Shade Increased Seed Yield and Quality of Incarvillea sinensis var. przewalskii
Abstract
:1. Introduction
2. Results
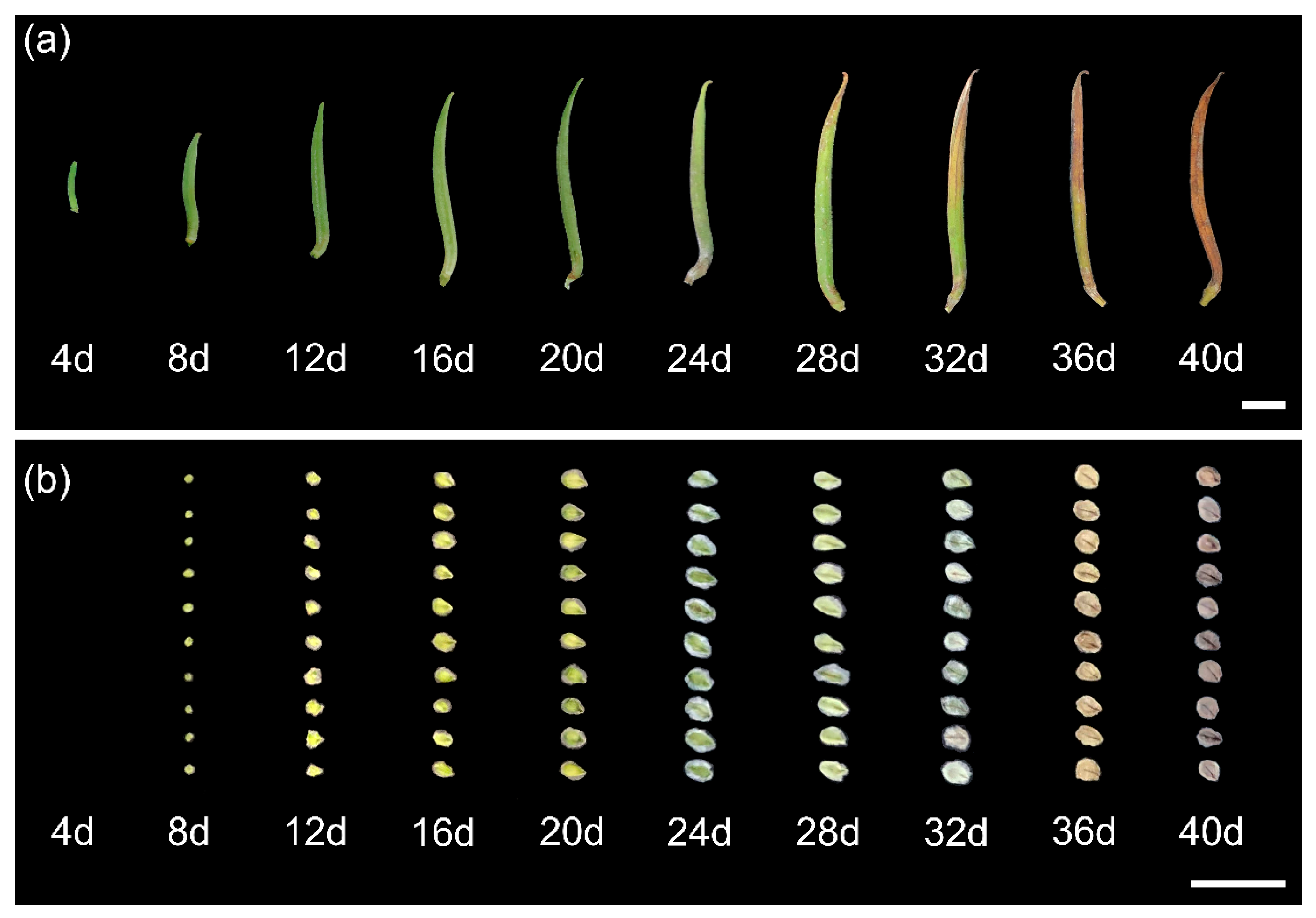
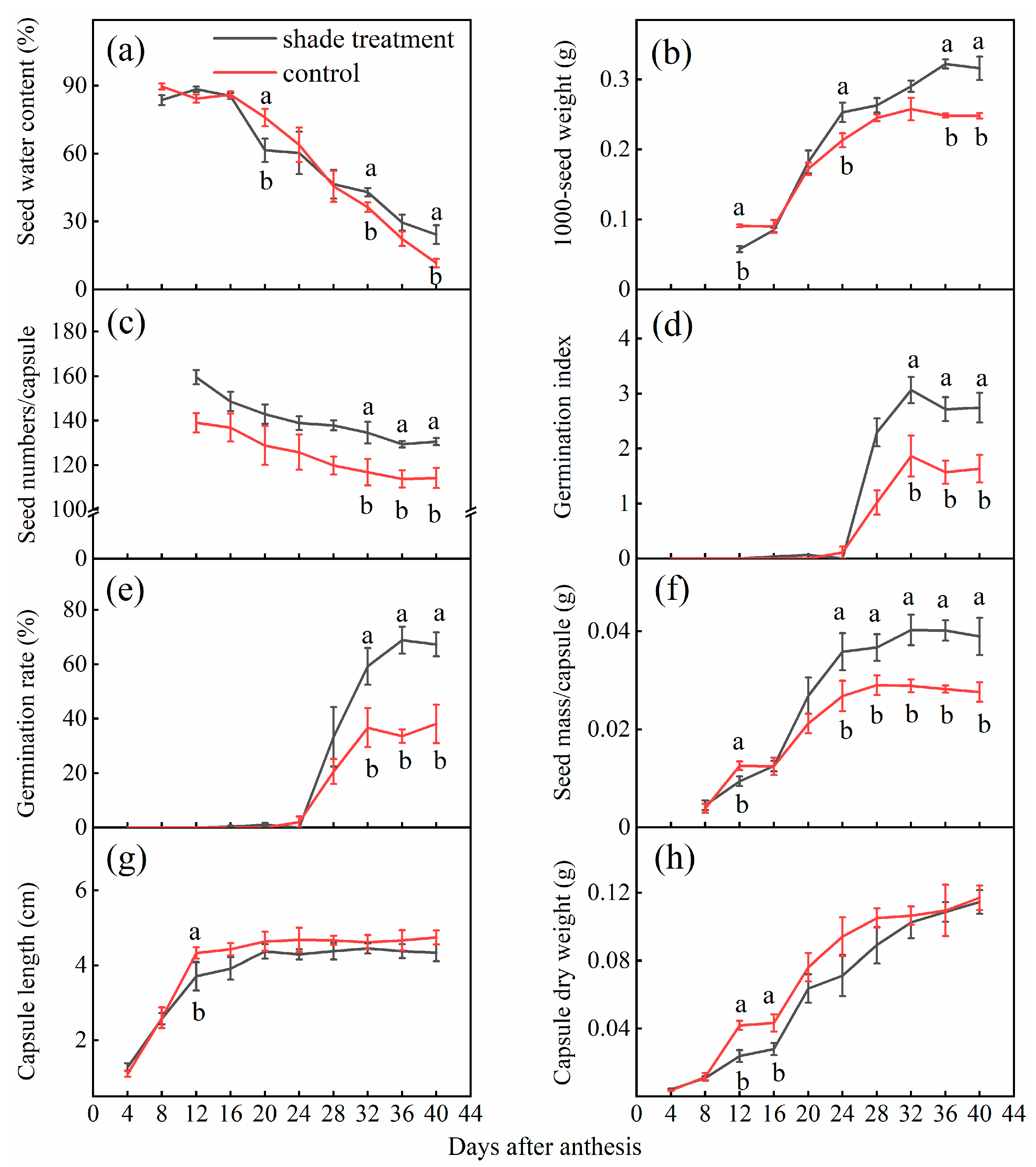
2.1. Effects of Shade on Seed Development
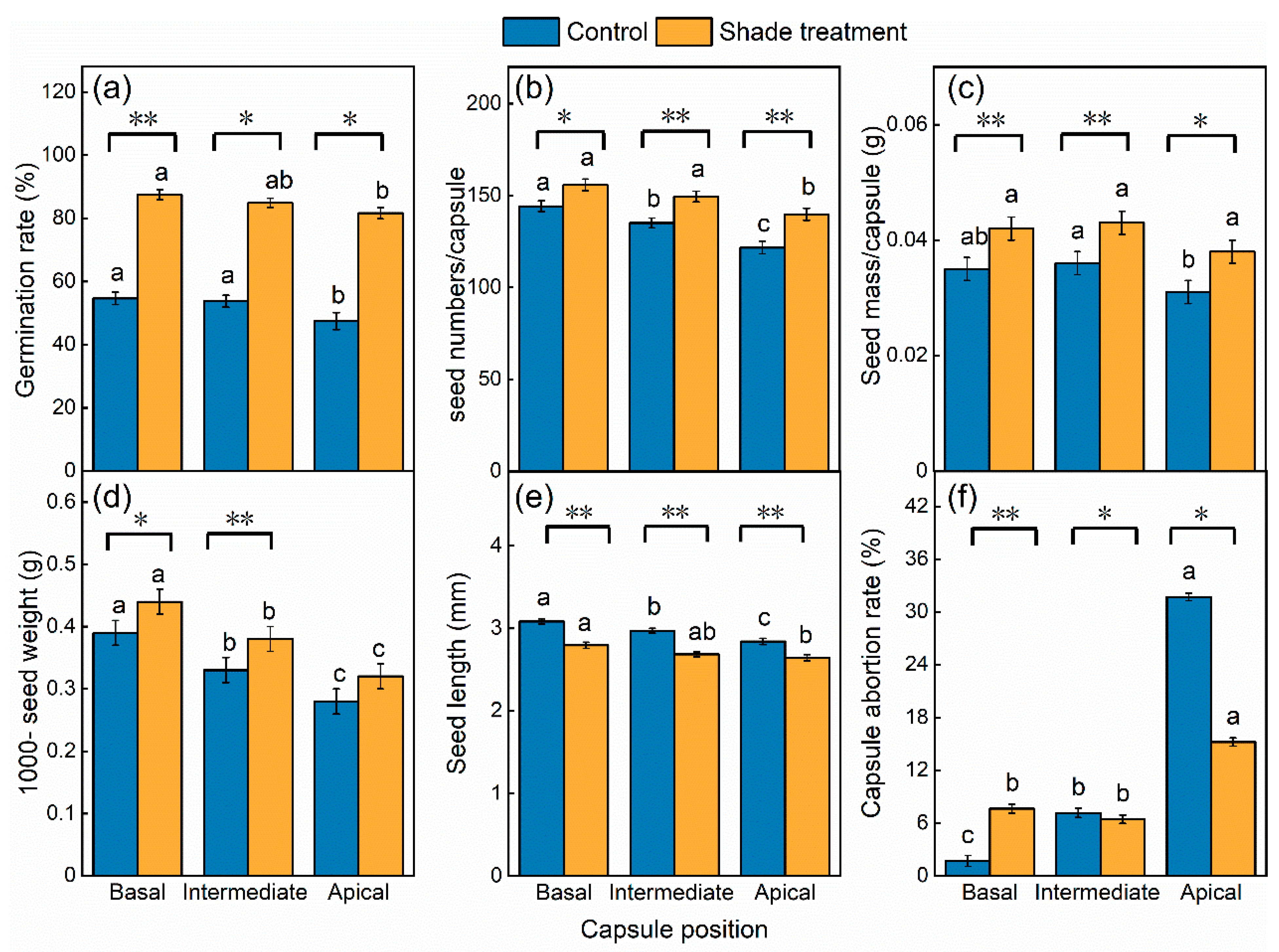
2.2. Effects of Shade and Capsule Position on Seed Quality
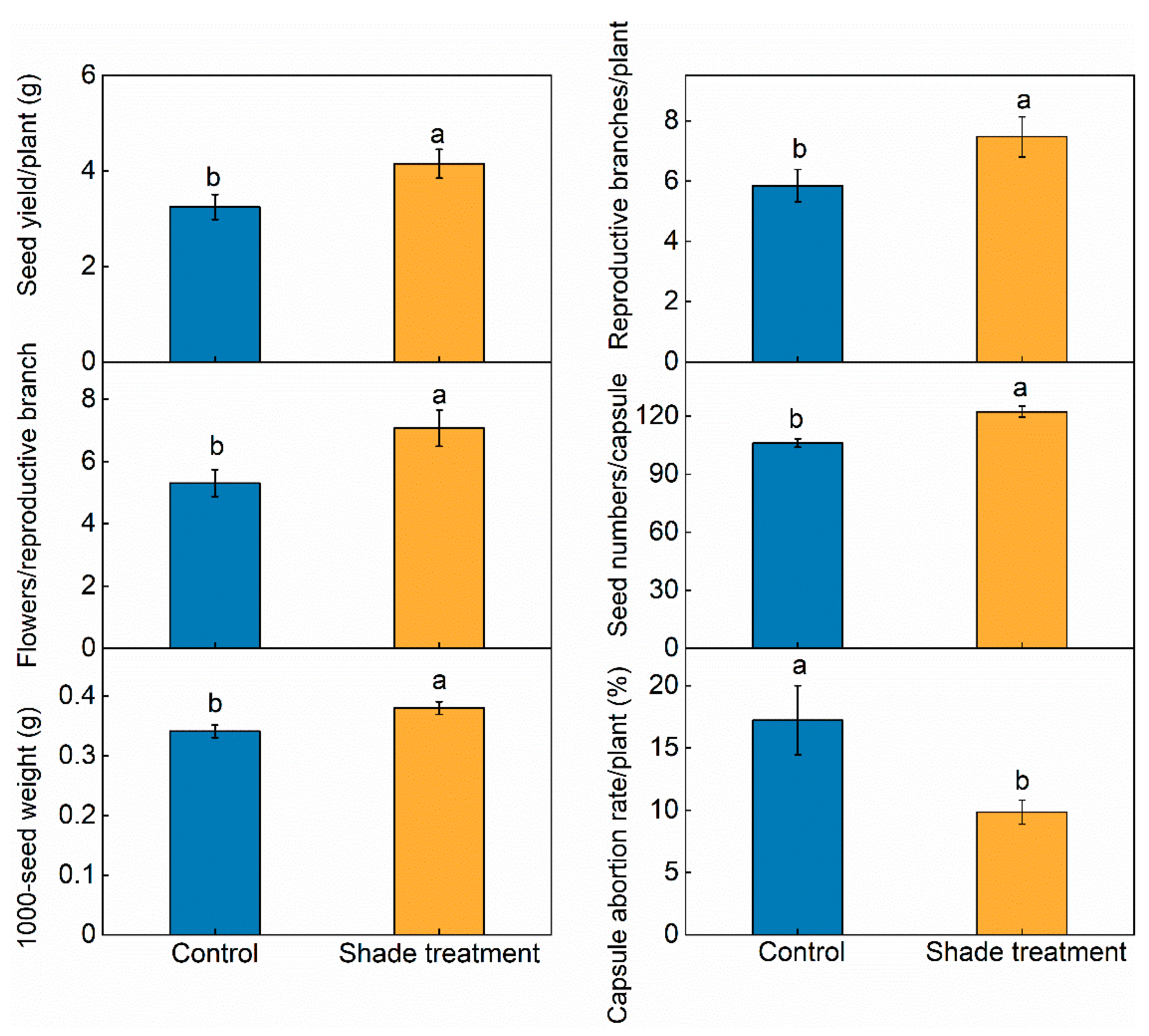
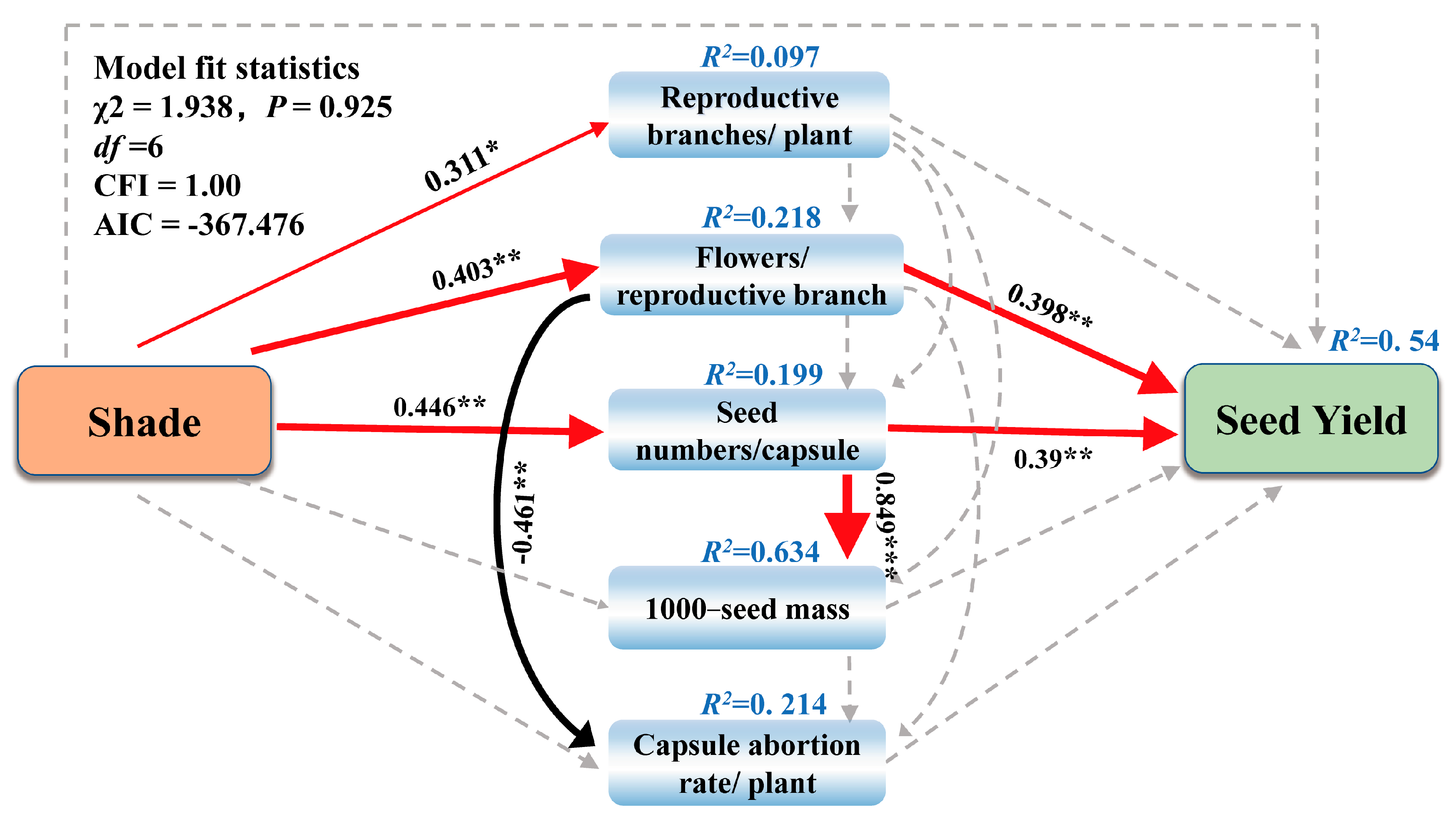
2.3. Effects of Shade on Seed Yield and Yield Components
3. Discussion
4. Materials and Methods
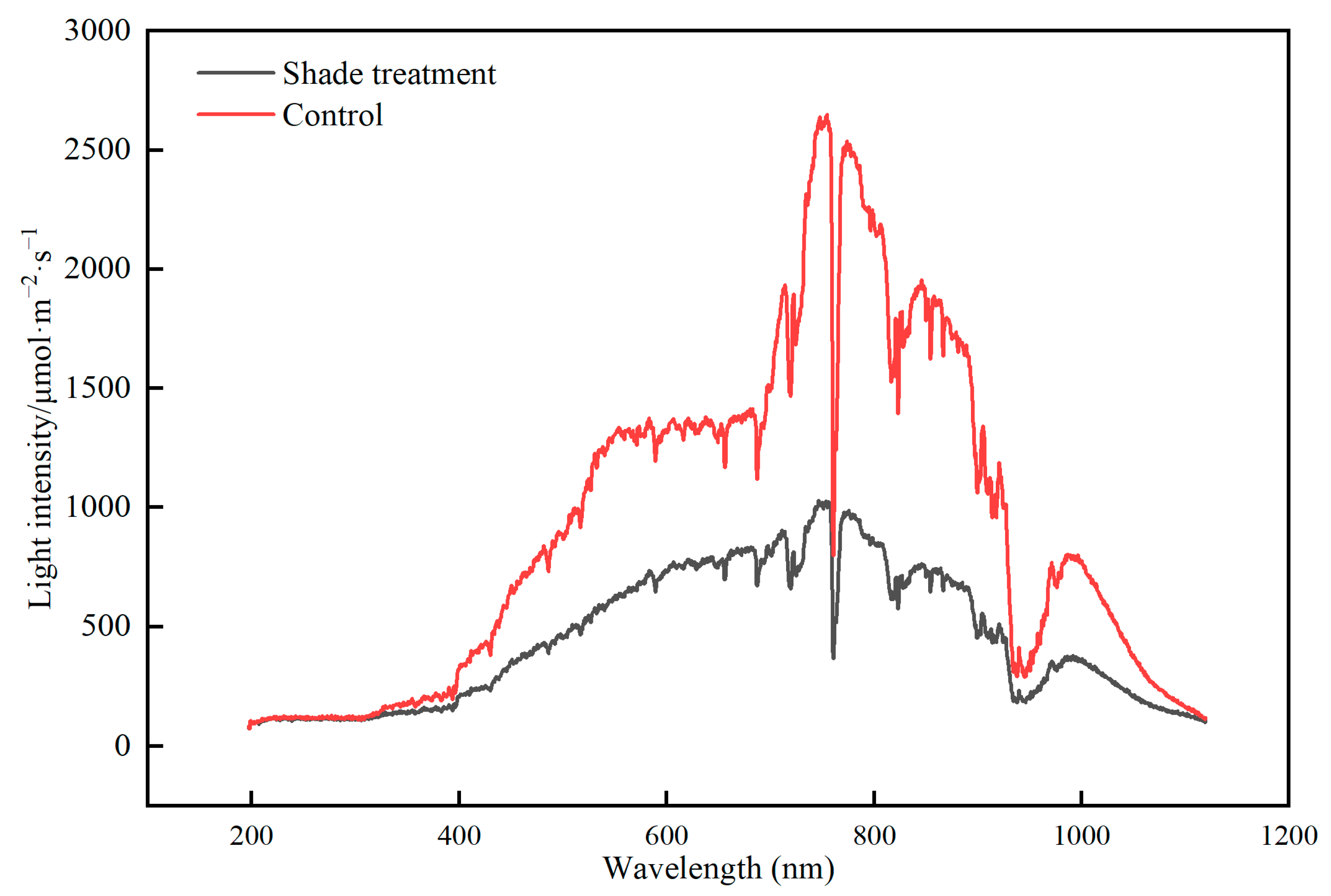
4.1. Plant Material, Growing Conditions, and Shade Treatments
4.2. Experimental Design
4.2.1. Effects of Shade on Seed Development
4.2.2. Effects of Shade and Capsule Position on Seed Quality
4.2.3. Effects of Shade on Seed Yield and Yield Components
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, S.T.; Xing, Y.W.; Su, T.; Zhou, Z.K.; Dilcher, E.D.L.; Soltis, D.E. Phylogeographic analysis reveals significant spatial genetic structure of Incarvillea sinensisas a product of mountain building. BMC Plant Biol. 2012, 12, 58. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.T.; Zhou, Z.K.; Guan, K.Y.; Nakata, M. Karyomorphology of Incarvillea (Bignoniaceae) and its implications in distribution and taxonomy. Bot. J. Linn. Soc. 2004, 144, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.P.; Zhong, G.Y.; Shen, Y.H. Chemical constituents from Incarvillea sinensis var. przewalskii. Chin. Tradit. Herbal Drugs 2016, 47, 712–716. [Google Scholar]
- Li, S.; Chen, H.; Li, C.Y.; Wang, M.W.; Wei, X.M.; Chang, Y.C. Orthogonal optimization of extraction process of total alkaloid in Incarvillea sinensis Lam. var. przewalskii. J. Gansu Univ. Chin. Med. 2012, 29, 34–37. [Google Scholar]
- Han, C.J.; Wang, Q.; Zhang, H.B.; Wang, S.H.; Song, H.D.; Hao, J.M.; Dong, H.Z. Light shading improves the yield and quality of seed in oil-seed peony (Paeonia ostii Feng Dan). J. Integr. Agr. 2018, 17, 1631–1640. [Google Scholar] [CrossRef]
- Cavagnaro, J.B.; Trione, S.O. Physiological, morphological and biochemical responses to shade of Trichloris crinita, a forage grass from the arid zone of Argentina. J. Arid. Environ. 2007, 68, 337–347. [Google Scholar] [CrossRef]
- Zhao, D.Q.; Hao, Z.J.; Tao, J. Effects of shade on plant growth and flower quality in the herbaceous peony (Paeonia lactiflora Pall.). Plant Physiol. Bioch. 2012, 61, 187–196. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Laanisto, L.; Niinemets, Ü. Polytolerance to abiotic stresses: How universal is the shade–drought tolerance trade-off in woody species? Global Ecol. Biogeogr. 2015, 24, 571–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koller, D. Germination-regulating mechanisms in some desert seeds, IV. Atriplex dimorphostegia Kar. et Kir. Ecology 1957, 38, 2–13. [Google Scholar] [CrossRef]
- Kollwr, D.; Roth, N. Studies on the ecological and physiological significance of amphicarpy in Gymnarrhena micrantha (Compositae). Am. J. Bot. 1964, 51, 26–35. [Google Scholar] [CrossRef]
- Shinomura, T.; Nagatani, A.; Hanzawa, H.; Kubota, M.; Watanabe, M.; Furuya, M. Action spectra for phytochrome A-and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1996, 93, 8129–8133. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhou, W.G.; Yin, H.; Luo, X.F.; Chen, W.; Liu, X.; Wang, X.; Meng, Y.; Feng, L.; Qin, Y.; et al. Shading of the mother plant during seed development promotes subsequent seed germination in soybean. J. Exp. Bot. 2020, 71, 2072–2084. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.C.; Evenari, M.; Gutterman, Y. The heteroblasty of Aegilops ovata. Israel J. Bot. 1970, 19, 463–483. [Google Scholar]
- Gray, D.; Thomas, T.H. Seed germination and seedling emergence as influenced by the position of development of the seed on, and chemical applications to, the parent plant. In Physiology and Biochemistry of Seed Development, Dormancy and Germination; Elsevier: New York, NY, USA, 1982; pp. 81–110. [Google Scholar]
- Rylski, I.; Spigelman, M. Effect of shading on plant development, yield and fruit quality of sweet pepper grown under conditions of high temperature and radlation. Sci. Hortic. 1986, 29, 31–35. [Google Scholar] [CrossRef]
- Steiman, S.; Idol, T.; Bittenbender, H.C.; Gautz, L. Shade coffee in Hawai‘i—Exploring some aspects of quality, growth, yield, and nutrition. Sci. Hortic. 2011, 128, 152–158. [Google Scholar] [CrossRef]
- Board, J.E.; Harville, B.G. Growth dynamics during the vegetative period affects yield of narrow-row, late-planted soybean. Agron. J. 1996, 88, 567–572. [Google Scholar] [CrossRef]
- Myers, R.L.; Brun, W.A.; Brenner, M.L. Effect of raceme-localized supplemental light on soybean reproductive abscission. Crop Sci. 1987, 27, 273–277. [Google Scholar] [CrossRef]
- Schou, J.B.; Jeffers, D.L.; Streeter, J.G. Effects of reflectors, black boards, or shades applied at different stages of plant development on yield of soybeans. Crop Sci. 1978, 18, 29–34. [Google Scholar] [CrossRef]
- Mathew, J.P.; Herbert, S.J.; Zhang, S.H.; Rautenkranz, A.A.F.; Litchfield, G.V. Differential response of soybean yield components to the timing of light enrichment. Agron. J. 2000, 92, 1156–1161. [Google Scholar] [CrossRef] [Green Version]
- Han, C.J.; Wang, Q.; Zhang, H.B.; Dong, H.Z. Seed development and nutrient accumulation as affected by light shading in oilseed peony (Paeonia ostii ‘Feng Dan’). Sci. Hortic. 2019, 251, 25–31. [Google Scholar] [CrossRef]
- Marin, M.; Blandino, C.; Laverack, G.; Toorop, P.; Powell, A.A. Responses of Primula vulgaris to light quality in the maternal and germination environments. Plant Biology 2019, 21, 439–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and, Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Gray, D.; Steckel, J.R.A. Parsnip (Pastinaca sativa) seed production: Effects of seed crop plant density, seed position on the mother plant, harvest date and method, and seed grading on embryo and seed size and seedling performance. Ann. Appl. Biol. 1985, 107, 559–570. [Google Scholar] [CrossRef]
- Hendrix, S.D. Variation in seed weight and its effects on germination in Pastinaca sativa L. (Umbelliferae). Am. J. Bot. 1984, 71, 795–802. [Google Scholar] [CrossRef] [Green Version]
- Koller, D. Analysis of the dual action of white light on germination of Atriplex dimorphostegia (Chenopodiaceae). Israel J. Bot. 1970, 19, 499–516. [Google Scholar]
- Keiller, D.R.; Morgan, D.J. Distribution of 14carbon-labelled assimilates in flowering plants of oilseed rape (Brassica napus L.). J. Agr. Sci. 1988, 111, 347–355. [Google Scholar] [CrossRef]
- Evans, E.J. Pre-anthesis growth and its influence on seed yield in winter oilseed rape. Aspects Appl. Biol. 1984, 6, 81–90. [Google Scholar]
- Inanaga, S.; Kumura, A.; Etho, K.; Tsunoda, K.J. Studies on matter production of rape plants: VIII. Effects of shading, leaf cutting and pod thinning treatments on yield and its components. Jpn. J. Crop Sci. 1986, 55, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Tayo, T.O.; Morgan, D.G. Factors influencing flower and pod development in oil-seed rape (Brassica napus L.). J. Agr. Sci. 1979, 92, 363–373. [Google Scholar] [CrossRef]
- Svečnjak, Z.; Varga, B.; Butorac, J. Yield components of apical and subapical ear contributing to the grain yield responses of prolific maize at high and low plant populations. J. Agron. Crop Sci. 2006, 192, 37–42. [Google Scholar] [CrossRef]
- Habekotté, B. Quantitative analysis of pod formation, seed set and seed filling in winter oilseed rape (Brassica napus L.) under field conditions. Field Crop Res. 1993, 35, 21–33. [Google Scholar] [CrossRef]
- Imru, N.O.; Wogderess, M.D.; Gidada, T.V. A study of the effects of shade on growth, production and quality of coffee (Coffea arabica) in Ethiopia. Int. J. Agric. Stat. Sci. 2015, 5, 748–752. [Google Scholar]
- Doust, J.L.; Doust, L.L. Plant Reproductive Ecology: Patterns and Strategies; Oxford University Press on Demand: Oxford, UK, 1988. [Google Scholar]
- Kohri, K.; Saitoh, K.; Kuroda, T.; Kumano, S. Significance of Flower Differentiation and Development in the Process of Determining Soybean Yield: Effects of shading treatment on the number of floral buds and pod sets. Jpn. J. Crop Sci. 1998, 67, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Bello, I.A.; Owen, M.D.K.; Hatterman-Valentp, H.M. Effect of shade on velvetleaf (Abutilon theophrasti) growth, seed production, and dormancy. Weed Technol. 1995, 9, 452–455. [Google Scholar] [CrossRef]
- Katepa-Mupondwa, F.M.; Smith, J.S.R.; Barnes, D.K. Influence of parent and temperature during pollination on alfalfa seed weight and number of seeds per pod. Can. J. Plant Sci. 1996, 76, 259–262. [Google Scholar] [CrossRef]
- Wulff, R.D. Seed size variation in Desmodium paniculatum: I. Factors affecting seed size. J. Ecol. 1986, 74, 87–97. [Google Scholar] [CrossRef]
- Almekinders, C.J.M.; Neuteboom, J.H.; Struik, P.C. Relation between berry weight, number of seeds per berry and 100-seed weight in potato inflorescences. Sci. Hortic. 1995, 61, 177–184. [Google Scholar] [CrossRef]
- Georgieva, N. Seed Heterogeneity in Dependence of Their Position on the Mother Plant in Lupinus albus L. Banats J. Biotechnol. 2020, 11, 76–82. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, X.M.; Qu, D.N. Relationship among seed size from different seed positions at several-seeded pod in soybean. J. Food Agric. Environ. 2012, 10, 768–771. [Google Scholar]
- Diggle, P.K. Architectural effects and the interpretation of patterns of fruit and seed development. Annu. Rev. Ecol. Syst. 1995, 26, 531–552. [Google Scholar] [CrossRef]
- Board, J.E.; Harville, B.G. Explanations for greater light interception in narrow-vs. wide-row. Crop Sci. 1992, 32, 198–202. [Google Scholar] [CrossRef]
- Taylor, H.W.; Mason, W.K.; Bennie, A.T.P.; Rowse, H.R. Responses of soybeans to two row spacings and two soil water levels. I. An analysis of biomass accumulation, canopy development, solar radiation interception and components of seed yield. Field Crop Res. 1982, 5, 1–14. [Google Scholar] [CrossRef]
- Willcott, J.; Herbert, S.J.; Liu, Z.Y. Leaf area display and light interception in short-season soybeans. Field Crop Res. 1984, 9, 173–182. [Google Scholar] [CrossRef]
- Jiang, H.; Egli, D. Shade induced changes in flower and pod number and flower and fruit abscission in soybean. Agron. J. 1993, 85, 221–225. [Google Scholar] [CrossRef]
- Iannucci, A.; Di Fonzo, N.; Martiniello, P. Alfalfa (Medicago sativa L.) seed yield and quality under different forage management systems and irrigation treatments in a Mediterranean environment. Field Crop Res. 2002, 78, 65–74. [Google Scholar] [CrossRef]
- Sengul, S. Using path analysis to determine lucerne (Medicago sativa L.) seed yield and its components. N. Z. J. Agr. Res. 2006, 49, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.; Walia, N. Growth and Yield of Soybean Glycine max (L.) Merill as Influenced by Light Intensity and Cytokinin. Environ. Ecol. 1996, 14, 307–310. [Google Scholar]
- Norberg, O.S.; Fiez, T.E.; Jolliff, G.D.; Seddigh, M.; Crane, J.M. Shading and Crop-Cover Effects on Meadowfoam Oil Yield. Agron. J. 1993, 85, 183–187. [Google Scholar] [CrossRef]
- Lippmann, R.; Babben, S.; Menger, A.; Delker, C.; Quint, M. Development of wild and cultivated plants under global warming conditions. Curr. Biol. 2019, 29, R1326–R1338. [Google Scholar] [CrossRef]
- Grace, J.B. Structural Equation Modeling and Natural Systems; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]

| Traits | Shade Treatment (ST) | Days after Anthesis (DAA) | ST×*DAA | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Seed traits | ||||||
| Germination rate | 33.69 | <0.01 | 55.52 | <0.01 | 5.86 | <0.01 |
| Germination index | 26.74 | <0.01 | 40.73 | <0.01 | 3.73 | <0.01 |
| Seed water content | 12.28 | <0.01 | 152.76 | <0.01 | 1.81 | 0.08 |
| 1000-seed weight | 29.34 | <0.01 | 139.45 | <0.01 | 7.10 | <0.01 |
| Seed numbers/capsule | 11.15 | <0.01 | 2.164 | 0.041 | 0.335 | 0.93 |
| Seed mass/capsule | 33.01 | <0.01 | 33.53 | <0.01 | 3.52 | <0.01 |
| Capsule traits | ||||||
| Capsule dry weight | 5.46 | 0.02 | 52.69 | <0.01 | 0.20 | 0.99 |
| Capsule length | 7.18 | 0.01 | 15.17 | <0.01 | 0.23 | 0.82 |
| Traits | Shade Treatment (ST) | Capsule Position (CP) | ST×*CP | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Germination rate | 408.09 | <0.001 | 5.59 | 0.007 | 0.49 | 0.616 |
| Seed numbers/capsule | 18.77 | <0.001 | 6.69 | <0.001 | 0.30 | 0.601 |
| Seed mass/capsule | 34.68 | <0.001 | 3.67 | 0.027 | 0.06 | 0.939 |
| 1000-seed weight | 41.76 | <0.001 | 30.76 | <0.001 | 0.81 | 0.446 |
| Seed length | 87.54 | <0.001 | 15.41 | <0.001 | 1.14 | 0.323 |
| Capsule abortion rate | 95.70 | <0.001 | 1369.83 | <0.001 | 122.36 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, J.; Chen, D.; Hui, Z.; Hu, X. Shade Increased Seed Yield and Quality of Incarvillea sinensis var. przewalskii. Plants 2023, 12, 2934. https://doi.org/10.3390/plants12162934
Wang Y, Wang J, Chen D, Hui Z, Hu X. Shade Increased Seed Yield and Quality of Incarvillea sinensis var. przewalskii. Plants. 2023; 12(16):2934. https://doi.org/10.3390/plants12162934
Chicago/Turabian StyleWang, Yan, Jingjing Wang, Dali Chen, Zhenning Hui, and Xiaowen Hu. 2023. "Shade Increased Seed Yield and Quality of Incarvillea sinensis var. przewalskii" Plants 12, no. 16: 2934. https://doi.org/10.3390/plants12162934





