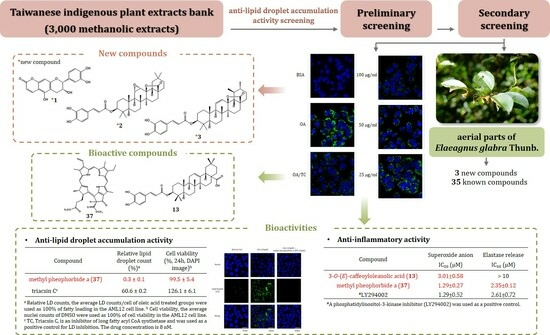
Chemical Constituents with Anti-Lipid Droplet Accumulation and Anti-Inflammatory Activity from Elaeagnus glabra
Abstract
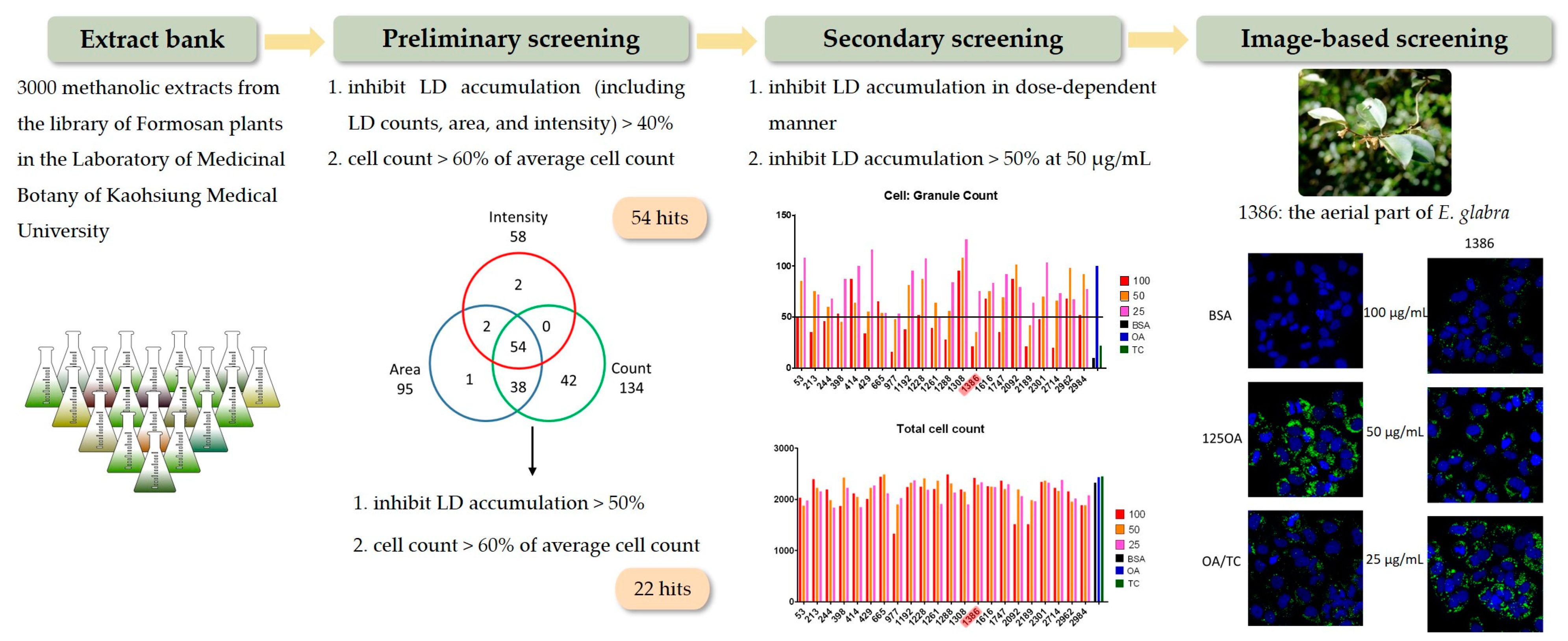
1. Introduction
2. Results and Discussion
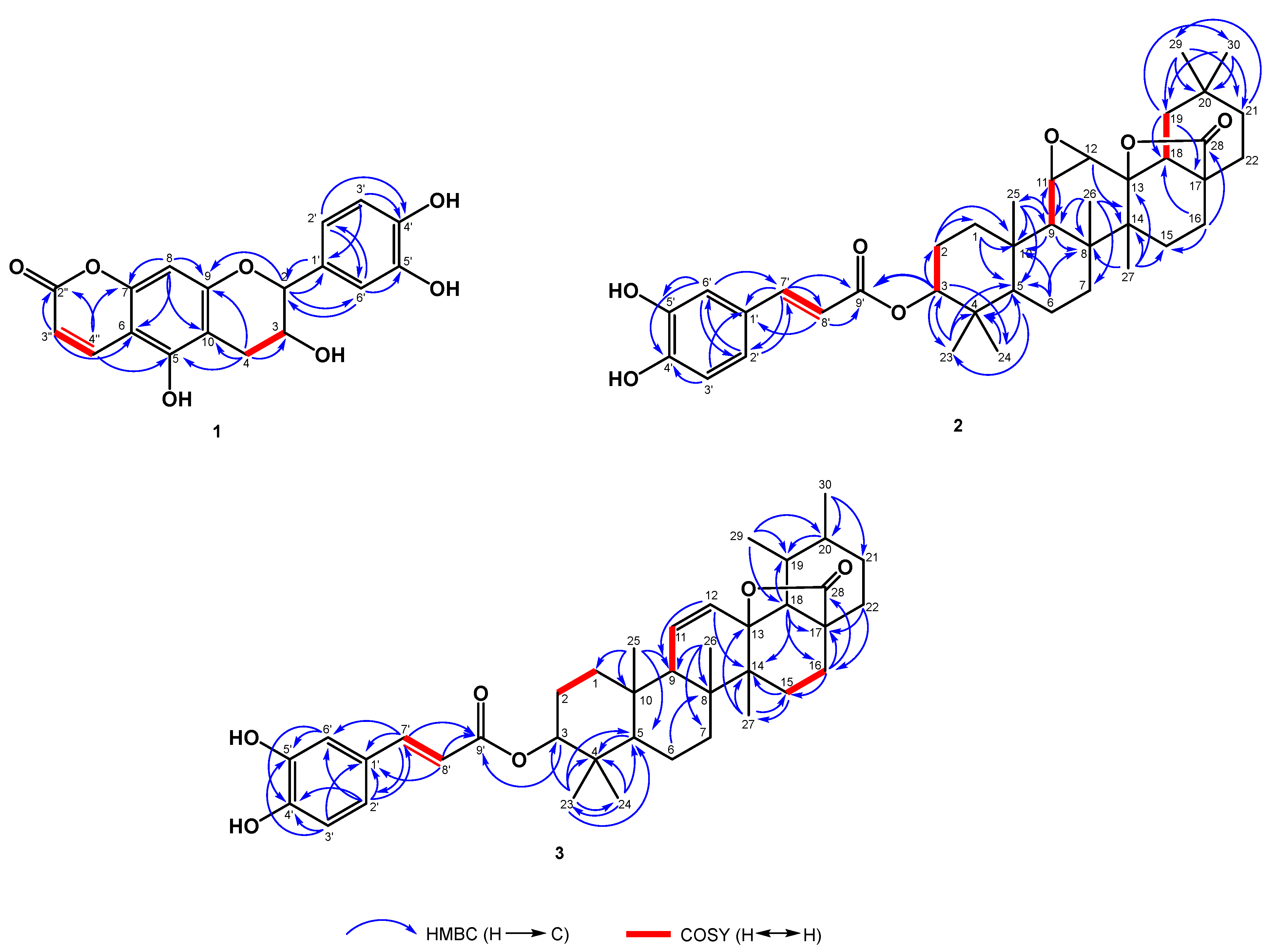
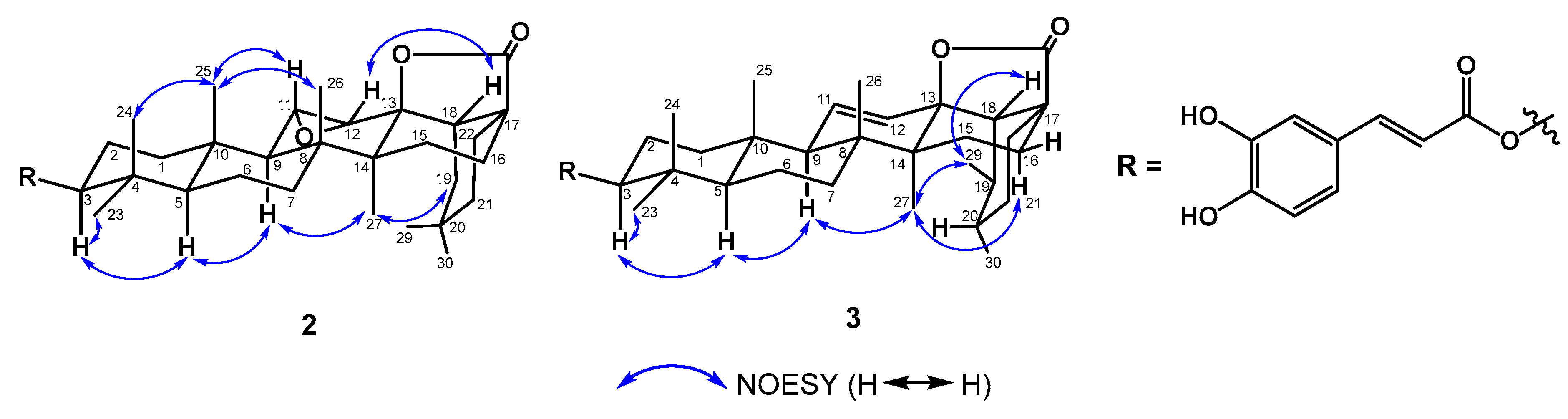
2.1. Structure Elucidation of Compounds 1−3
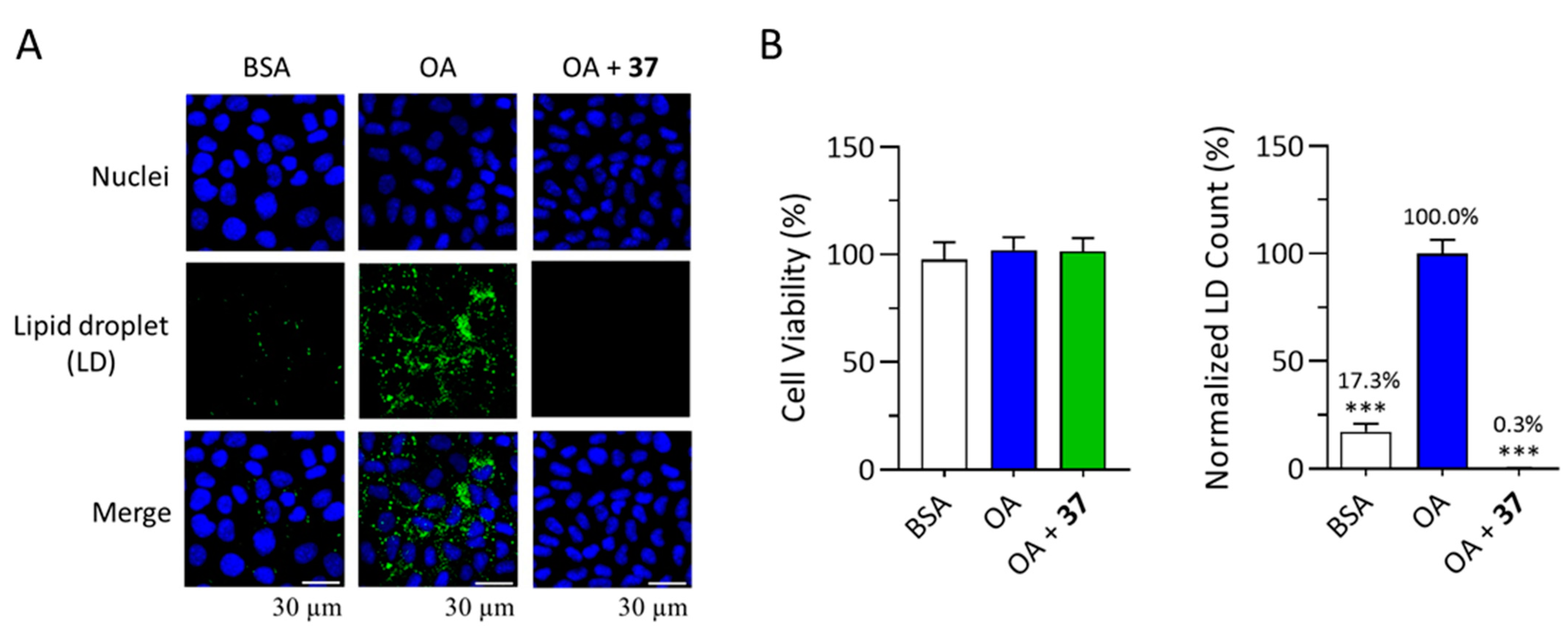
2.2. Anti-LD Accumulation Activity of Compounds Isolated from Aerial Parts of E. glabra
2.3. Anti-Inflammatory Activity of Compounds Isolated from Aerial Parts of E. glabra
3. Materials and Methods
3.1. General Experiment Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectroscopic Data of New Compounds
3.4.1. Elaeagncoumarin (1)
[(7R,8R)-8-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-7,8-dihydro-2H,6H-pyrano[3,2-g]chromen-2-one]
3.4.2. Elaeagterpene A (2)
[(3S,4aR,6aR,6bS,8aS,12aR,12bS,12cS,13aS,13bR,13cS)-4,4,6a,6b,11,11,13c-Heptamethyl-15-oxooctadecahydro-1H,9H-12b,8a-(epoxymethano)piceno [13,14-b]oxiren-3-yl(E)-3-(3,4-dihydroxyphenyl)acrylate]
3.4.3. Elaeagterpene B (3)
[(3S,4aR,6aR,6bS,8aS,11R,12S,12aR,12bS,14aR,14bS)-4,4,6a,6b,11,12,14b-Heptamethyl-16-oxo-2,3,4,4a,5,6,6a,6b,7,8,10,11,12,12a,14a,14b-hexadecahydro-1H,9H-12b,8a-(epoxymethano)picen-3-yl(E)-3-(3,4-dihydroxyphenyl)acrylate]
3.5. Cell Line
3.6. LD Assay
3.7. Superoxide Anion and Elastase Release Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yen, C.H.; Chang, H.S.; Yang, T.H.; Wang, S.F.; Wu, H.C.; Chen, Y.C.; Lin, K.J.; Wang, S. High-content screening of a Taiwanese indigenous plant extract library identifies Syzygium simile leaf extract as an inhibitor of fatty acid uptake. Int. J. Mol. Sci. 2018, 19, 2130. [Google Scholar] [CrossRef] [PubMed]
- Ashtari, S.; Pourhoseingholi, M.A.; Zali, M.R. Non-alcohol fatty liver disease in Asia: Prevention and planning. World J. Hepatol. 2015, 7, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; Roelstraete, B.; Hartjes, K.; Shah, U.; Khalili, H.; Arnell, H.; Ludvigsson, J.F. Non-alcoholic fatty liver disease in children and young adults is associated with increased long-term mortality. J. Hepatol. 2021, 75, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Cholongitas, E.; Pavlopoulou, I.; Papatheodoridi, M.; Markakis, G.E.; Bouras, E.; Haidich, A.B.; Papatheodoridis, G. Epidemiology of nonalcoholic fatty liver disease in Europe: A systematic review and meta-analysis. Ann. Gastroenterol. 2021, 34, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; De, A.; Chowdhury, A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl. Gastroenterol. Hepatol. 2020, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American association for the study of liver diseases, American college of gastroenterology, and the American gastroenterological association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- Cigrovski Berkovic, M.; Bilic-Curcic, I.; Mrzljak, A.; Cigrovski, V. NAFLD and physical exercise: Ready, steady, go! Front. Nutr. 2021, 8, 734859. [Google Scholar] [CrossRef]
- Gluchowski, N.L.; Becuwe, M.; Walther, T.C.; Farese, R.V., Jr. Lipid droplets and liver disease: From basic biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 343–355. [Google Scholar] [CrossRef]
- Welte, M.A. Expanding roles for lipid droplets. Curr. Biol. 2015, 25, R470–R481. [Google Scholar] [CrossRef]
- Walther, T.C.; Farese, R.V., Jr. Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 2012, 81, 687–714. [Google Scholar] [CrossRef]
- Nishino, C.; Enoki, N.; Tawata, S.; Mori, A.; Kobayashi, K.; Fukushima, M. Antibacterial activity of flavonoids against Staphylococcus epidermidis, a skin bacterium. J. Agric. Food Chem. 1987, 51, 139–143. [Google Scholar] [CrossRef]
- Mori, A.; Nishino, C.; Enoki, N.; Tawata, S. Antibacterial activity and mode of action of plant flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry 1987, 26, 2231–2234. [Google Scholar] [CrossRef]
- Tagahara, K.; Kato, A.; Hashimoto, Y.; Suzuta, Y. Constituents of the genus Elaeagnus (III): On the constituents of Elaeagnus glabra Thunb. Shoyakugaku Zasshi 1984, 3, 131. [Google Scholar]
- Sisa, M.; Bonnet, S.L.; Ferreira, D.; Van der Westhuizen, J.H. Photochemistry of flavonoids. Molecules 2010, 15, 5196–5245. [Google Scholar] [CrossRef] [PubMed]
- Salim, F.; Zain, M.M.; Ridzuan, M.S.M.; Langat, M.K.; Mulholland, D.A.; Ahmad, R. Flavan-3-ols from the leaves of Malaysian Uncaria longiflora var. pteropoda (Miq.) Ridsd. Phytochem. Lett. 2013, 6, 236–240. [Google Scholar] [CrossRef][Green Version]
- Tobiasona, F.L.; Fronczek, F.R.; Steynberg, J.P.; Steynberg, E.C.; Hemingway, R.W. Crystal structure, conformational analyses, and charge density distributions for ent-epifisetinidol: An explanation for regiospecific electrophilic aromatic substitution of 5-deoxyflavans. Tetrahedron 1993, 49, 5927–5940. [Google Scholar] [CrossRef]
- Abdel-Monem, A.R.; Kandil, Z.A.; Abdel-Naim, A.B.; Abdel-Sattar, E. A new triterpene and protective effect of Periploca somaliensis Browicz fruits against CCl4-induced injury on human hepatoma cell line (Huh7). Nat. Prod. Res. 2015, 29, 423–429. [Google Scholar] [CrossRef]
- Tkachev, A.V.; Denisov, A.Y.; Gatilov, Y.V.; Bagryanskaya, I.Y.; Shevtsov, S.A.; Rybalova, T.V. Stereochemistry of hydrogen peroxide—Acetic acid oxidation of ursolic acid and related compounds. Tetrahedron 1994, 50, 11459–11488. [Google Scholar] [CrossRef]
- Lalith, J.G.; Wannigama, P.; Macleod, J.K. Triterpenoids from Anamirta cocculus. Phytochemistry 1993, 34, 1111–1116. [Google Scholar] [CrossRef]
- Raju, R.; Gunawardena, D.; Ahktar, M.A.; Low, M.; Reddell, P.; Münch, G. Anti-inflammatory chemical profiling of the Australian rainforest tree Alphitonia petriei (Rhamnaceae). Molecules 2016, 21, 1521. [Google Scholar] [CrossRef]
- Kahnt, M.; Heller, L.; Al-Harrasi, A.; Schäfer, R.; Kluge, R.; Wagner, C.; Otgonbayar, C.; Csuk, R. Platanic acid-derived methyl 20-amino-30-norlupan-28-oates are potent cytotoxic agents acting by apoptosis. Med. Chem. Res. 2018, 27, 1757–1769. [Google Scholar] [CrossRef]
- Wang, C.; Wu, P.; Tian, S.; Xue, J.; Xu, L.; Li, H.; Wei, X. Bioactive Pentacyclic Triterpenoids from the Leaves of Cleistocalyx operculatus. J. Nat. Prod. 2016, 79, 2912–2923. [Google Scholar] [CrossRef]
- Fomogne-Fodjo, M.C.Y.; Ndinteh, D.T.; Olivier, D.K.; Kempgens, P.; van Vuuren, S.; Krause, R.W.M. Secondary metabolites from Tetracera potatoria stem bark with anti-mycobacterial activity. J. Ethnopharmacol. 2017, 195, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.L.; Cao, X.P. Pomolic acid derivatives from the root of Sanguisorba officinalis. Phytochemistry 1992, 31, 1317–1320. [Google Scholar] [CrossRef]
- Wu, C.; Cui, X.; Yu, P.; Yang, M.; Zhang, Y.; Liu, X.; Qu, G. Triterpenic Acids from Sorbaria sorbifolia. Chem. Nat. Compd. 2019, 55, 580–582. [Google Scholar] [CrossRef]
- Fuchino, H.; Satoh, T.; Tanaka, N. Chemical evaluation of Betula species in Japan. I.Constituents of Betula ermanii. Chem. Pharm. Bull. 1995, 43, 1937–1942. [Google Scholar] [CrossRef]
- Tanachatchairatana, T.; Bremner, J.B.; Chokchaisiri, R.; Suksamrarn, A. Antimycobacterial activity of cinnamate-based esters of the triterpenes betulinic, oleanolic and ursolic acids. Chem. Pharm. Bull. 2008, 56, 194–198. [Google Scholar] [CrossRef]
- Van Der westhuizen, J.H.; Steenkamp, J.A.; Ferreira, D. An unusual reaction of flavan-3-ols with acetone of relevance to the formation of the tetracyclic ring system in peltogynoids. Tetrahedron 1990, 46, 7849–7854. [Google Scholar] [CrossRef]
- Yue, J.M.; Zhao, Y.; Zhao, Q.S.; Lin, Z.W.; Sun, H.D.; Wu, H.M.; Xu, J.F. Phenolics from Ceratostigma minus. J. Integr. Plant Biol. 1998, 40, 1035–1039. [Google Scholar]
- Huang, A.C.; Wilde, A.; Ebmeyer, J.; Skouroumounis, G.K.; Taylor, D.K. Examination of the phenolic profile and antioxidant activity of the leaves of the Australian native plant Smilax glyciphylla. J. Nat. Prod. 2013, 76, 1930–1936. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Yue, J.; Sun, Y.; Zhang, X.; Zhao, Y. Polyphenols from acorn aeaves (Quercus liaotungensis) protect pancreatic beta cells and their inhibitory activity against α-glucosidase and protein tyrosine phosphatase 1B. Molecules 2018, 23, 2167. [Google Scholar] [CrossRef] [PubMed]
- Lage, G.A.; Medeiros, F.d.S.; Furtado, W.d.L.; Takahashi, J.A.; Filho, J.D.d.S.; Pimenta, L.P.S. The first report on flavonoid isolation from Annona crassiflora Mart. Nat. Prod. Res. 2014, 28, 808–811. [Google Scholar] [CrossRef]
- Lin, G.; Chang, L.; Liu, Y.; Xiang, Z.; Chen, J.; Yang, Z. Enantioselective total syntheses of (+)-gallocatechin, (−)-epigallocatechin, and 8-C-ascorbyl-(−)-epigallocatechin. Asian J. Chem. 2013, 8, 700–704. [Google Scholar] [CrossRef]
- Terfassi, S.; Dauvergne, X.; Cérantola, S.; Lemoine, C.; Bensouici, C.; Fadila, B.; Christian, M.; Marchioni, E.; Benayache, S. First report on phytochemical investigation, antioxidant and antidiabetic activities of Helianthemum getulum. Nat. Prod. Res. 2022, 36, 2806–2813. [Google Scholar] [CrossRef] [PubMed]
- Meena, H.; Hnamte, S.; Siddhardha, B. Secondary metabolites from endophytic fungi: Chemical diversity and application. In Advances in Endophytic Fungal Research: Present Status and Future Challenges; Singh, B.P., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 145–169. [Google Scholar]
- Madikizela, B.; Aderogba, M.A.; Finnie, J.F.; Van Staden, J. Isolation and characterization of antimicrobial compounds from Terminalia phanerophlebia Engl. & Diels leaf extracts. J. Ethnopharmacol. 2014, 156, 228–234. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Liu, Q.; Sun, C.; Li, J.; Yang, P.; Wang, X. Preparative separation of phenolic compounds from Chimonanthus praecox flowers by high-speed counter-current chromatography using a stepwise elution mode. Molecules 2016, 21, 1016. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Zhang, Z.Z.; Yang, L.P.; Zhai, Y.Y.; Li, S.N.; Hao, S.Y.; Chen, X.Y.; Wang, J.H.; Wang, Z.H. Chemical constituents of Curculigo orchioides. Chem. Nat. Compd. 2020, 56, 957–959. [Google Scholar] [CrossRef]
- Li, A.; Mishra, Y.; Malik, M.; Wang, Q.; Li, S.; Taylor, M.; Reichert, D.E.; Luedtke, R.R.; Mach, R.H. Evaluation of N-phenyl homopiperazine analogs as potential dopamine D3 receptor selective ligands. Bioorg. Med. Chem. 2013, 21, 2988–2998. [Google Scholar] [CrossRef]
- Shu, P.; Sun, M.; Li, J.; Zhang, L.; Xu, H.; Lou, Y.; Ju, Z.; Wei, X.; Wu, W.; Sun, N. Chemical constituents from Ginkgo biloba leaves and their cytotoxicity activity. J. Nat. Med. 2020, 74, 269–274. [Google Scholar] [CrossRef]
- Tan, S.P.; Ali, A.M.; Nafiah, M.A.; Amna, U.; Ramli, S.A.; Ahmad, K. Terpenes and phenolic compounds of Murraya koenigii. Chem. Nat. Compd. 2017, 53, 980–981. [Google Scholar] [CrossRef]
- Tang, C.; Tao, G.; Wang, Y.; Liu, Y.; Li, J. Identification of α-tocopherol and its oxidation products by ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J. Agric. Food Chem. 2020, 68, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.C.; Lai, M.H.; Hsu, K.P.; Kuo, Y.H.; Chen, J.; Tsai, M.C.; Li, C.X.; Yin, X.J.; Jeyashoke, N.; Chao, L.K.P. Identification of β-sitosterol as in vitro anti-inflammatory constituent in Moringa oleifera. J. Agric. Food. Chem. 2018, 66, 10748–10759. [Google Scholar] [CrossRef]
- Huang, C.M.; Sung, P.J.; Kuo, Y.H.; Chang, T.H.; Chen, C.L.; Cheng, M.J.; Chen, J.J. A new dihydroagarofuranoid sesquiterpene and cytotoxic constituents of Microtropis fokienensis. Chem. Nat. Compd. 2020, 56, 440–444. [Google Scholar] [CrossRef]
- Yodsing, N.; Lekphrom, R.; Sangsopha, W.; Aimi, T.; Boonlue, S. Secondary metabolites and their biological activity from Aspergillus aculeatus KKU-CT2. Curr. Microbiol. 2018, 75, 513–518. [Google Scholar] [CrossRef]
- Sribuhom, T.; Sriphana, U.; Thongsri, Y.; Yenjai, C. Chemical constituents from the stems of Alyxia schlechteri. Phytochem. Lett. 2015, 11, 80–84. [Google Scholar] [CrossRef]
- Zou, G.A.; Wang, Y.; Zou, Z.M.; Chen, S. Lignans from stems of Croton caudatus var. tomentosus. Chem. Nat. Compd. 2013, 49, 93–94. [Google Scholar] [CrossRef]
- Gudmundsson, H.G.; Linderborg, K.M.; Kallio, H.; Yang, B.; Haraldsson, G.G. Synthesis of enantiopure ABC-type triacylglycerols. Tetrahedron 2020, 76, 130813. [Google Scholar] [CrossRef]
- Xiao, W.L.; Chen, W.H.; Zhang, J.Y.; Song, X.P.; Chen, G.Y.; Han, C.R. Ionone-type sesquiterpenoids from the stems of Ficus pumila. Chem. Nat. Compd. 2016, 52, 531–533. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chiu, H.L.; Lu, T.L.; Tzeng, C.Y.; Lee, T.H.; Lee, C.K.; Shao, Y.Y.; Chen, C.R.; Chang, C.I.; Kuo, Y.H. Ficusmicrochlorin A—C, two new methoxy lactone chlorins and an anhydride chlorin from the leaves of Ficus microcarpa. Chem. Pharm. Bull. 2011, 59, 113–116. [Google Scholar] [CrossRef]
- Hurtado-Díaz, I.; Sánchez-Carranza, J.N.; Romero-Estrada, A.; González-Maya, L.; González-Christen, J.; Herrera-Ruiz, M.; Alvarez, L. 16-Hydroxy-lycopersene, a polyisoprenoid alcohol isolated from Tournefortia hirsutissima, inhibits nitric oxide production in RAW 264.7 cells and induces apoptosis in Hep3B Cells. Molecules 2019, 24, 2366. [Google Scholar] [CrossRef]
- Das, P.; Mandal, S.; Gangopadhyay, S.; Das, K.; Mitra, A.G.; Dasgupta, S.; Mukhopadhyay, S.; Mukhopadhyay, A. Antioxidative and anticarcinogenic activities of methylpheophorbide a, isolated from wheat grass (Triticum aestivum Linn). Nat. Prod. Res. 2016, 30, 474–477. [Google Scholar] [CrossRef]
- Wongsinkongman, P.; Brossi, A.; Wang, H.K.; Bastow, K.F.; Lee, K.H. Antitumor agents. Part 209: Pheophorbide-a derivatives as photo-independent cytotoxic agents. Bioorg. Med. Chem. 2002, 10, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Rushdi, M.I.; Abdel-Rahman, I.A.M.; Saber, H.; Attia, E.Z.; Abdelraheem, W.M.; Madkour, H.A.; Abdelmohsen, U.R. The genus Turbinaria: Chemical and pharmacological diversity. Nat. Prod. Res. 2021, 35, 4560–4578. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.H.; Lai, C.C.; Shia, T.H.; Chen, M.; Yu, H.C.; Liu, Y.P.; Chang, F.R. Gynura divaricata attenuates tumor growth and tumor relapse after cisplatin therapy in HCC xenograft model through suppression of cancer stem cell growth and Wnt/β-catenin signalling. J. Ethnopharmacol. 2018, 213, 366–375. [Google Scholar] [CrossRef]
- Kao, T.I.; Chen, P.J.; Wang, Y.H.; Tseng, H.H.; Chang, S.H.; Wu, T.S.; Yang, S.H.; Lee, Y.T.; Hwang, T.L. Bletinib ameliorates neutrophilic inflammation and lung injury by inhibiting Src family kinase phosphorylation and activity. Br. J. Pharmacol. 2021, 178, 4069–4084. [Google Scholar] [CrossRef] [PubMed]
- Korinek, M.; Hsieh, P.S.; Chen, Y.L.; Hsieh, P.W.; Chang, S.H.; Wu, Y.H.; Hwang, T.L. Randialic acid B and tomentosolic acid block formyl peptide receptor 1 in human neutrophils and attenuate psoriasis-like inflammation in vivo. Biochem. Pharmacol. 2021, 190, 114596. [Google Scholar] [CrossRef]


| Position | 1 | |
|---|---|---|
| δH (J in Hz) | δC | |
| 1 | – | – |
| 2 | 5.02, br s | 80.7 |
| 3 | 4.30, br dd (4.2, 2.7) | 66.6 |
| 4a | 2.96, dd (17.0, 4.2) | 29.4 |
| 4b | 2.87, dd (17.0, 2.7) | |
| 5 | – | 153.4 |
| 6 | – | 103.4 |
| 7 | – | 156.2 |
| 8 | 6.35, s | 95.7 |
| 9 | – | 163.1 |
| 10 | – | 105.6 |
| 1′ | – | 131.4 |
| 2′ | 7.04, d (2.3) | 115.2 |
| 3′ | – | 146.2 |
| 4′ | – | 146.1 |
| 5′ | 6.80, d (8.3) | 116.1 |
| 6′ | 6.86, dd (8.3, 2.3) | 119.3 |
| 2″ | – | 164.5 |
| 3″ | 6.07, d (9.6) | 109.4 |
| 4″ | 8.14, d (9.6) | 141.2 |
| Position | 2 | 3 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 1.60, m | 38.9 | 1.12, m/1.93, m | 39.0 |
| 2 | 1.60, m/1.81, m | 24.4 | 1.50, m/1.76, m | 32.5 |
| 3 | 4.62, dd (11.4, 4.8) | 82.0 | 4.60, dd (11.4, 4.8) | 82.0 |
| 4 | – | 39.1 | – | 39.3 |
| 5 | 0.94, m | 56.0 | 0.99, m | 56.0 |
| 6 | 1.60, m | 18.6 | 1.67, m | 18.7 |
| 7 | 1.20, m/1.41, m | 32.2 | 1.30, m | 32.3 |
| 8 | – | 42.6 | – | 43.2 |
| 9 | 1.66, br s | 52.0 | 2.11, br s | 54.3 |
| 10 | – | 37.7 | – | 37.5 |
| 11 | 3.12, dd (3.9, 1.8) | 53.8 | 5.60, dd (10.5, 3.3) | 130.0 |
| 12 | 3.06, d (3.9) | 58.2 | 6.06, dd (10.5, 1.5) | 134.8 |
| 13 | – | 89.4 | – | 91.9 |
| 14 | – | 41.8 | – | 43.0 |
| 15 | 1.15, m | 27.8 | 1.67, m | 26.6 |
| 16 | 1.29, m/2.28, m | 22.3 | 1.30, m/2.30, ddd (18.9, 13.5, 5.7) | 23.9 |
| 17 | – | 45.4 | – | 46.6 |
| 18 | 2.40, dd (13.8, 3.0) | 50.8 | 1.70, d (11.4) | 61.8 |
| 19 | 1.29, m/1.99, m | 38.9 | 1.12, m | 39.2 |
| 20 | – | 32.3 | 0.95, m | 41.5 |
| 21 | 1.29, m/1.47, ddd (18.6, 14.0, 4.4) | 35.2 | 1.35, m/1.59, m | 31.8 |
| 22 | 1.60, m/1.70, dd (14.0, 4.4) | 28.2 | 1.76, m/1.81, m | 24.5 |
| 23 | 0.92, s | 16.9 | 0.92, s | 16.7 |
| 24 | 0.99, s | 28.3 | 0.98, s | 28.3 |
| 25 | 1.14, s | 17.7 | 1.01, s | 15.8 |
| 26 | 1.08, s | 20.7 | 1.06, s | 19.6 |
| 27 | 1.17, s | 19.3 | 1.25, s | 16.6 |
| 28 | – | 181.6 | – | 182.6 |
| 29 | 0.96, s | 24.0 | 1.04, d (6.3) | 18.3 |
| 30 | 1.02, s | 33.5 | 0.96, d (6.3) | 19.4 |
| 1′ | – | 127.7 | – | 127.7 |
| 2′ | 7.04, d (2.3) | 115.1 | 7.04, d (2.4) | 115.1 |
| 3′ | – | 146.85 | – | 146.85 |
| 4′ | – | 149.6 | – | 149.6 |
| 5′ | 6.78, d (8.6) | 116.5 | 6.78, d (8.4) | 116.5 |
| 6′ | 6.95, dd (8.6, 2.3) | 122.9 | 6.95, dd (8.4, 2.4) | 122.9 |
| 7′ | 7.54, d (15.9) | 146.78 | 7.53, d (15.9) | 146.75 |
| 8′ | 6.26, d (15.9) | 115.5 | 6.25, d (15.9) | 115.5 |
| 9′ | – | 169.1 | – | 169.2 |
| Compound (100 μg/mL) | Relative Lipid Droplet Count (%) a | Cell Viability (%, 24h, DAPI Image) b |
|---|---|---|
| lupeol (9) | 95.2 ± 1.8 | 113.7 ± 3.6 |
| ursolic acid (11) | 99.2 ± 4 | 146.5 ± 4.5 |
| plumbocatechins B (15) | 105.9 ± 7.8 | 132.5 ± 11.8 |
| (−)-catechin (16) | 110.7 ± 5.1 | 154 ± 12.3 |
| (−)-epicatechin (18) | 115.9 ± 6 | 145.9 ± 10.3 |
| (−)-epigallocatechin (19) | 93.5 ± 6.6 | 127.2 ± 22.6 |
| (−)-gallocatechin (20) | 116.7 ± 7.8 | 153.9 ± 6.2 |
| plumbocatechin A (22) | 118.2 ± 3.1 | 147.8 ± 9.6 |
| syringaldehyde (25) | 102 ± 5 | 137.8 ± 5.7 |
| β-sitosterol (30) | 112.7 ± 7.6 | 128.5 ± 9.1 |
| β-sitosterone (31) | 121.5 ± 4.3 | 135.3 ± 7.3 |
| methyl pheophorbide a (37) | 0.3 ± 0.1 | 99.5 ± 5.4 |
| TC c | 60.6 ± 0.2 | 126.1 ± 6.1 |
| Compound | Superoxide Anion IC50 (μM) | Elastase Release IC50 (μM) |
|---|---|---|
| elaeagterpene A (2) | >10 | >10 |
| cleistocalyxic acid E (7) | >10 | >10 |
| 2α,3β,23-trihydroxy-11α,12α-epoxyolean-28,13β-olide (12) | >10 | >10 |
| 3-O-(E)-caffeoyloleanolic acid (13) | 3.01 ± 0.58 | >10 |
| (+)-3β-O-trans-caffeoyl betulinic acid (14) | >10 | >10 |
| syringaldehyde (25) | >10 | >10 |
| 4-(2-hydroxyethyl)benzoic acid (26) | >10 | >10 |
| methyl pheophorbide a (37) | 1.29 ± 0.27 | 2.35 ± 0.12 |
| #LY294002 | 1.29 ± 0.52 | 2.61 ± 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.-H.; Wu, H.-C.; Yen, C.-H.; Hwang, T.-L.; Ko, H.-H.; Chang, H.-S. Chemical Constituents with Anti-Lipid Droplet Accumulation and Anti-Inflammatory Activity from Elaeagnus glabra. Plants 2023, 12, 2943. https://doi.org/10.3390/plants12162943
Cheng J-H, Wu H-C, Yen C-H, Hwang T-L, Ko H-H, Chang H-S. Chemical Constituents with Anti-Lipid Droplet Accumulation and Anti-Inflammatory Activity from Elaeagnus glabra. Plants. 2023; 12(16):2943. https://doi.org/10.3390/plants12162943
Chicago/Turabian StyleCheng, Ju-Hsin, Ho-Cheng Wu, Chia-Hung Yen, Tsong-Long Hwang, Horng-Huey Ko, and Hsun-Shuo Chang. 2023. "Chemical Constituents with Anti-Lipid Droplet Accumulation and Anti-Inflammatory Activity from Elaeagnus glabra" Plants 12, no. 16: 2943. https://doi.org/10.3390/plants12162943
APA StyleCheng, J.-H., Wu, H.-C., Yen, C.-H., Hwang, T.-L., Ko, H.-H., & Chang, H.-S. (2023). Chemical Constituents with Anti-Lipid Droplet Accumulation and Anti-Inflammatory Activity from Elaeagnus glabra. Plants, 12(16), 2943. https://doi.org/10.3390/plants12162943