Phosphorus Addition Reduces Seedling Growth and Survival for the Arbuscular Mycorrhizal Tree Cinnamomum camphora (Lauraceae) and Ectomycorrhizal Tree Castanopsis sclerophylla (Fagaceae) in Fragmented Forests in Eastern China
Abstract
1. Introduction
2. Results
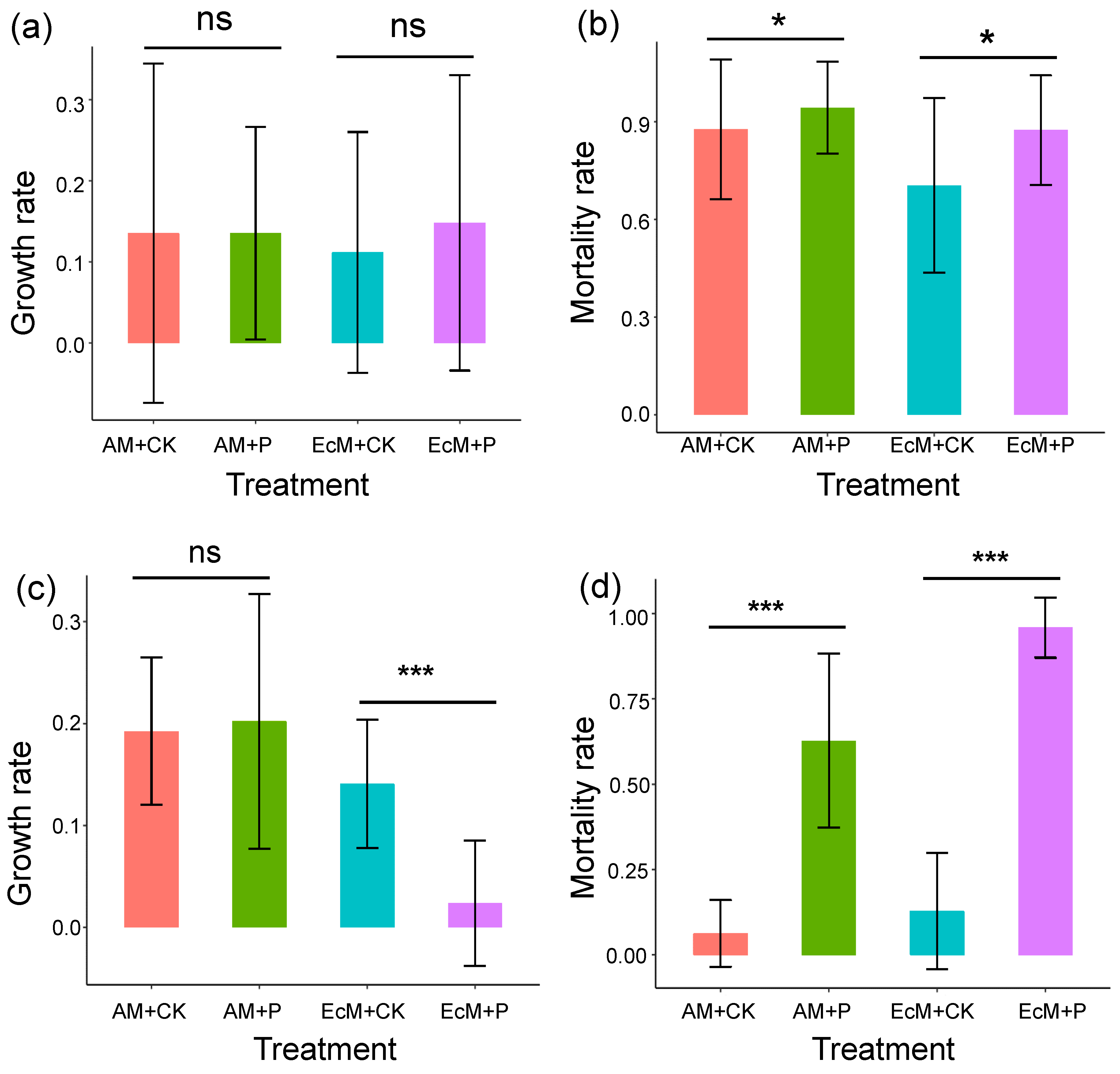
2.1. Effect of Phosphorus Addition on the Growth and Mortality of Seedlings
2.2. Relationships between Island Area, Relative Growth Rate, and Relative Mortality Rate
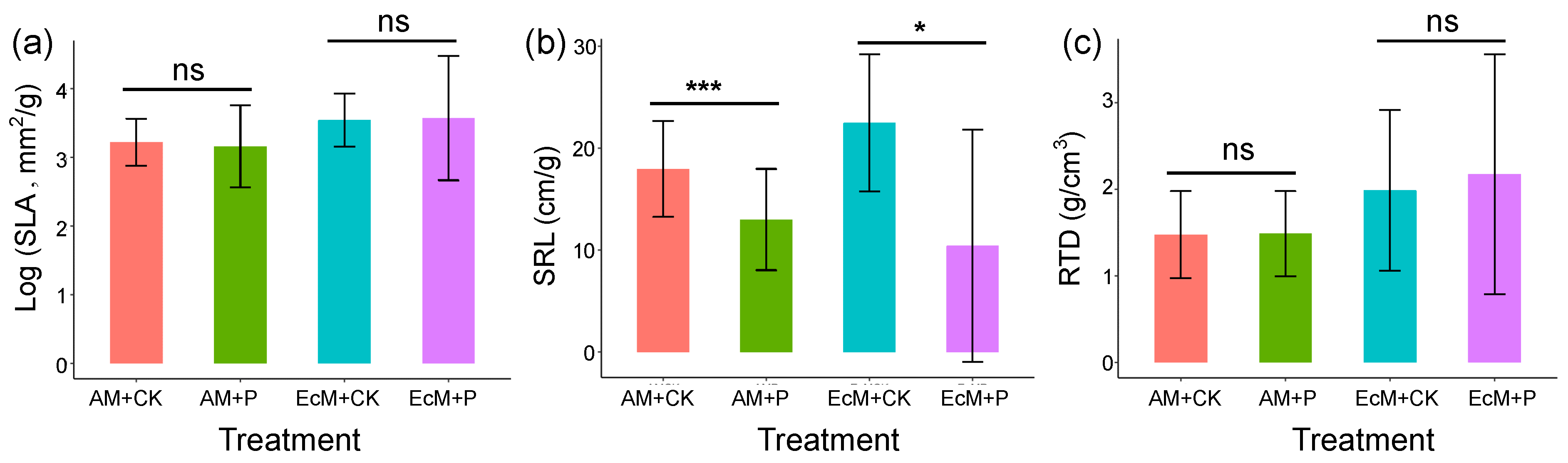
2.3. Effects of Phosphorus Addition on Functional Traits
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Selection of Experimental Islands and Sampling Sites
4.3. Phosphorus Addition Experiment on Islands
4.4. Phosphorus Addition Experiment in the Greenhouse
4.5. Measurement of Functional Traits
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chase, J.M.; Blowes, S.A.; Knight, T.M.; Gerstner, K.; May, F. Ecosystem decay exacerbates biodiversity loss with habitat loss. Nature 2020, 584, 238–243. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967. [Google Scholar]
- Whittaker, R.J.; Fernández-Palacios, J.M. Island Biogeography: Ecology, Evolution, and Conservation; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e15000522015. [Google Scholar] [CrossRef]
- Burgos, A.; Grez, A.A.; Bustamante, R.O. Seed production, pre-dispersal seed predation and germination of Nothofagus glauca (Nothofagaceae) in a temperate fragmented forest in Chile. For. Ecol. Manag. 2008, 255, 1226–1233. [Google Scholar] [CrossRef]
- Stride, G.; Thomas, C.D.; Benedick, S.; Hodgson, J.A.; Jelling, A.; Senior, M.J.M.; Hill, J.K.; Ibáñez, I. Divergent tree seedling communities indicate different trajectories of change among rain forest remnants. Divers. Distrib. 2019, 25, 1751–1762. [Google Scholar] [CrossRef]
- Benítez-Malvido, J.; Lázaro, A.; Ferraz, I.D.K. Effect of distance to edge and edge interaction on seedling regeneration and biotic damage in tropical rainforest fragments: A long-term experiment. J. Ecol. 2018, 106, 2204–2217. [Google Scholar] [CrossRef]
- Nathan, R.; Muller-Landau, H.C. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol. Evolut. 2000, 15, 278–285. [Google Scholar] [CrossRef]
- Ruffell, J.; Didham, R.K. Towards a better mechanistic understanding of edge effects. Landsc. Ecol. 2016, 31, 2205–2213. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Terrer, C.; Pellegrini, A.F.A.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Wassen, M.J.; Venterink, H.O.; Lapshina, E.D.; Tanneberger, F. Endangered plants persist under phosphorus limitation. Nature 2005, 437, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Ceulemans, T.; Stevens, C.J.; Duchateau, L.; Jacquemyn, H.; Gowing, D.J.; Merckx, R.; Wallace, H.; van Rooijen, N.; Goethem, T.; Bobbink, R.; et al. Soil phosphorus constrains biodiversity across European grasslands. Glob. Chang. Biol. 2014, 20, 3814–3822. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Townsend, A.R.; Taylor, P.; Alvarez-Clare, S.; Bustamante, M.M.; Chuyong, G.; Dobrowski, S.Z.; Grierson, P.; Harms, K.E.; Houlton, B.Z.; et al. Relationships among net primary productivity, nutrients and climate in tropical rain forest: A pan-tropical analysis. Ecol. Lett. 2011, 14, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M. Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecology 1984, 65, 285–298. [Google Scholar] [CrossRef]
- Condit, R.; Engelbrecht, B.M.; Pino, D.; Perez, R.; Turner, B.L. Species distributions in response to individual soil nutrients and seasonal drought across a community of tropical trees. Proc. Natl. Acad. Sci. USA 2013, 110, 5064–5068. [Google Scholar] [CrossRef]
- Sanchez, P.A. Properties and Management of Tropical Soils; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Veldkamp, E.; Schmidt, M.; Powers, J.S.; Corre, M.D. Deforestation and reforestation impacts on soils in the tropics. Nat. Rev. Earth Environ. 2020, 1, 590–605. [Google Scholar] [CrossRef]
- Sugiyama, A.; Peterson, C.J. Edge Effects Act Differentially on Multiple Early Regeneration Stages of a Shade-tolerant Tree Tapirira mexicana. Biotropica 2013, 45, 37–44. [Google Scholar] [CrossRef]
- Liu, J.; Matthews, T.J.; Zhong, L.; Liu, J.; Wu, D.; Yu, M. Environmental filtering underpins the island species–area relationship in a subtropical anthropogenic archipelago. J. Ecol. 2020, 108, 424–432. [Google Scholar] [CrossRef]
- Ji, P.; Chen, J.; Zhou, A.; Chen, R.; Ding, G.; Wang, H.; Chen, S.; Chen, F. Anthropogenic atmospheric deposition caused the nutrient and toxic metal enrichment of the enclosed lakes in North China. J. Hazard. Mater. 2023, 448, 130972. [Google Scholar] [CrossRef]
- Penuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; van der Velde, M.; Bopp, L.; Boucher, O.; Godderis, Y.; Hinsinger, P.; Llusia, J.; et al. Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 4, 2934. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: London, UK, 2008. [Google Scholar]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Joner, E.J.; Jakobsen, I. Contribution by two arbuscular mycorrhizal fungi to P uptake by cucumber (Cucumis sativus L.) from 32P-labelled organic matter during mineralization in soil. Plant Soil 1994, 163, 203–209. [Google Scholar] [CrossRef]
- Munkvold, L.; Kjoller, R.; Vestberg, M.; Rosendahl, S.; Jakobsen, I. High functional diversity within species of arbuscular mycorrhizal fungi. New Phytol. 2004, 164, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.J.; Dewbre, G.R.; Liu, J. A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. Plant Cells 2002, 14, 2413–2429. [Google Scholar] [CrossRef] [PubMed]
- Alexander, I.J.; Lee, S.S. Mycorrhizas and ecosystem processes in tropical rain forest: Implications for diversity. In Biotic Interactions in the Tropics: Their Role in the Maintenance of Species Diversity; Burslem, D., Pinard, M.A., Hartley, S.E., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 165–203. [Google Scholar]
- Liu, X.; Burslem, D.; Taylor, J.D.; Taylor, A.F.S.; Khoo, E.; Majalap-Lee, N.; Helgason, T.; Johnson, D. Partitioning of soil phosphorus among arbuscular and ectomycorrhizal trees in tropical and subtropical forests. Ecol. Lett. 2018, 21, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, J.F.; Camenzind, T.; Roy, J.; Hempel, S.; Homeier, J.; Suarez, J.P.; Rillig, M.C. Moderate phosphorus additions consistently affect community composition of arbuscular mycorrhizal fungi in tropical montane forests in southern Ecuador. New Phytol. 2020, 227, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Wang, P.; Chen, Y.; Wilson, M.C.; Yang, X.; Ma, C.; Lu, J.; Chen, X.Y.; Wu, J.; Shu, W.S.; et al. Island biogeography of soil bacteria and fungi: Similar patterns, but different mechanisms. ISME J. 2020, 14, 1886–1896. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Lü, X.-T.; Hartmann, H.; Keller, A.; Han, X.-G.; Trumbore, S.; Phillips, R.P. Foliar nutrient resorption differs between arbuscular mycorrhizal and ectomycorrhizal trees at local and global scales. Glob. Ecol. Biogeogr. 2018, 27, 875–885. [Google Scholar] [CrossRef]
- Jiang, F.; Lutz, J.A.; Guo, Q.; Hao, Z.; Wang, X.; Gilbert, G.S.; Mao, Z.; Orwig, D.A.; Parker, G.G.; Sang, W.; et al. Mycorrhizal type influences plant density dependence and species richness across 15 temperate forests. Ecology 2021, 102, e032592021. [Google Scholar] [CrossRef] [PubMed]
- Delavaux, C.S.; Weigelt, P.; Dawson, W.; Duchicela, J.; Essl, F.; van Kleunen, M.; Konig, C.; Pergl, J.; Pysek, P.; Stein, A.; et al. Mycorrhizal fungi influence global plant biogeography. Nat. Ecol. Evolut. 2019, 3, 424–429. [Google Scholar] [CrossRef]
- Li, X.; Li, T.; Zhong, L.; Guo, J.; Yu, M.; Liu, J. Arbuscular Mycorrhizal Plants Are More Susceptible to Habitat Fragmentation than Ectomycorrhizal Plants Due to Environmental Filtering. Plant Soil. 2023, in press. [Google Scholar] [CrossRef]
- Patiño, J.; Whittaker, R.J.; Borges, P.A.V.; Fernández-Palacios, J.M.; Ah-Peng, C.; Araújo, M.B.; Ávila, S.P.; Cardoso, P.; Cornuault, J.; de Boer, E.J.; et al. A roadmap for island biology: 50 fundamental questions after 50 years of the Theory of Island Biogeography. J. Biogeogr. 2017, 44, 963–983. [Google Scholar] [CrossRef]
- Vellend, M.; Baeten, L.; Becker-Scarpitta, A.; Boucher-Lalonde, V.; McCune, J.L.; Messier, J.; Myers-Smith, I.H.; Sax, D.F. Plant biodiversity change across scales during the Anthropocene. Annu. Rev. Plant Biol. 2017, 68, 563–586. [Google Scholar] [CrossRef] [PubMed]
- Lázaro-Nogal, A.; Matesanz, S.; Gimeno, T.E.; Escudero, A.; Valladares, F. Fragmentation modulates the strong impact of habitat quality and plant cover on fertility and microbial activity of semiarid gypsum soils. Plant Soil 2012, 358, 213–223. [Google Scholar] [CrossRef]
- Tng, D.Y.; Janos, D.P.; Jordan, G.J.; Weber, E.; Bowman, D.M. Phosphorus limits Eucalyptus grandis seedling growth in an unburnt rain forest soil. Front. Plant Sci. 2014, 5, 527. [Google Scholar] [CrossRef] [PubMed]
- Newbery, D.M.; Chuyong, G.B.; Green, J.J.; Songwe, N.C.; Tchuenteu, F.; Zimmermann, L. Does low phosphorus supply limit seedling establishment and tree growth in groves of ectomycorrhizal trees in a central African rainforest? New Phytol. 2002, 156, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Manu, R.; Corre, M.D.; Aleeje, A.; Mwanjalolo, M.J.G.; Babweteera, F.; Veldkamp, E.; van Straaten, O. Responses of tree growth and biomass production to nutrient addition in a semi-deciduous tropical forest in Africa. Ecology 2022, 103, e3659. [Google Scholar] [CrossRef]
- Mao, Q.; Chen, H.; Gurmesa, G.A.; Gundersen, P.; Ellsworth, D.S.; Gilliam, F.S.; Wang, C.; Zhu, F.; Ye, Q.; Mo, J.; et al. Negative effects of long-term phosphorus additions on understory plants in a primary tropical forest. Sci. Total Environ. 2021, 798, 149306. [Google Scholar] [CrossRef]
- Kadowaki, K.; Yamamoto, S.; Sato, H.; Tanabe, A.S.; Hidaka, A.; Toju, H. Mycorrhizal fungi mediate the direction and strength of plant-soil feedbacks differently between arbuscular mycorrhizal and ectomycorrhizal communities. Commun. Biol. 2018, 1, 196. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, Y.-P.; Yang, Y.; Yu, M.; Wang, C.; Yan, J. Interactive effects of nitrogen and phosphorus additions on plant growth vary with ecosystem type. Plant Soil 2019, 440, 523–537. [Google Scholar] [CrossRef]
- Wright, S.J.; Yavitt, J.B.; Wurzburger, N.; Turner, B.L.; Tanner, E.V.; Sayer, E.J.; Santiago, L.S.; Kaspari, M.; Hedin, L.O.; Harms, K.E.; et al. Potassium, phosphorus, or nitrogen limit root allocation, tree growth, or litter production in a lowland tropical forest. Ecology 2011, 92, 1616–1625. [Google Scholar] [CrossRef]
- Zalamea, P.C.; Turner, B.L.; Winter, K.; Jones, F.A.; Sarmiento, C.; Dalling, J.W. Seedling growth responses to phosphorus reflect adult distribution patterns of tropical trees. New Phytol. 2016, 212, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Treseder, K.K. The extent of mycorrhizal colonization of roots and its influence on plant growth and phosphorus content. Plant Soil 2013, 371, 1–13. [Google Scholar] [CrossRef]
- Wright, S.J.; Turner, B.L.; Yavitt, J.B.; Harms, K.E.; Kaspari, M.; Tanner, E.V.J.; Bujan, J.; Griffin, E.A.; Mayor, J.R.; Pasquini, S.C.; et al. Plant responses to fertilization experiments in lowland, species-rich, tropical forests. Ecology 2018, 99, 1129–1138. [Google Scholar] [CrossRef]
- Wurzburger, N.; Wright, S.J. Fine-root responses to fertilization reveal multiple nutrient limitation in a lowland tropical forest. Ecology 2015, 96, 2137–2146. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Song, M.; Kou, L.; Li, Q.; Wang, H. Contrasting effects of nitrogen and phosphorus additions on nitrogen competition between coniferous and broadleaf seedlings. Sci. Total Environ. 2023, 861, 160661. [Google Scholar] [CrossRef]
- Soudzilovskaia, N.A.; Vaessen, S.; Barcelo, M.; He, J.; Rahimlou, S.; Abarenkov, K.; Brundrett, M.C.; Gomes, S.I.F.; Merckx, V.; Tedersoo, L. FungalRoot: Global online database of plant mycorrhizal associations. New Phytol. 2020, 227, 955–966. [Google Scholar] [CrossRef]
- Raven, J.A.; Lambers, H.; Smith, S.E.; Westoby, M. Costs of acquiring phosphorus by vascular land plants: Patterns and implications for plant coexistence. New Phytol. 2018, 217, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, X.; Wu, C.; Zhong, Y.; Yu, M.; Liu, J. Herbivory rather than root competition and environmental factors determines plant establishment in fragmented forests. Forests 2022, 13, 767. [Google Scholar] [CrossRef]
- Liu, J.; Coomes, D.A.; Hu, G.; Liu, J.; Yu, J.; Luo, Y.; Yu, M. Larger fragments have more late-successional species of woody plants than smaller fragments after 50 years of secondary succession. J. Ecol. 2019, 107, 582–594. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, Y.; Mou, Z.; Kuang, L.; Wu, W.; Zhang, J.; Wang, F.; Hui, D.; Penuelas, J.; Sardans, J.; et al. Phosphorus addition decreases microbial residual contribution to soil organic carbon pool in a tropical coastal forest. Glob. Chang. Biol. 2021, 27, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Didham, R.K.; Yu, M.; Webber, B.L. Species-level CWM values mask contrasting intra-versus interspecific trait shifts at subtropical forest edges. Ecography 2022, 2022, e05837. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multi-Model Inference: A Practical Information Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhou, M.; Li, X.; Li, T.; Jiang, H.; Zhao, L.; Chen, S.; Tian, J.; Han, W. Phosphorus Addition Reduces Seedling Growth and Survival for the Arbuscular Mycorrhizal Tree Cinnamomum camphora (Lauraceae) and Ectomycorrhizal Tree Castanopsis sclerophylla (Fagaceae) in Fragmented Forests in Eastern China. Plants 2023, 12, 2946. https://doi.org/10.3390/plants12162946
Liu J, Zhou M, Li X, Li T, Jiang H, Zhao L, Chen S, Tian J, Han W. Phosphorus Addition Reduces Seedling Growth and Survival for the Arbuscular Mycorrhizal Tree Cinnamomum camphora (Lauraceae) and Ectomycorrhizal Tree Castanopsis sclerophylla (Fagaceae) in Fragmented Forests in Eastern China. Plants. 2023; 12(16):2946. https://doi.org/10.3390/plants12162946
Chicago/Turabian StyleLiu, Jinliang, Mengsi Zhou, Xue Li, Tianxiang Li, Haoyue Jiang, Luping Zhao, Shuman Chen, Jingying Tian, and Wenjuan Han. 2023. "Phosphorus Addition Reduces Seedling Growth and Survival for the Arbuscular Mycorrhizal Tree Cinnamomum camphora (Lauraceae) and Ectomycorrhizal Tree Castanopsis sclerophylla (Fagaceae) in Fragmented Forests in Eastern China" Plants 12, no. 16: 2946. https://doi.org/10.3390/plants12162946
APA StyleLiu, J., Zhou, M., Li, X., Li, T., Jiang, H., Zhao, L., Chen, S., Tian, J., & Han, W. (2023). Phosphorus Addition Reduces Seedling Growth and Survival for the Arbuscular Mycorrhizal Tree Cinnamomum camphora (Lauraceae) and Ectomycorrhizal Tree Castanopsis sclerophylla (Fagaceae) in Fragmented Forests in Eastern China. Plants, 12(16), 2946. https://doi.org/10.3390/plants12162946






