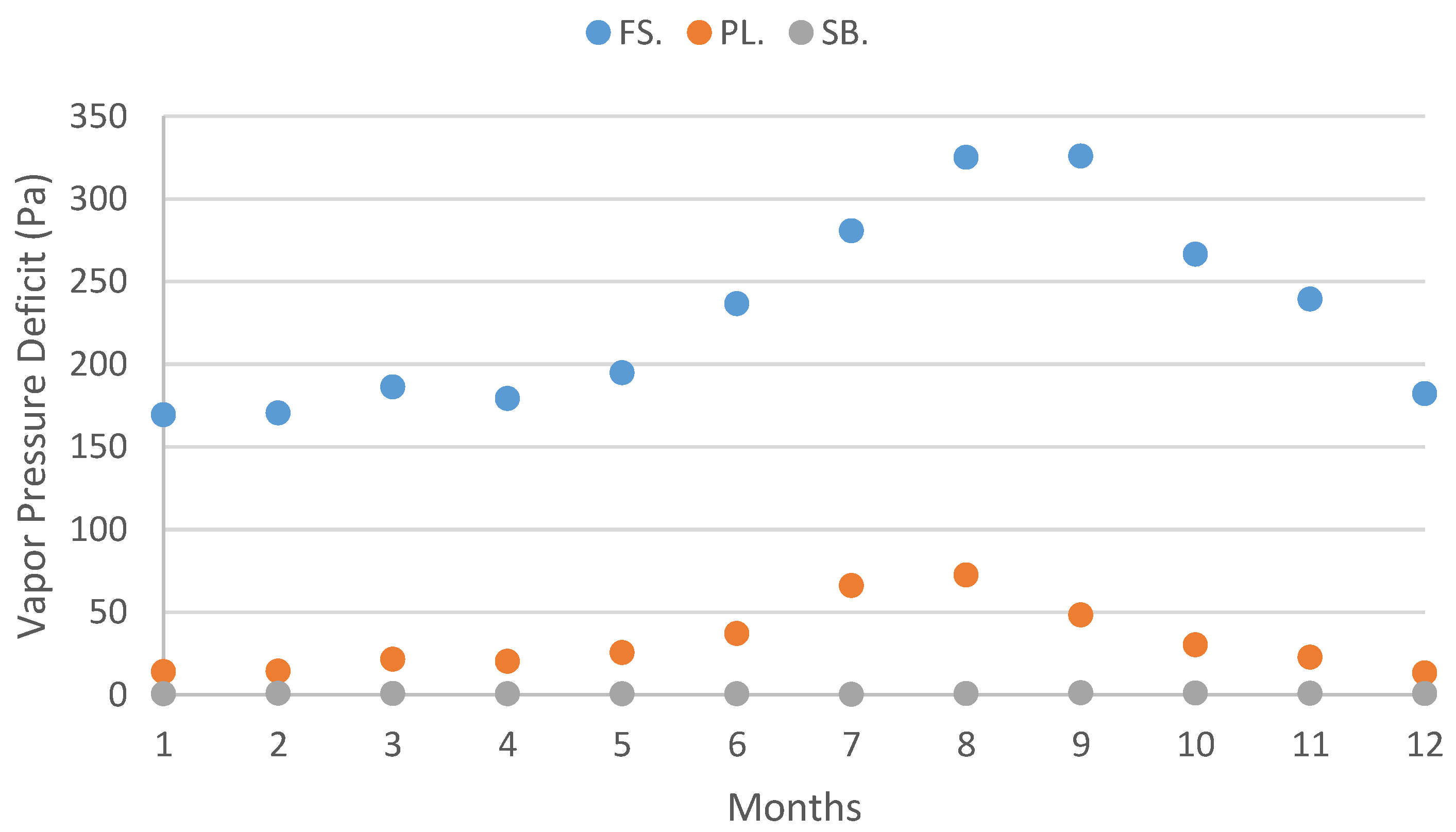
| Vapor Pressure Deficit (Pa) | 169.39 | 170.51 | 186.17 | 179.20 | 194.89 | 236.62 | 280.67 | 325.09 | 325.88 | 266.50 | 239.41 | 182.26 |
| Pico da Lagoínha (PL) | ||||||||||||
| Precipitation (mm) | 363.84 | 327.07 | 288.13 | 252.51 | 136.73 | 131.35 | 99.28 | 160.50 | 283.66 | 379.19 | 392.11 | 376.58 |
| Temperature—min (°C) | 6.76 | 6.30 | 6.87 | 7.52 | 9.03 | 11.33 | 13.34 | 14.70 | 14.34 | 11.50 | 9.16 | 7.87 |
| Temperature—max (°C) | 11.68 | 11.33 | 12.01 | 12.87 | 14.70 | 17.21 | 20.06 | 21.30 | 19.76 | 16.92 | 14.16 | 12.57 |
| Relative Humidity—min (%) | 97.85 | 97.77 | 96.64 | 97.09 | 96.83 | 95.92 | 93.98 | 93.69 | 95.56 | 96.61 | 97.11 | 98.09 |
| Relative Humidity—max (%) | 99.72 | 99.75 | 99.62 | 99.61 | 99.53 | 99.44 | 99.21 | 99.28 | 99.47 | 99.60 | 99.66 | 99.76 |
| Vapor Pressure Deficit (Pa) | 14.01 | 14.24 | 21.47 | 20.26 | 25.52 | 37.07 | 66.05 | 72.45 | 48.23 | 30.20 | 22.71 | 13.26 |
| Serra de Santa Bárbara (SB) | ||||||||||||
| Precipitation (mm) | 480.38 | 443.27 | 354.58 | 314.11 | 165.10 | 144.92 | 100.19 | 160.59 | 309.25 | 455.95 | 485.22 | 504.00 |
| Temperature—min (°C) | 4.85 | 4.36 | 4.87 | 5.54 | 7.05 | 9.37 | 11.17 | 12.56 | 12.41 | 9.55 | 7.24 | 6.00 |
| Temperature—max (°C) | 9.94 | 9.57 | 10.18 | 11.07 | 12.88 | 15.42 | 18.04 | 19.31 | 17.97 | 15.12 | 12.40 | 10.87 |
| Relative Humidity—min (%) | 99.93 | 99.91 | 99.93 | 99.94 | 99.95 | 99.96 | 99.98 | 99.96 | 99.93 | 99.92 | 99.92 | 99.92 |
| Relative Humidity—max (%) | 99.94 | 99.92 | 99.93 | 99.94 | 99.96 | 99.96 | 99.98 | 99.96 | 99.93 | 99.93 | 99.92 | 99.93 |
| Vapor Pressure Deficit (Pa) | 0.60 | 0.90 | 0.75 | 0.64 | 0.61 | 0.56 | 0.34 | 0.73 | 1.19 | 1.12 | 0.98 | 0.75 |
References
- Gabriel, R.; Sjögren, E.; Schumacker, R.; Sérgio, C.; Aranda, S.C.; Claro, D.; Homem, N.; Martins, B. List of Bryophytes (Anthocerotophyta, Marchantiophyta, Bryophyta). In A List of the Terrestrial and Marine Biota of the Azores; Princípia: Cascais, Portugal, 2010; pp. 99–115. [Google Scholar]
- Borges, P.A.V.; Bried, J.; Costa, A.; Cunha, R.; Gabriel, R.; Gonçalves, V.; Martins, A.F.; Melo, I.; Parente, M.; Raposeiro, P.; et al. (Eds.) Description of the Terrestrial and Marine Azorean Biodiversity. In A List of the Terrestrial and Marine Biota from the Azores; Princípia: Cascais, Portugal, 2010; pp. 9–33. [Google Scholar]
- Silva, L.; Moura, M.; Schaefer, H.; Rumsey, F.; Dias, E.F. List of Vascular Plants (Tracheobionta). In A List of the Terrestrial and Marine Biota from the Azores; Borges, P.A.V., Costa, A., Cunha, R., Gabriel, R., Gonçalves, V., Martins, A.F., Melo, I., Parente, M., Raposeiro, P., Rodrigues, P., et al., Eds.; Princípia: Cascais, Portugal, 2010; pp. 117–146. [Google Scholar]
- Henriques, D.S.; Elias, R.B.; Coelho, M.C.; Hérnandez, R.H.; Pereira, F.; Gabriel, R. Long-Term Monitoring across Elevational Gradients (III): Vascular Plants on Terceira Island (Azores) Transect. Arquipel. Life Mar. Sci. 2017, 34, 1–20. [Google Scholar]
- Coelho, M.C.M.; Gabriel, R.; Hespanhol, H.; Borges, P.A.V.; Ah-Peng, C. Bryophyte Diversity along an Elevational Gradient on Pico Island (Azores, Portugal). Diversity 2021, 13, 162. [Google Scholar] [CrossRef]
- Gabriel, R.; Coelho, M.; Henriques, D.; Borges, P.; Elias, R.; Kluge, J.; Ah-Peng, C. Long-term Monitoring across Elevational Gradients to Assess Ecological Hypothesis: A Description of Standardized Sampling Methods in Oceanic Islands and First Results. Arquipel. Life Mar. Sci. 2014, 31, 45–67. [Google Scholar]
- Coelho, M.; Elias, R.; Kluge, J.; Pereira, F.; Henriques, D.; Aranda, S.; Borges, P.; Ah-Peng, C.; Gabriel, R. Long-Term Monitoring across Elevational Gradients (II): Vascular Plants on Pico Island (Azores) Transect. Arquipel. Life Mar. Sci. 2016, 33, 21–44. [Google Scholar]
- Alpert, P. Distribution Quantified by Microtopography in an Assemblage of Saxicolous Mosses. Vegetatio 1985, 64, 131–139. [Google Scholar] [CrossRef]
- McCune, B. Gradients in Epiphyte Biomass in Three Pseudotsuga-Tsuga Forests of Different Ages in Western Oregon and Washington. Bryologist 1993, 96, 405–411. [Google Scholar] [CrossRef]
- Sillett, S.C.; Neitlich, P.N. Emerging Themes in Epiphyte Research in Westside Forests with Special Reference to Cyanolichens. Northwest Sci. 1996, 70, 54–60. [Google Scholar]
- Wolseley, P.A.; Aguirre-Hudson, B. The Ecology and Distribution of Lichens in Tropical Deciduous and Evergreen Forests of Northern Thailand. J. Biogeogr. 1997, 24, 327–343. [Google Scholar] [CrossRef]
- Batty, K.; Bates, J.W.; Bell, J.N.B. A Transplant Experiment on the Factors Preventing Lichen Colonization of Oak Bark in Southeast England under Declining SO2 Pollution. Can. J. Bot. 2003, 81, 439–451. [Google Scholar] [CrossRef]
- Pitkin, P.H. Variability and Seasonality of the Growth of Some Corticolous Pleurocarpous Mosses. J. Bryol. 1975, 8, 337–356. [Google Scholar] [CrossRef]
- Sveinbjörnsson, B.; Oechel, W.C. Controls on Growth and Productivity of Bryophytes: Environmental Limitations under Current and Anticipated Conditions. In Bryophytes and Lichens in a Changing Environment; Bates, J.W., Farmer, A.M., Eds.; Cambridge University Press, Clarendon Press: Oxford, UK, 1992; pp. 77–102. [Google Scholar]
- Azevedo, E.B.; Rodrigues, M.C.; Fernandes, J.F. O Clima Dos Açores. Climatologia: Introdução. In Atlas Básico dos Açores; Forjaz, V.H., Ed.; Observatório Vulcanológico e Geotérmico dos Açores: Ponta Delgada, Portugal, 2004. [Google Scholar]
- Sjögren, E. Bryophyte Vegetation in the Azores Islands. Mem. Soc. Broteriana 1978, 26, 1–273. [Google Scholar]
- Sjögren, E. Epiphyllous Bryophytes in the Azores Islands. Arquipél. Life Mar. Sci. 1997, 15, 1–49. [Google Scholar]
- Gabriel, R.; Bates, J.W. Bryophyte Community Composition and Habitat Specificity in the Natural Forests of Terceira, Azores. Plant Ecol. 2005, 177, 125–144. [Google Scholar] [CrossRef]
- Gimingham, C.H.; Birse, E.M. Ecological Studies on Growth-Form in Bryophytes: I. Correlations Between Growth-Form and Habitat. J. Ecol. 1957, 45, 533–545. [Google Scholar] [CrossRef]
- Noailles, M.-C. Les Frontières Des Plantes Vasculaires. Bull. Soc. Bot. Fr. Actual. Bot. 1987, 134, 53–61. [Google Scholar] [CrossRef][Green Version]
- Proctor, M.C.F.; Oliver, M.J.; Andrew, J.W.; Alpert, P. Desiccation-Tolerance in Bryophytes: A Review. Bryologist 2007, 110, 595–621. [Google Scholar] [CrossRef]
- Lakatos, M.; Hartard, B.; Máguas, C. The Stable Isotopes Δ13C and Δ18O of Lichens Can Be Used as Tracers of Microenvironmental Carbon and Water Sources. In Terrestrial Ecology; Dawson, T.E., Siegwolf, R.T.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 1, pp. 77–92. ISBN 9780123736277. [Google Scholar]
- Gonzalez, L.; Gonzalez-Vilar, M. Determination of Relative Water Content. In Handbook of Plant Ecophysiology Techniques; Reigosa Roger, M.J., Ed.; Springer: Dordrecht, The Netherlands, 2001; pp. 207–212. [Google Scholar]
- Watson, W. Xerophytic Adaptations of Bryophytes in Relation to Habitat. New Phytol. 1914, 13, 149–169. [Google Scholar] [CrossRef]
- Watson, W. Xerophytic Adaptations of Bryophytes in Relation to Habitat (Continued). New Phytol. 1914, 13, 181–190. [Google Scholar] [CrossRef]
- Dilks, T.J.K.; Proctor, M.C.F. Photosynthesis Respiration and Water Content in Bryophytes. New Phytol. 1979, 82, 97–114. [Google Scholar] [CrossRef]
- Vitt, D.H.; Crandall-Stotler, B.; Wood, A. Bryophytes: Survival in a Dry World through Tolerance and Avoidance. In Plant Ecology and Evolution in Harsh Enviroments; Rajakaruna, N.R.B., Harris, T., Eds.; Nova Science: New York, NY, USA, 2014; pp. 267–295. [Google Scholar]
- Proctor, M.C.F. The Physiological Basis of Bryophyte Production. Bot. J. Linn. Soc. 1990, 104, 61–77. [Google Scholar] [CrossRef]
- Cruz de Carvalho, R.; Maurício, A.; Pereira, M.F.; Marques da Silva, J.; Branquinho, C. All for One: The Role of Colony Morphology in Bryophyte Desiccation Tolerance. Front. Plant Sci. 2019, 10, 1360. [Google Scholar] [CrossRef] [PubMed]
- Bayfield, N.G. Notes on Water Relations of Polytrichum Commune Hedw. J. Bryol. 1973, 7, 607–617. [Google Scholar] [CrossRef]
- Proctor, M.C.F. Diffusion Resistances in Bryophytes. In Plants and Their Atmospheric Environment; Grace, J., Ford, E.D., Jarvis, P.G., Eds.; Blackwell Scientific Publications: Oxford, UK, 1981; pp. 219–227. [Google Scholar]
- Proctor, M.C.F. Structure and Eco-Physiological Adaptations in Bryophytes. In Bryophyte Systematics, Systematic Association; Clarke, G.C.S., Duckett, J.G., Eds.; Academic Press: London, UK, 1979; pp. 479–509. [Google Scholar]
- Proctor, M.C.F. Physiological Ecology: Water Relations, Light and Temperature Responses, Carbon Balance. In Bryophyte Ecology; Smith, A.G.E., Ed.; Chapman and Hall: London, UK, 1982; pp. 333–381. [Google Scholar]
- Proctor, M.C.F. Physiological Ecology. In Bryophyte Biology; Goffinet, B., Shaw, A.J., Eds.; Cambridge University Press: Cambridge, MA, 2009; pp. 238–268. ISBN 978-0-08-045405-4. [Google Scholar]
- Pentecost, A. The Lichens and Bryophytes of Rhyolite and Pumice-Tuff Rock Outcrops in Snowdonia, and Some Factors Affecting Their Distribution. J. Ecol. 1980, 68, 251–267. [Google Scholar] [CrossRef]
- Cleavitt, N.L. Stress Tolerance of Rare and Common Moss Species in Relation to Their Occupied Environments and Asexual Dispersal Potential. J. Ecol. 2002, 90, 785–795. [Google Scholar] [CrossRef]
- Barr, H.D.; Weatherley, P.E. Are-Examination of the Relative Turgidity Technique for Estimating Water Deficit in Leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Köhler, L.; Tobón, C.; Frumau, K.F.A.; Bruijnzeel, L.A. (Sampurno) Biomass and Water Storage Dynamics of Epiphytes in Old-Growth and Secondary Montane Cloud Forest Stands in Costa Rica. Plant Ecol. 2007, 193, 171–184. [Google Scholar] [CrossRef]
- Blum, O.B. Water Relations. In The Lichens; Ahmadjian, V., Hale, M.E., Eds.; Academic Press: New York, NY, USA; London, UK, 1973; pp. 381–400. [Google Scholar]
- Kershaw, K.A. Physiological Ecology of Lichens; Cambridge University Press: Cambridge, UK, 1985; p. 293. [Google Scholar]
- Pypker, T.G.; Unsworth, M.H.; Bond, B.J. The Role of Epiphytes in Rainfall Interception by Forests in the Pacific Northwest. I. Laboratory Measurements of Water Storage. Can. J. For. Res. 2006, 36, 809–818. [Google Scholar] [CrossRef]
- Pypker, T.G.; Unsworth, M.H.; Bond, B.J. The Role of Epiphytes in Rainfall Interception by Forests in the Pacific Northwest. II. Field Measurements at the Branch and Canopy Scale. Can. J. For. Res. 2006, 36, 819–832. [Google Scholar] [CrossRef]
- Ah-Peng, C.; Cardoso, A.W.; Flores, O.; West, A.; Wilding, N.; Strasberg, D.; Hedderson, T.A.J. The Role of Epiphytic Bryophytes in Interception, Storage, and the Regulated Release of Atmospheric Moisture in a Tropical Montane Cloud Forest. J. Hydrol. 2017, 548, 665–673. [Google Scholar] [CrossRef]
- Mullan, D.; Pietragalla, J. Leaf Relative Water Content; Pask, A., Pietragalla, J., Mullan, D., Reynolds, M., Eds.; International Maize and Wheat Improvement Center: El Batán, Mexico, 2012. [Google Scholar]
- Vanderpoorten, A.; Goffinet, B. Introduction to Bryophytes; Cambridge University Press: Cambridge, UK, 2009; ISBN 9780521877121. [Google Scholar]
- Oishi, Y. Evaluation of the Water-Storage Capacity of Bryophytes along an Altitudinal Gradient from Temperate Forests to the Alpine Zone. Forests 2018, 9, 433. [Google Scholar] [CrossRef]
- Song, L.; Zhang, Y.J.; Chen, X.; Li, S.; Lu, H.Z.; Wu, C.S.; Tan, Z.H.; Liu, W.Y.; Shi, X.M. Water Relations and Gas Exchange of Fan Bryophytes and Their Adaptations to Microhabitats in an Asian Subtropical Montane Cloud Forest. J. Plant Res. 2015, 128, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Henriques, D.S.G.; Borges, P.A.V.; Ah-Peng, C.; Gabriel, R. Mosses and Liverworts Show Contrasting Elevational Distribution Patterns in an Oceanic Island (Terceira, Azores): The Influence of Climate and Space. J. Bryol. 2016, 38, 183–194. [Google Scholar] [CrossRef]
- Coelho, M.C.M.; Gabriel, R.; Ah-Peng, C. Characterizing and Quantifying Water Content in 14 Species of Bryophytes Present in Azorean Native Vegetation. Diversity 2023, 15, 295. [Google Scholar] [CrossRef]
- Brown, D.H.; Bates, J.W. Bryophytes and Nutrient Cycling. Bot. J. Linn. Soc. 1990, 104, 129–147. [Google Scholar] [CrossRef]
- Gall, C.; Nebel, M.; Quandt, D.; Scholten, T.; Seitz, S. Pioneer Biocrust Communities Prevent Soil Erosion in Temperate Forests after Disturbances. Biogeosciences 2022, 19, 3225–3245. [Google Scholar] [CrossRef]
- Gabriel, R.; Borges, P.A.V. (Eds.) Field Guide of the Azorean Native Flora; Instituto Açoriano de Cultura: Angra do Heroísmo, Portugal, 2022; ISBN 978-989-8225-74-0. [Google Scholar]
- Gabriel, R.; Homem, N.; Couto, A.B.; Aranda, S.C.; Borges, P.A.V. Azorean Bryophytes: A Preliminary Review of Rarity Patterns. Açoreana 2011, 144, 149–206. [Google Scholar]
- Veneklaas, E.J.; Zagt, R.J.; Leerdam, A.; Ek, R.; Broekhoven, A.J.; Genderen, M. Hydrological Properties of the Epiphyte Mass of a Montane Tropical Rain Forest, Colombia. Vegetatio 1990, 89, 183–192. [Google Scholar] [CrossRef]
- Elumeeva, T.G.; Soudzilovskaia, N.A.; During, H.J.; Cornelissen, J.H.C. The Importance of Colony Structure versus Shoot Morphology for the Water Balance of 22 Subarctic Bryophyte Species. J. Veg. Sci. 2011, 22, 152–164. [Google Scholar] [CrossRef]
- Patidar, K.C.; Kaul, A. Response in Growth to Soil Moisture: A Laboratory and Field Investigation of Riccia Discolor L. J. Bryol. 1984, 13, 269–318. [Google Scholar] [CrossRef]
- Frahm, J.P.; Pócs, T. Phytomass and Water Storage Capacity. Trop. Bryol. 2003, 23, 53–56. [Google Scholar]
- Köhler, L.; Hölscher, D.; Bruijnzeel, L.A.; Leuschner, C. Epiphyte Biomass in Costa Rican Oldgrowth and Secondary Montane Rain Forests and Its Hydrologic Significance. In Tropical Montane Cloud Forests: Science for Conversation and Mangement; Bruijnzeel, L.A., Scatena, F.N., Hamilton, L.S., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 268–274. [Google Scholar]
- Elias, R.B.; Gil, A.; Silva, L.; Fernández-Palacios, J.M.; Azevedo, E.B.; Reis, F. Natural Zonal Vegetation of the Azores Islands: Characterization and Potential Distribution. Phytocoenologia 2016, 46, 107–123. [Google Scholar] [CrossRef]
- Schonbeck, M.W.; Bewley, J.D. Responses of the Moss Tortula Ruralis to Desiccation Treatments. I. Effects of Minimum Water Content and Rates of Dehydration and Rehydration. Canad. J. Bot. 1981, 59, 2698–2706. [Google Scholar] [CrossRef]
- Proctor, M.C.F. The Bryophyte Paradox: Tolerance of Desiccation, Evasion of Drought. Plant Ecol. 2000, 151, 41–49. [Google Scholar] [CrossRef]
- Stark, L.R.; Greenwood, J.L.; Slate, M.L.; Brinda, J.C. Syntrichia Norvegica Shoots Exhibit a Complex Inducible Response to Desiccation: Separating the Effects of Rate of Drying and Water Content. Botany 2016, 95, 481–491. [Google Scholar] [CrossRef]
- Proctor, M.C.F. Structure and Ecological Adaptation. In The Experimental Biology of Bryophytes; Dyer, A.F., Duckett, J.G., Eds.; Academic Press: London, UK, 1984; pp. 9–38. [Google Scholar]
- Bates, J.W. Comparative Growth Patterns of the Thalloid Liverworts Pallavicinia Lyellii and Pellia Epiphylla at Silwood Park, Southern England. J. Bryol. 1993, 17, 439–445. [Google Scholar] [CrossRef]
- Rice, S.K.; Collins, D.; Anderson, A.M. Functional Significance of Variation in Bryophyte Canopy Structure. Am. J. Bot. 2001, 88, 1568–1576. [Google Scholar] [CrossRef]
- Glime, J.M. (Ed.) Water Relations: Reydration and Repair. In Bryophyte Ecology; Physiological Ecology; Michigan Technological University and the International Association of Bryologists: Houghton, MI, USA, 2007; Volume 1. [Google Scholar]
- Hölscher, D.; Köhler, L.; van Dijk, A.I.J.M.; Bruijnzeel, L.A. (Sampurno) The Importance of Epiphytes to Total Rainfall Interception by a Tropical Montane Rain Forest in Costa Rica. J. Hydrol. 2004, 292, 308–322. [Google Scholar] [CrossRef]
- Gabriel, R. Ecophysiology of Azorean Forest Bryophytes. Ph.D. Thesis, University of London, London, UK, 2000. [Google Scholar]
- Löbs, N.; Walter, D.; Barbosa, C.G.G.; Brill, S.; Cerqueira, G.R.; de Oliveira Sá, M.; de Araújo, A.C.; de Oliveira, L.R.; Ditas, F.; Moran-Zuloaga, D.; et al. Microclimatic and Ecophysiological Conditions Experienced by Epiphytic Bryophytes in an Amazonian Rain Forest. Biogeosci. Discuss. 2019, 2019, 5399–5416. [Google Scholar] [CrossRef]
- Löbs, N.; Walter, D.; Barbosa, C.G.G.; Brill, S.; Alves, R.P.; Cerqueira, G.R.; De Oliveira Sá, M.; De Araújo, A.C.; De Oliveira, L.R.; Ditas, F.; et al. Microclimatic Conditions and Water Content Fluctuations Experienced by Epiphytic Bryophytes in an Amazonian Rain Forest. Biogeosciences 2020, 17, 5399–5416. [Google Scholar] [CrossRef]
- Glime, J.M. Bryophyte Ecology; Michigan Technological University and the International Association of Bryologists: Houghton, MI, USA, 2021. [Google Scholar]
- Schipperges, B.; Rydin, H. Response of Photosynthesis of Sphagnum Species from Contrasting Microhabitats to Tissue Water Content and Repeated Desiccation. New Phytol. 1998, 140, 677–684. [Google Scholar] [CrossRef]
- Jauhiainen, J.; Silvola, J.; Vasander, H. Effects of Increased Carbon Dioxide and Nitrogen Supply on Mosses. In Bryology for the Twenty-First Century; Bates, J.W., Ashton, N.W., Duckett, J.G., Eds.; British Bryological Society: London, UK, 1998; pp. 343–360. [Google Scholar]
- Proctor, M.C.F. Trait Correlations in Bryophytes: Exploring an Alternative World. New Phytol. 2010, 185, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Henriques, D. Bryophyte Taxonomic and Functional Diversity in Terceira Island (Azores): Patterns, Drivers and Future Challenges. Ph.D. Thesis, Universidade dos Açores, Angra do Heroísmo, Portugal, 2017. [Google Scholar]
- Rydin, H.; Clymo, R.S. Transport of Carbon and Phosphorus Compounds about Sphagnum. Proc. R. Soc. Lond. B Biol. Sci. 1989, 237, 63–84. [Google Scholar]
- Thomas, R.J.; Ryder, S.H.; Gardner, M.I.; Sheetz, J.P.; Nichipor, S.D. Photosynthetic Function of Leaf Lamellae in Polytrichum Commune. Bryologist 1996, 99, 6–11. [Google Scholar] [CrossRef]
- Azevedo, E.B.; Pereira, L.S.; Itier, B. Modelling the Local Climate in Island Environments: Water Balance Applications. Agric. Water Manag. 1999, 40, 393–403. [Google Scholar] [CrossRef]
- Patiño, J.; Mateo, R.G.; Zanatta, F.; Marquet, A.; Aranda, S.C.; Borges, P.A.V.; Dirkse, G.; Gabriel, R.; Gonzalez-Mancebo, J.M.; Guisan, A.; et al. Climate Threat on the Macaronesian Endemic Bryophyte Flora. Sci. Rep. 2016, 6, 29156. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.T.; Cardoso, P.; Borges, P.A.V.; Gabriel, R.; de Azevedo, E.B.; Reis, F.; Araujo, M.B.; Elias, R.B. Effects of Climate Change on the Distribution of Indigenous Species in Oceanic Islands (Azores). Clim. Change 2016, 138, 603–615. [Google Scholar] [CrossRef]
- Hodgetts, N.; Calix, M.; Englefield, E.; Fettes, N.; Garcia Criado, M.; Patin, L.; Nieto, A.; Bergamini, A.; Bisang, I.; Baisheva, E.; et al. A Miniature World in Decline: European Red List of Mosses, Liverworts and Hornworts; IUCN: Brussels, Belgium, 2019; ISBN 9782831719931/9782831719948. [Google Scholar]
- Azevedo, J.M.M. Geologia e Hidrogeologia da Ilha das Flores. Ph.D. Thesis, Universidade de Coimbra, Coimbra, Portugal, 1998. [Google Scholar]
- Anderson, M.; Lambrinos, J.; Schroll, E. The Potential Value of Mosses for Stormwater Management in Urban Environments. Urban Ecosyst. 2010, 13, 319–332. [Google Scholar] [CrossRef]
- Monteith, J.L.; Unsworth, M.H. Principles of Environmental Physics, 2nd ed.; Arnold, E., Ed.; Academic Press: London, UK, 1990; ISBN 9780123869104. [Google Scholar]
- Forjaz, V.H. Atlas Básico dos Açores; OVGA: Ponta Delgada, Portugal, 2004. [Google Scholar]
- PBA. (2022, Janeiro, 22). Portal da Biodiversidade dos Açores. Available online: https://azoresbioportal.uac.pt/ (accessed on 1 August 2023).
- Magdefrau, K. Life Forms of Bryophytes. In Bryophyte Ecology; Smith, A.J.E., Ed.; Chapman and Hall: London, UK, 1982; pp. 45–58. [Google Scholar] [CrossRef]
- Paton, J.A. The Liverwort Fora of the British Isles; Paton, J.A., Ed.; Harley Books: Colchester, UK, 1999; p. 626. [Google Scholar]
- Smith, A.J.E. The Moss Flora of Britain and Ireland, 2nd ed.; Smith, A.J.E., Ed.; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Schumacker, R.; Váňa, J. Identification Keys to the Liverworts and Hornworts of Europe and Macaronesia (Distribution and Status), 2nd ed.; Sorus: Poznan, Poland, 2005. [Google Scholar]
- Casas, C.; Brugués, M.; Cros, R.M.; Sérgio, C. Handbook of Mosses of the Iberian Peninsula and the Balearic Islands; Institut d’Estudis Catalans: Barcelona, Spain, 2006; ISBN 847283865X. [Google Scholar]
- Casas, C.; Brugués, M.; Cros, R.M.; Sérgio, C.; Infante, M. Handbook of Liverworts of the Iberian Peninsula and the Balearic Islands; Institut d’Estudis Catalans: Barcelona, Spain, 2009; ISBN 978-84-92583-55-3. [Google Scholar]
- Atherton, I.; Bosanquet, S.; Lawley, M. Mosses and Liverworts of Britain and Ireland a Field Guide; British Bryological Society: Plymouth, UK, 2010; ISBN 9780956131010. [Google Scholar]
- Ros, R.M.; Mazimpaka, V.; Abou-Salama, U.; Aleffi, M.; Blockeel, T.L.; Brugués, M.; Cros, R.M.; Dia, M.G.; Dirkse, G.M.; Draper, I.; et al. Mosses of the Mediterranean, an Annotated Checklist. Cryptogam. Bryol. 2013, 34, 99–283. [Google Scholar] [CrossRef]
- Hodgetts, N.G.; Söderström, L.; Blockeel, T.L.; Caspari, S.; Ignatov, M.S.; Konstantinova, N.A.; Lockhart, N.; Papp, B.; Schröck, C.; Sim-Sim, M.; et al. An Annotated Checklist of Bryophytes of Europe, Macaronesia and Cyprus. J. Bryol. 2020, 42, 1–116. [Google Scholar] [CrossRef]
- Watkins, J.E., Jr.; Mack, M.; Sinclair, T.; Mulkey, S. Ecological and Evolutionary Consequences of Desiccation Tolerance in Fern Gametophytes. New Phytol. 2007, 176, 708–717. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics Corner: A Guide to Appropriate Use of Correlation Coefficient in Medical Research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar] [PubMed]


| Plant Species | Type III | Omnibus Test a | |||||
|---|---|---|---|---|---|---|---|
| Indicator Variable | Wald Chi-Square | df | Sig. | Likelihood Ratio Chi-Square | df | Sig. | |
| Frullania acicularis | (Intercept) | 4.41 | 1 | 0.036 | 30.44 | 3 | <0.000 |
| Precipitation | 6.13 | 1 | 0.013 | ||||
| Temperature | 0.03 | 1 | 0.871 | ||||
| Relative Humidity | 4.91 | 1 | 0.027 | ||||
| Campylopus brevipilus | (Intercept) | 2.08 | 1 | 0.149 | 32.98 | 3 | <0.000 |
| Precipitation | 10.09 | 1 | 0.001 | ||||
| Temperature | 0.15 | 1 | 0.702 | ||||
| Relative Humidity | 2.32 | 1 | 0.128 | ||||
| Trichostomum brachydontium | (Intercept) | 3.21 | 1 | 0.073 | 37.29 | 3 | <0.000 |
| Precipitation | 12.40 | 1 | <0.000 | ||||
| Temperature | 0.05 | 1 | 0.828 | ||||
| Relative Humidity | 3.45 | 1 | 0.063 | ||||
| Plant Species | Type III | Omnibus Test a | |||||
|---|---|---|---|---|---|---|---|
| Indicator Variable | Wald Chi-Square | df | Sig. | Likelihood Ratio Chi-Square | df | Sig. | |
| Plagiochila bifaria | (Intercept) | 17.47 | 1 | 0.000 | 33.26 | 3 | <0.000 |
| Precipitation | 16.07 | 1 | 0.000 | ||||
| Temperature | 2.98 | 1 | 0.084 | ||||
| Relative Humidity | 18.46 | 1 | 0.000 | ||||
| Scapania gracilis | (Intercept) | 3.93 | 1 | 0.047 | 23.45 | 3 | <0.000 |
| Precipitation | 1.11 | 1 | 0.293 | ||||
| Temperature | 0.02 | 1 | 0.876 | ||||
| Relative Humidity | 4.32 | 1 | 0.038 | ||||
| Frullania acicularis | (Intercept) | 5.04 | 1 | 0.025 | 18.50 | 3 | <0.000 |
| Precipitation | 8.62 | 1 | 0.003 | ||||
| Temperature | 0.37 | 1 | 0.543 | ||||
| Relative Humidity | 5.36 | 1 | 0.021 | ||||
| Sphagnum subnitens | (Intercept) | 3.54 | 1 | 0.060 | 56.35 | 3 | <0.000 |
| Precipitation | 0.26 | 1 | 0.609 | ||||
| Temperature | 1.45 | 1 | 0.229 | ||||
| Relative Humidity | 5.03 | 1 | 0.025 | ||||
| Isothecium prolixum | (Intercept) | 11.27 | 1 | 0.001 | 32.27 | 3 | <0.000 |
| Precipitation | 12.44 | 1 | 0.000 | ||||
| Temperature | 0.93 | 1 | 0.335 | ||||
| Relative Humidity | 11.93 | 1 | 0.001 | ||||
| Myurium hochstetteri | (Intercept) | 7.92 | 1 | 0.005 | 41.50 | 3 | <0.000 |
| Precipitation | 5.13 | 1 | 0.023 | ||||
| Temperature | 0.02 | 1 | 0.901 | ||||
| Relative Humidity | 8.50 | 1 | 0.004 | ||||
| Thuidium tamariscinum | (Intercept) | 3.68 | 1 | 0.055 | 34.22 | 3 | <0.000 |
| Precipitation | 0.51 | 1 | 0.476 | ||||
| Temperature | 0.08 | 1 | 0.777 | ||||
| Relative Humidity | 3.96 | 1 | 0.047 | ||||
| Plant Species | Type III | Omnibus Test a | |||||
|---|---|---|---|---|---|---|---|
| Indicator Variable | Wald Chi-Square | df | Sig. | Likelihood Ratio Chi-Square | df | Sig. | |
| Bazzania azorica | (Intercept) | 1.28 | 1 | 0.258 | 58.89 | 3 | <0.000 |
| Precipitation | 4.38 | 1 | 0.036 | ||||
| Temperature | 18.29 | 1 | 0.000 | ||||
| Relative Humidity | 1.26 | 1 | 0.261 | ||||
| Herbertus azoricus | (Intercept) | 0.00 | 1 | 0.987 | 17.73 | 3 | 0.001 |
| Precipitation | 0.28 | 1 | 0.593 | ||||
| Temperature | 10.14 | 1 | 0.001 | ||||
| Relative Humidity | 0.00 | 1 | 0.990 | ||||
| Lepidozia cupressina | (Intercept) | 1.61 | 1 | 0.205 | 32.54 | 3 | <0.000 |
| Precipitation | 11.30 | 1 | 0.001 | ||||
| Temperature | 3.25 | 1 | 0.071 | ||||
| Relative Humidity | 1.62 | 1 | 0.203 | ||||
| Plagiochila bifaria | (Intercept) | 3.17 | 1 | 0.075 | 6.42 | 3 | 0.093 |
| Precipitation | 0.83 | 1 | 0.362 | ||||
| Temperature | 4.77 | 1 | 0.029 | ||||
| Relative Humidity | 3.18 | 1 | 0.074 | ||||
| Scapania gracilis | (Intercept) | 2.73 | 1 | 0.098 | 14.03 | 3 | 0.003 |
| Precipitation | 2.52 | 1 | 0.113 | ||||
| Temperature | 8.09 | 1 | 0.004 | ||||
| Relative Humidity | 2.75 | 1 | 0.097 | ||||
| Frullania acicularis | (Intercept) | 4.24 | 1 | 0.039 | 20.63 | 3 | <0.000 |
| Precipitation | 0.13 | 1 | 0.715 | ||||
| Temperature | 21.61 | 1 | 0.000 | ||||
| Relative Humidity | 4.27 | 1 | 0.039 | ||||
| Sphagnum subnitens | (Intercept) | 0.13 | 1 | 0.719 | 28.44 | 3 | <0.000 |
| Precipitation | 1.81 | 1 | 0.179 | ||||
| Temperature | 12.68 | 1 | 0.000 | ||||
| Relative Humidity | 0.14 | 1 | 0.708 | ||||
| Polytrichum commune | (Intercept) | 0.43 | 1 | 0.510 | 2.70 | 3 | 0.441 |
| Precipitation | 0.00 | 1 | 0.978 | ||||
| Temperature | 2.31 | 1 | 0.128 | ||||
| Relative Humidity | 0.44 | 1 | 0.505 | ||||
| Campylopus shawii | (Intercept) | 2.80 | 1 | 0.094 | 29.37 | 3 | <0.000 |
| Precipitation | 11.15 | 1 | 0.001 | ||||
| Temperature | 4.06 | 1 | 0.044 | ||||
| Relative Humidity | 2.82 | 1 | 0.093 | ||||
| Plant Species (Sites) | Type III | Omnibus Test a | |||||
|---|---|---|---|---|---|---|---|
| Indicator Variable | Wald Chi-Square | df | Sig. | Likelihood Ratio Chi-Square | df | Sig. | |
| Frullania acicularis (FS + PL + SB) | (Intercept) | 0.560 | 1 | 0.454 | 108.886 | 3 | <0.000 |
| Precipitation | 7.747 | 1 | 0.005 | ||||
| Temperature | 49.484 | 1 | 0.000 | ||||
| Relative Humidity | 9.467 | 1 | 0.002 | ||||
| Plagiochila bifaria (PL + SB) | (Intercept) | 1.242 | 1 | 0.265 | 25.899 | 3 | <0.000 |
| Precipitation | 4.806 | 1 | 0.028 | ||||
| Temperature | 13.758 | 1 | 0.000 | ||||
| Relative Humidity | 2.718 | 1 | 0.099 | ||||
| Scapania gracilis (PL + SB) | (Intercept) | 9.650 | 1 | 0.002 | 50.903 | 3 | <0.000 |
| Precipitation | 0.086 | 1 | 0.770 | ||||
| Temperature | 11.664 | 1 | 0.001 | ||||
| Relative Humidity | 13.632 | 1 | 0.000 | ||||
| Sphagnum subnitens (PL + SB) | (Intercept) | 1.741 | 1 | 0.187 | 71.776 | 3 | <0.000 |
| Precipitation | 4.406 | 1 | 0.036 | ||||
| Temperature | 36.920 | 1 | 0.000 | ||||
| Relative Humidity | 0.181 | 1 | 0.670 | ||||
| Farol da Serreta (FS) | Pico da Lagoínha (PL) | Serra Santa Bárbara (SSB) | ||
|---|---|---|---|---|
| Geographic Variables | ||||
| Coordinates (N)/(W) | 38°45′58.9″/27°22′32.4″ | 38°45′09.4″/27°19′53.7″ | 38°43′50.0″/27°19′18.7″ | |
| Altitude (a.s.l.) | 40 m | 683 m | 1012 m | |
| Exposure | 270° W | 150° S | 20° N | |
| Slope (°) | 15.5 | 13.2 | 5.1 | |
| Vascular Vegetation | ||||
| Type | Erica-Morella coastal woodland | Laurus submontane forest | Juniperus montane woodland | |
| Maximum height (cm) | 300 | 800 | 160 | |
| Dominant phorophyte | Erica azorica | Laurus azorica | Juniperus brevifolia | |
| Cover (%) | 75 | 40 | 85 | |
| Bryophytes | ||||
| Richness (S) | 22 | 56 | 50 | |
| Cover (%) | soil | 10 | 95 | 90 |
| rocks | 20 | na | na | |
| epiphytes | 5 | 70 | 90 | |
| Dominant species | Frullania acicularis | Myurium hochstetteri | Scapania gracilis | |
| Sites | Division | Class | Species | Abb. | LF | Substrate |
|---|---|---|---|---|---|---|
| FS | Marchantiophyta | Jungermanniopsida | Frullania acicularis Hentschel and von Konrat | Fa | mat | rock |
| Bryophyta | Bryopsida | Campylopus brevipilus Bruch et Schimp. | Cb | cushion | rock | |
| Trichostomum brachydontium Bruch | Tb | turf | rock | |||
| PL | Marchantiophyta | Jungermanniopsida | Plagiochila bifaria (Sw.) Lindenb. | Pb | turf | tree |
| Scapania gracilis Lindb. | Sg | weft | tree | |||
| Frullania acicularis Hentschel and von Konrat | Fa | mat | tree | |||
| Bryophyta | Sphagnopsida | Sphagnum subnitens Russow et Warnst. | Ss | tall turf-db | humus | |
| Bryopsida | Isothecium prolixum (Mitt.) Stech, Sim-Sim, Tangney et D.Quandt | Ip | weft | tree | ||
| Myurium hochstetteri (Schimp.) Kindb. | Mh | mat | tree | |||
| Thuidium tamariscinum (Hedw.) Schimp. | Tt | weft | soil | |||
| SB | Marchantiophyta | Jungermanniopsida | Bazzania azorica | Ba | weft | tree |
| Herbertus azoricus (Steph.) P.W.Richards | Ha | turf | tree | |||
| Lepidozia cupressina (Sw.) Lindenb. | Lc | weft | tree | |||
| Plagiochila bifaria (Sw.) Lindenb. | Pb | turf | tree | |||
| Scapania gracilis Lindb. | Sg | weft | tree | |||
| Frullania acicularis Hentschel and von Konrat | Fa | mat | tree | |||
| Bryophyta | Sphagnopsida | Sphagnum subnitens Russow et Warnst. | Ss | tall turf-db | humus | |
| Polytrichopsida | Polytrichum commune Hedw. | Pc | tall turf | humus | ||
| Bryopsida | Campylopus shawii Wilson | Cs | tall turf | soil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, M.C.M.; Gabriel, R.; Ah-Peng, C. Seasonal Hydration Status of Common Bryophyte Species in Azorean Native Vegetation. Plants 2023, 12, 2931. https://doi.org/10.3390/plants12162931
Coelho MCM, Gabriel R, Ah-Peng C. Seasonal Hydration Status of Common Bryophyte Species in Azorean Native Vegetation. Plants. 2023; 12(16):2931. https://doi.org/10.3390/plants12162931
Chicago/Turabian StyleCoelho, Márcia C. M., Rosalina Gabriel, and Claudine Ah-Peng. 2023. "Seasonal Hydration Status of Common Bryophyte Species in Azorean Native Vegetation" Plants 12, no. 16: 2931. https://doi.org/10.3390/plants12162931
APA StyleCoelho, M. C. M., Gabriel, R., & Ah-Peng, C. (2023). Seasonal Hydration Status of Common Bryophyte Species in Azorean Native Vegetation. Plants, 12(16), 2931. https://doi.org/10.3390/plants12162931






