Variation in the Drought Tolerance of Tropical Understory Plant Communities across an Extreme Elevation and Precipitation Gradient
Abstract
:1. Introduction
2. Methods
2.1. Vegetation Sampling
2.2. Statistical Analysis
3. Results
3.1. Floristic composition
3.2. Drought Tolerance Metrics
4. Discussion
4.1. Are the Three Metrics of Drought Tolerance Coordinated or Independent of Each Other?
4.2. How Are Drought Tolerance Metrics Related to Rainfall and Elevation across the Gradient?
4.3. Solute Leakage (SL) Is a Potentially Efficient and Effective Tool for Measuring Plant Drought Tolerances in the Field
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Von Humboldt, A.; Bonpland, A. Essai sur la Géographie des Plantes: Accompagne d’un Tableau Physique des Régions Équinoxiales, Fondé sur des Mesures Exécutées, Depuis le Dixième Degré la Latitude Boréale Juasqu’au Dixième Degré de Latitude Australe, Pendant les Années 1799, 1800, 1801, 1802 et 1803; Chez Levrault, Schoell et Cie: Paris, France, 1805. [Google Scholar]
- Whittaker, R.H.; Niering, W.A. Vegetation of the Santa Catalina Mountains, Arizona: A gradient analysis of the south slope. Ecology 1965, 46, 429–452. [Google Scholar] [CrossRef]
- Clark, D.B.; Palmer, M.W.; Clark, D.A. Edaphic factors and the landscape-scale distributions of tropical rain forest trees. Ecology 1999, 80, 2662–2675. [Google Scholar] [CrossRef]
- Harms, K.E.; Condit, R.; Hubbell, S.P.; Foster, R.B. Habitat associations of trees and shrubs in a 50-ha neotropical forest plot. J. Ecol. 2001, 89, 947–959. [Google Scholar] [CrossRef]
- Zuleta, D.; Russo, S.E.; Barona, A.; Barreto-Silva, J.S.; Cardenas, D.; Castaño, N.; Davies, S.J.; Detto, M.; Sua, S.; Turner, B.L. Importance of topography for tree species habitat distributions in a terra firme forest in the Colombian Amazon. Plant Soil 2020, 450, 133–149. [Google Scholar] [CrossRef]
- Valencia, R.; Foster, R.B.; Villa, G.; Condit, R.; Svenning, J.-C.; Hernandez, C.; Romoleroux, K.; Losos, E.; Magard, E.; Balslev, H. Tree species distributions and local habitat variation in the Amazon: Large forest plot in eastern Ecuador. J. Ecol. 2004, 92, 214–229. [Google Scholar] [CrossRef]
- Salinas, N.; Malhi, Y.; Meir, P.; Silman, M.; Roman Cuesta, R.; Huaman, J.; Salinas, D.; Huaman, V.; Gibaja, A.; Mamani, M. The sensitivity of tropical leaf litter decomposition to temperature: Results from a large-scale leaf translocation experiment along an elevation gradient in Peruvian forests. New Phytol. 2011, 189, 967–977. [Google Scholar] [CrossRef]
- Malhi, Y.; Girardin, C.A.; Goldsmith, G.R.; Doughty, C.E.; Salinas, N.; Metcalfe, D.B.; Huaraca Huasco, W.; Silva-Espejo, J.E.; del Aguilla-Pasquell, J.; Farfán Amézquita, F. The variation of productivity and its allocation along a tropical elevation gradient: A whole carbon budget perspective. New Phytol. 2017, 214, 1019–1032. [Google Scholar] [CrossRef]
- Feeley, K.J.; Silman, M.R.; Bush, M.B.; Farfan, W.; Cabrera, K.G.; Malhi, Y.; Meir, P.; Revilla, N.S.; Quisiyupanqui, M.N.R.; Saatchi, S. Upslope migration of Andean trees. J. Biogeogr. 2011, 38, 783–791. [Google Scholar] [CrossRef]
- Feeley, K.J.; Hurtado, J.; Saatchi, S.; Silman, M.R.; Clark, D.B. Compositional shifts in Costa Rican forests due to climate-driven species migrations. Glob. Chang. Biol. 2013, 19, 3472–3480. [Google Scholar] [CrossRef]
- Duque, A.; Stevenson, P.; Feeley, K.J. Thermophilization of adult and juvenile tree communities in the northern tropical Andes. Proc. Natl. Acad. Sci. USA 2015, 112, 10744–10749. [Google Scholar] [CrossRef]
- Girardin, C.A.J.; Malhi, Y.; Aragao, L.; Mamani, M.; Huaraca Huasco, W.; Durand, L.; Feeley, K.; Rapp, J.; Silva-Espejo, J.; Silman, M. Net primary productivity allocation and cycling of carbon along a tropical forest elevational transect in the Peruvian Andes. Glob. Chang. Biol. 2010, 16, 3176–3192. [Google Scholar] [CrossRef]
- Rapp, J.; Silman, M. Diurnal, seasonal, and altitudinal trends in microclimate across a tropical montane cloud forest. Clim. Res. 2012, 55, 17–32. [Google Scholar] [CrossRef]
- Malizia, A.; Blundo, C.; Carilla, J.; Osinaga Acosta, O.; Cuesta, F.; Duque, A.; Aguirre, N.; Aguirre, Z.; Ataroff, M.; Baez, S.; et al. Elevation and latitude drives structure and tree species composition in Andean forests: Results from a large-scale plot network. PLoS ONE 2020, 15, e0231553. [Google Scholar] [CrossRef]
- Homeier, J.; Breckle, S.W.; Günter, S.; Rollenbeck, R.T.; Leuschner, C. Tree diversity, forest structure and productivity along altitudinal and topographical gradients in a species-rich Ecuadorian montane rain forest. Biotropica 2010, 42, 140–148. [Google Scholar] [CrossRef]
- Báez, S.; Malizia, A.; Carilla, J.; Blundo, C.; Aguilar, M.; Aguirre, N.; Aquirre, Z.; Álvarez, E.; Cuesta, F.; Duque, Á.; et al. Large-scale patterns of turnover and basal area change in Andean forests. PLoS ONE 2015, 10, e0126594. [Google Scholar] [CrossRef]
- Quiroga, M.P.; Pacheco, S.; Malizia, L.R.; Premoli, A.C. Shrinking forests under warming: Evidence of Podocarpus parlatorei (pino del cerro) from the subtropical Andes. J. Hered. 2012, 103, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Krömer, T.; Kessler, M.; Gradstein, S.R. Vertical stratification of vascular epiphytes in submontane and montane forest of the Bolivian Andes: The importance of the understory. Plant Ecol. 2007, 189, 261–278. [Google Scholar] [CrossRef]
- Fadrique, B.; Báez, S.; Duque, Á.; Malizia, A.; Blundo, C.; Carilla, J.; Osinaga-Acosta, O.; Malizia, L.; Silman, M.; Farfán-Ríos, W.; et al. Widespread but heterogeneous responses of Andean forests to climate change. Nature 2018, 564, 207–212. [Google Scholar] [CrossRef]
- Feeley, K.J.; Silman, M.R. Land-use and climate change effects on population size and extinction risk of Andean plants. Glob. Chang. Biol. 2010, 16, 3215–3222. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Araujo, M.B.; Sykes, M.T.; Prentice, I.C. Climate change threats to plant diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef]
- Crimmins, S.M.; Dobrowski, S.Z.; Greenberg, J.A.; Abatzoglou, J.T.; Mynsberge, A.R. Changes in climatic water balance drive downhill shifts in plant species’ optimum elevations. Science 2011, 331, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.E.; Goulden, M.L. Rapid shifts in plant distribution with recent climate change. Proc. Natl. Acad. Sci. USA 2008, 105, 11823–11826. [Google Scholar] [CrossRef] [PubMed]
- Wieczynski, D.J.; Boyle, B.; Buzzard, V.; Duran, S.M.; Henderson, A.N.; Hulshof, C.M.; Kerkhoff, A.J.; McCarthy, M.C.; Michaletz, S.T.; Swenson, N.G. Climate shapes and shifts functional biodiversity in forests worldwide. Proc. Natl. Acad. Sci. USA 2019, 116, 587–592. [Google Scholar] [CrossRef]
- Feeley, K.J.; Davies, S.J.; Perez, R.; Hubbell, S.P.; Foster, R.B. Directional changes in the species composition of a tropical forest. Ecology 2011, 92, 871–882. [Google Scholar] [CrossRef]
- Esquivel-Muelbert, A.; Baker, T.R.; Dexter, K.G.; Lewis, S.L.; Brienen, R.J.; Feldpausch, T.R.; Lloyd, J.; Monteagudo-Mendoza, A.; Arroyo, L.; Álvarez-Dávila, E. Compositional response of Amazon forests to climate change. Glob. Chang. Biol. 2019, 25, 39–56. [Google Scholar] [CrossRef]
- Esquivel-Muelbert, A.; Baker, T.R.; Dexter, K.G.; Lewis, S.L.; ter Steege, H.; Lopez-Gonzalez, G.; Monteagudo Mendoza, A.; Brienen, R.; Feldpausch, T.R.; Pitman, N.; et al. Seasonal drought limits tree species across the Neotropics. Ecography 2017, 40, 618–629. [Google Scholar] [CrossRef]
- Feeley, K.J.; Bravo-Avila, C.; Fadrique, B.; Perez, T.M.; Zuleta, D. Climate-driven changes in the composition of New World plant communities. Nat. Clim. Chang. 2020, 10, 965–970. [Google Scholar] [CrossRef]
- Tovar, C.; Arnillas, C.A.; Cuesta, F.; Buytaert, W. Diverging Responses of Tropical Andean Biomes under Future Climate Conditions. PLoS ONE 2013, 8, e63634. [Google Scholar] [CrossRef]
- Vuille, M.; Bradley, R.S.; Werner, M.; Keimig, F. 20th century climate change in the tropical Andes: Observations and model results. In Climate Variability and Change in High Elevation Regions: Past, Present & Future; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003; pp. 75–99. [Google Scholar]
- Urrutia, R.; Vuille, M. Climate change projections for the tropical Andes using a regional climate model: Temperature and precipitation simulations for the end of the 21st century. J. Geophys. Res. 2009, 114. [Google Scholar] [CrossRef]
- Nepstad, D.C.; Moutinho, P.; Dias-Filho, M.B.; Davidson, E.; Cardinot, G.; Markewitz, D.; Figueiredo, R.; Vianna, N.; Chambers, J.; Ray, D.; et al. The effects of partial throughfall exclusion on canopy processes, aboveground production, and biogeochemistry of an Amazon forest. J. Geophys. Res. Atmos. 2002, 107, LBA 53-1–LBA 53-18. [Google Scholar] [CrossRef]
- Lloyd, J.; Farquhar, G.D. Effects of rising temperatures and [CO2] on the physiology of tropical forest trees. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1811–1817. [Google Scholar] [CrossRef] [PubMed]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.W.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef] [PubMed]
- Bruijnzeel, L.; Mulligan, M.; Scatena, F.N. Hydrometeorology of tropical montane cloud forests: Emerging patterns. Hydrol. Process. 2011, 25, 465–498. [Google Scholar] [CrossRef]
- Martin, P.H.; Sherman, R.E.; Fahey, T.J. Tropical montane forest ecotones: Climate gradients, natural disturbance, and vegetation zonation in the Cordillera Central, Dominican Republic. J. Biogeogr. 2007, 34, 1792–1806. [Google Scholar] [CrossRef]
- Santiago, L.S.; Schuur, E.A.; Silvera, K. Nutrient cycling and plant–soil feedbacks along a precipitation gradient in lowland Panama. J. Trop. Ecol. 2005, 21, 461–470. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Whitaker, J.; Turner, B.L.; Salinas, N.; Zimmermann, M.; Malhi, Y.; Meir, P. Climate warming and soil carbon in tropical forests: Insights from an elevation gradient in the Peruvian Andes. Bioscience 2015, 65, 906–921. [Google Scholar] [CrossRef]
- Gotsch, S.G.; Dawson, T.E.; Draguljić, D. Variation in the resilience of cloud forest vascular epiphytes to severe drought. New Phytol. 2018, 219, 900–913. [Google Scholar] [CrossRef]
- Horwath, A.B.; Royles, J.; Tito, R.; Gudiño, J.A.; Salazar Allen, N.; Farfan-Rios, W.; Rapp, J.M.; Silman, M.R.; Malhi, Y.; Swamy, V. Bryophyte stable isotope composition, diversity and biomass define tropical montane cloud forest extent. Proc. R. Soc. B 2019, 286, 20182284. [Google Scholar] [CrossRef]
- Zimmermann, M.; Meir, P.; Silman, M.; Fedders, A.; Gibbon, A.; Malhi, Y.; Urrego, D.; Bush, M.; Feeley, K.; Garcia, K.; et al. No differences in soil carbon stocks across the tree line in the Peruvian Andes. Ecosystems 2010, 13, 62–74. [Google Scholar] [CrossRef]
- Rehm, E.M.; Feeley, K.J. Forest patches and the upward migration of timberline in the southern Peruvian Andes. For. Ecol. Manag. 2013, 305, 204–211. [Google Scholar] [CrossRef]
- Venturas, M.D.; Sperry, J.S.; Hacke, U.G. Plant xylem hydraulics: What we understand, current research, and future challenges. J. Integr. Plant Biol. 2017, 59, 356–389. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, M.K.; Klein, T.; Jansen, S.; Choat, B.; Sack, L. The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. Proc. Natl. Acad. Sci. USA 2016, 113, 13098–13103. [Google Scholar] [CrossRef]
- Su, R.; Liu, H.; Wang, C.; Zhang, H.; Cui, J. Leaf turgor loss point is one of the best predictors of drought-induced tree mortality in tropical forest. Front. Ecol. Evol. 2022, 10. [Google Scholar] [CrossRef]
- Kerstiens, G. Cuticular water permeability and its physiological significance. J. Exp. Bot. 1996, 47, 1813–1832. [Google Scholar] [CrossRef]
- Slot, M.; Nardwattanawong, T.; Hernández, G.G.; Bueno, A.; Riederer, M.; Winter, K. Large differences in leaf cuticle conductance and its temperature response among 24 tropical tree species from across a rainfall gradient. New Phytol. 2021, 232, 1618–1631. [Google Scholar] [CrossRef]
- Dzialak, M.R.; Lacki, M.J.; Larkin, J.L.; Carter, K.M.; Vorisek, S. Corridors affect dispersal initiation in reintroduced peregrine falcons. Anim. Conserv. 2005, 8, 421. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; López, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef]
- Volaire, F. A unified framework of plant adaptive strategies to drought: Crossing scales and disciplines. Glob. Chang. Biol. 2018, 24, 2929–2938. [Google Scholar] [CrossRef] [PubMed]
- Agarie, S.; Hanaoka, N.; Kubota, F.; Agata, W.; Kaufman, P.B. Measurement of cell membrane stability evaluated by electrolyte leakage as a drought and heat tolerance test in rice (Oryza sativa L.). J. Fac. Agric. Kyushu Univ. 1995, 40, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat 1. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Premachandra, G.S.; Shimada, T. The measurement of cell membrane stability using polyethylene glycol as a drought tolerance test in wheat. Jpn. J. Crop Sci. 1987, 56, 92–98. [Google Scholar] [CrossRef]
- Premachandra, G.S.; Saneoka, H.; Ogata, S. Cell membrane stability, an indicator of drought tolerance, as affected by applied nitrogen in soyabean. J. Agric. Sci. 1990, 115, 63–66. [Google Scholar] [CrossRef]
- George, R.; Sujatha, K.B. Screening of chilli genotypes for drought tolerance. J. Agric. Ecol. 2019, 8, 38–45. [Google Scholar] [CrossRef]
- Fadrique, B.; Baraloto, C.; Bravo-Avila, C.H.; Feeley, K.J. Bamboo climatic tolerances are decoupled from functional traits across an Andean elevation gradient. Oikos 2022, 2022, e09229. [Google Scholar] [CrossRef]
- Zuleta, D.; Muller-Landau, H.C.; Duque, A.; Caro, N.; Cardenas, D.; Castaño, N.; León-Peláez, J.D.; Feeley, K.J. Interspecific and intraspecific variation of tree branch, leaf and stomatal traits in relation to topography in an aseasonal Amazon forest. Funct. Ecol. 2022, 36, 2955–2968. [Google Scholar] [CrossRef]
- Santiago, L.S.; De Guzman, M.E.; Baraloto, C.; Vogenberg, J.E.; Brodie, M.; Hérault, B.; Fortunel, C.; Bonal, D. Coordination and trade-offs among hydraulic safety, efficiency and drought avoidance traits in Amazonian rainforest canopy tree species. New Phytol. 2018, 218, 1015–1024. [Google Scholar] [CrossRef]
- Killeen, T.J.; Douglas, M.; Consiglio, T.; Jørgensen, P.M.; Mejia, J. Dry spots and wet spots in the Andean hotspot. J. Biogeogr. 2007, 34, 1357–1373. [Google Scholar] [CrossRef]
- Lenz, T.I.; Wright, I.J.; Westoby, M. Interrelations among pressure–volume curve traits across species and water availability gradients. Physiol. Plant. 2006, 127, 423–433. [Google Scholar] [CrossRef]
- Pearcy, R.W.; Schulze, E.-D.; Zimmermann, R. Measurement of transpiration and leaf conductance. In Plant Physiological Ecology; Springer: New York, NY, USA, 2000; pp. 137–160. [Google Scholar]
- Bajji, M.; Kinet, J.-M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Whitlow, T.H.; Bassuk, N.L.; Ranney, T.G.; Reichert, D.L. An improved method for using electrolyte leakage to assess membrane competence in plant tissues. Plant Physiol. 1992, 98, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Premachandra, G.; Saneoka, H.; Ogata, S. Nutrio-physiological evaluation of the polyethylene glycol test of cell membrane stability in maize. Crop Sci. 1989, 29, 1287–1292. [Google Scholar] [CrossRef]
- França, M.G.C.; Thi, A.T.P.; Pimentel, C.; Rossiello, R.O.P.; Zuily-Fodil, Y.; Laffray, D. Differences in growth and water relations among Phaseolus vulgaris cultivars in response to induced drought stress. Environ. Exp. Bot. 2000, 43, 227–237. [Google Scholar] [CrossRef]
- Saneoka, H.; Moghaieb, R.E.; Premachandra, G.S.; Fujita, K. Nitrogen nutrition and water stress effects on cell membrane stability and leaf water relations in Agrostis palustris Huds. Environ. Exp. Bot. 2004, 52, 131–138. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Pivovaroff, A.L.; Cook, V.M.W.; Santiago, L.S. Stomatal behaviour and stem xylem traits are coordinated for woody plant species under exceptional drought conditions. Plant Cell Environ. 2018, 41, 2617–2626. [Google Scholar] [CrossRef]
- Guillemot, J.; Martin-StPaul, N.K.; Bulascoschi, L.; Poorter, L.; Morin, X.; Pinho, B.X.; le Maire, G.; Bittencourt, P.R.L.; Oliveira, R.S.; Bongers, F.; et al. Small and slow is safe: On the drought tolerance of tropical tree species. Glob. Chang. Biol. 2022, 28, 2622–2638. [Google Scholar] [CrossRef]
- Tobin, M.F.; Lopez, O.R.; Kursar, T.A. Responses of Tropical Understory Plants to a Severe Drought: Tolerance and Avoidance of Water Stress 1. Biotropica 1999, 31, 570–578. [Google Scholar] [CrossRef]
- Jane, G.; Green, T. Patterns of stomatal conductance in six evergreen tree species from a New Zealand cloud forest. Bot. Gaz. 1985, 146, 413–420. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Scoffoni, C.; Ardy, R.; Zhang, Y.; Sun, S.; Cao, K.; Sack, L. Rapid determination of comparative drought tolerance traits: Using an osmometer to predict turgor loss point. Methods Ecol. Evol. 2012, 3, 880–888. [Google Scholar] [CrossRef]
- Cavelier, J. Tissue water relations in elfin cloud forest tree species of Serrania de Macuira, Guajira, Colombia. Trees 1990, 4, 155–163. [Google Scholar] [CrossRef]
- Fetcher, N.; Oberbauer, S.F.; Strain, B.R. Vegetation effects on microclimate in lowland tropical forest in Costa Rica. Int. J. Biometeorol. 1985, 29, 145–155. [Google Scholar] [CrossRef]
- Brum, M.; Vadeboncoeur, M.; Asbjornsen, H.; Puma Vilca, B.L.; Galiano, D.; Horwath, A.B.; Metcalfe, D.B. Ecophysiological controls on water use of tropical cloud forest trees in response to experimental drought. Tree Physiol. 2023. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Eller, C.B.; Bittencourt, P.R.L.; Mulligan, M. The hydroclimatic and ecophysiological basis of cloud forest distributions under current and projected climates. Ann. Bot. 2014, 113, 909–920. [Google Scholar] [CrossRef]
- Goldsmith, G.R.; Matzke, N.J.; Dawson, T.E. The incidence and implications of clouds for cloud forest plant water relations. Ecol. Lett. 2013, 16, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Schreel, J.D.M.; Steppe, K. Foliar water uptake changes the world of tree hydraulics. npj Clim. Atmos. Sci. 2019, 2, 1. [Google Scholar] [CrossRef]
- Eller, C.B.; Lima, A.L.; Oliveira, R.S. Foliar uptake of fog water and transport belowground alleviates drought effects in the cloud forest tree species, Drimys brasiliensis (Winteraceae). New Phytol. 2013, 199, 151–162. [Google Scholar] [CrossRef]
- Berry, Z.C.; White, J.C.; Smith, W.K. Foliar uptake, carbon fluxes and water status are affected by the timing of daily fog in saplings from a threatened cloud forest. Tree Physiol. 2014, 34, 459–470. [Google Scholar] [CrossRef]
- Eller, C.; Lima, A.; Oliveira, R. Cloud forest trees with higher foliar water uptake capacity and anisohydric behavior are more vulnerable to drought and climate change. New Phytol. 2016, 211, 489–501. [Google Scholar] [CrossRef]
- Premachandra, G.S.; Saneoka, H.; Fujita, K.; Ogata, S. Leaf water relations, osmotic adjustment, cell membrane stability, epicuticular wax load and growth as affected by increasing water deficits in sorghum. J. Exp. Bot. 1992, 43, 1569–1576. [Google Scholar] [CrossRef]
- Yang, G.; Rhodes, D.; Joly, R. Effects of High Temperature on Membrane Stability and Chlorophyll Fluorescence in Glycinebetaine-Deficient and Glycinebetaine-Containing Maize Lines. Funct. Plant Biol. 1996, 23, 437–443. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Haylock, M.R.; Peterson, T.C.; Alves, L.M.; Ambrizzi, T.; Anunciação, Y.M.T.; Baez, J.; Barros, V.R.; Berlato, M.A.; Bidegain, M.; Coronel, G.; et al. Trends in Total and Extreme South American Rainfall in 1960–2000 and Links with Sea Surface Temperature. J. Clim. 2006, 19, 1490–1512. [Google Scholar] [CrossRef]
- Halladay, K.; Malhi, Y.; New, M. Cloud frequency climatology at the Andes/Amazon transition: 2. Trends and variability. J. Geophys. Res. Atmos. 2012, 117. [Google Scholar] [CrossRef]
- Draper, F.C.; Costa, F.R.C.; Arellano, G.; Phillips, O.L.; Duque, A.; Macía, M.J.; ter Steege, H.; Asner, G.P.; Berenguer, E.; Schietti, J.; et al. Amazon tree dominance across forest strata. Nat. Ecol. Evol. 2021, 5, 757–767. [Google Scholar] [CrossRef] [PubMed]
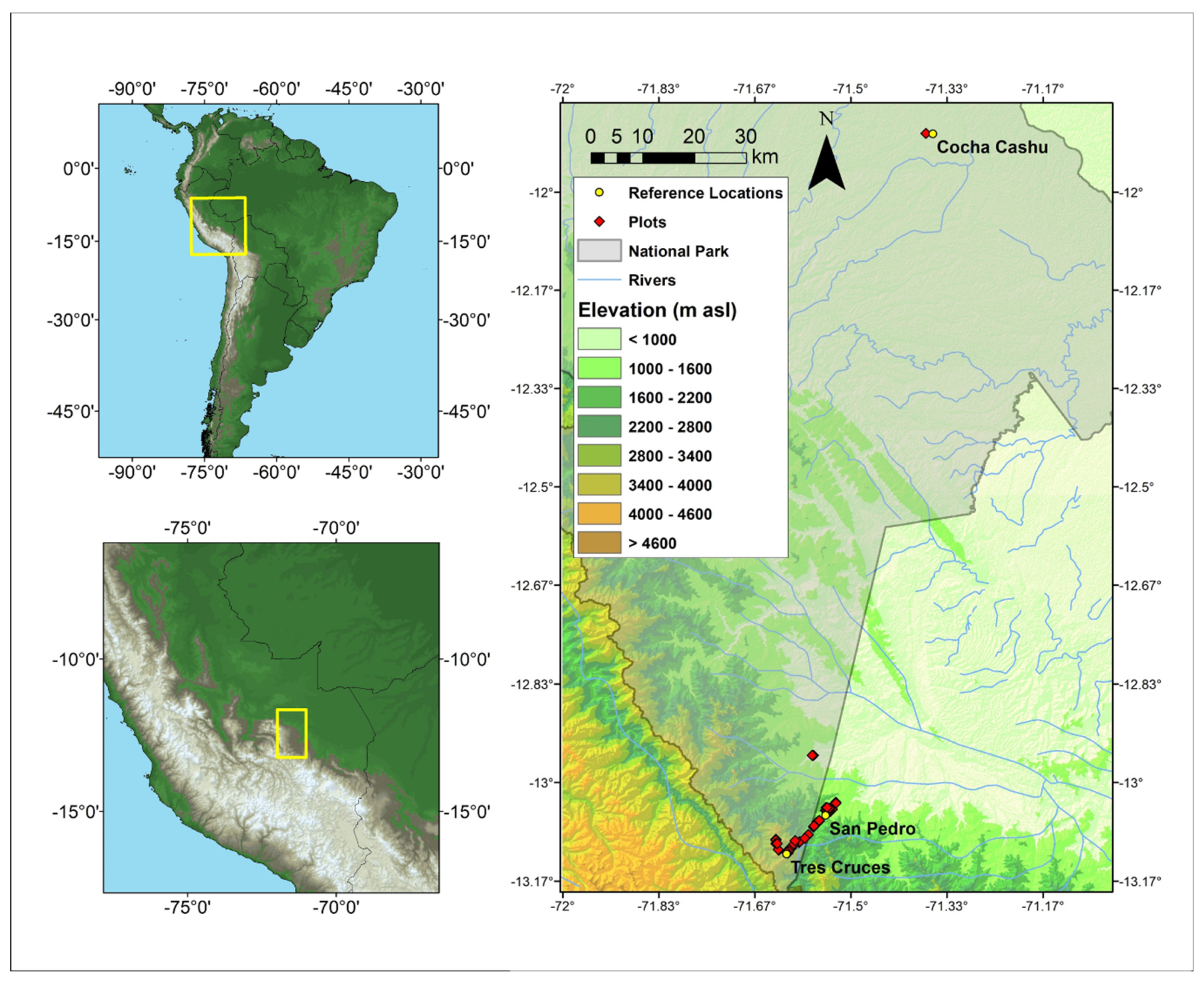
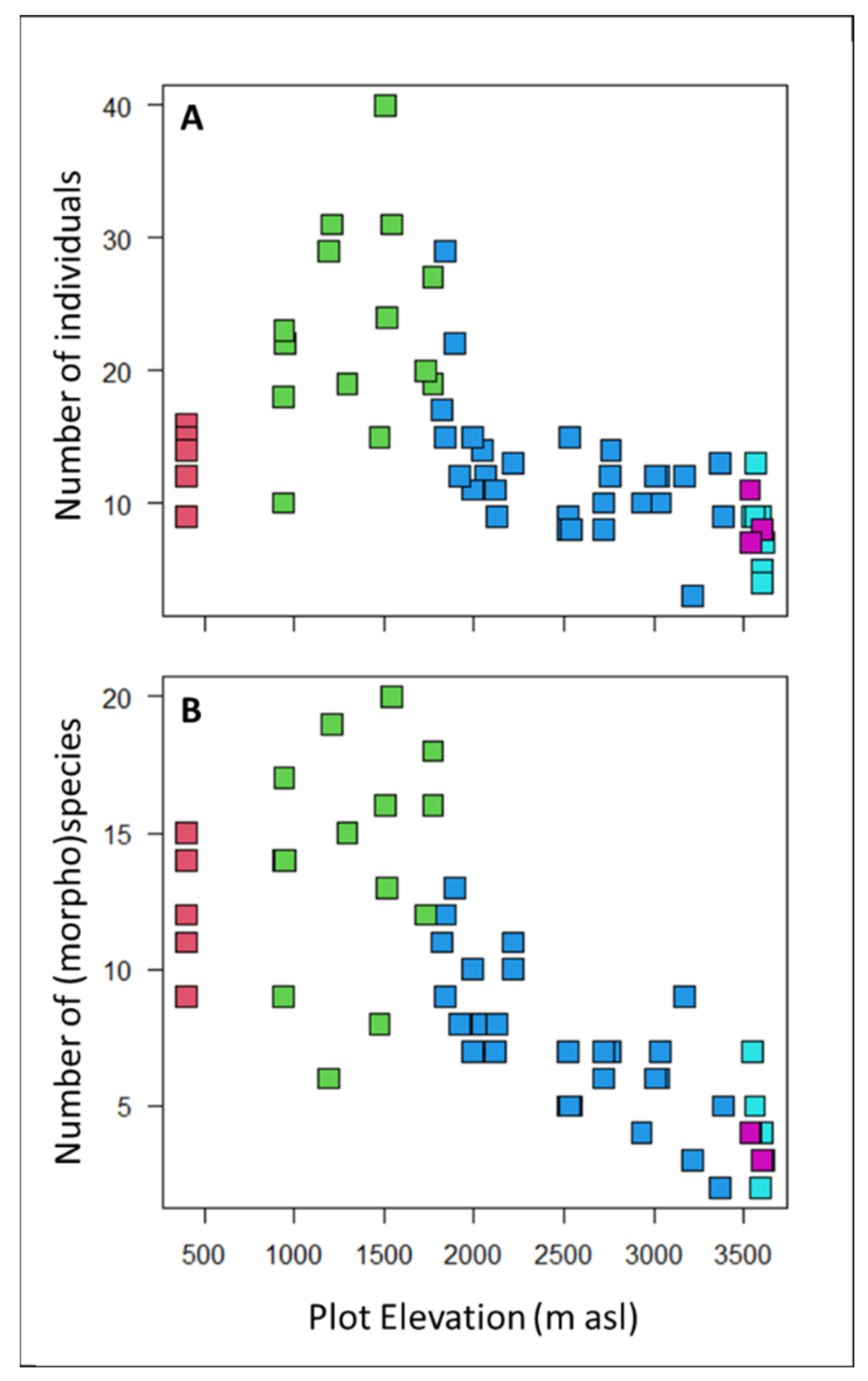
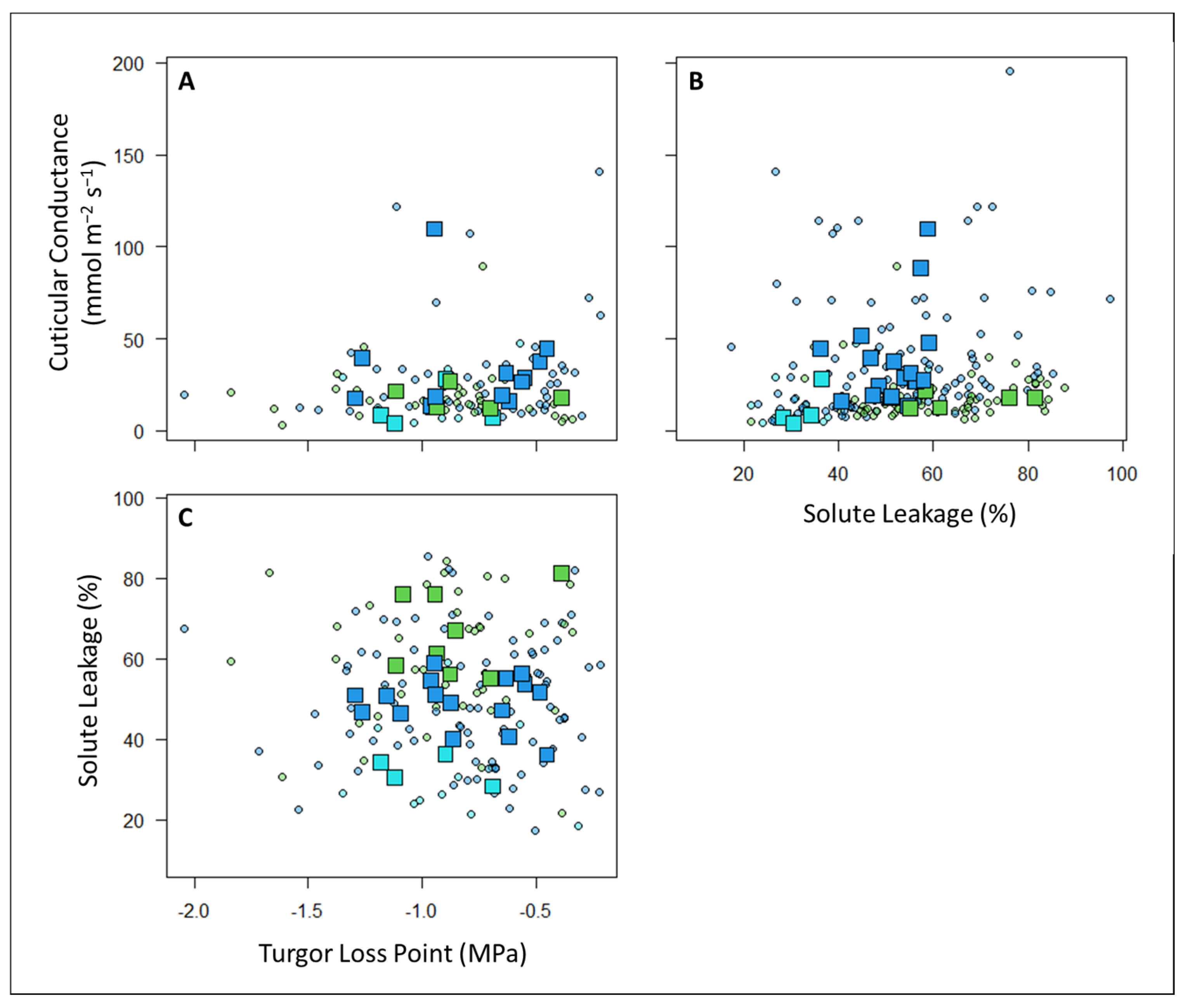
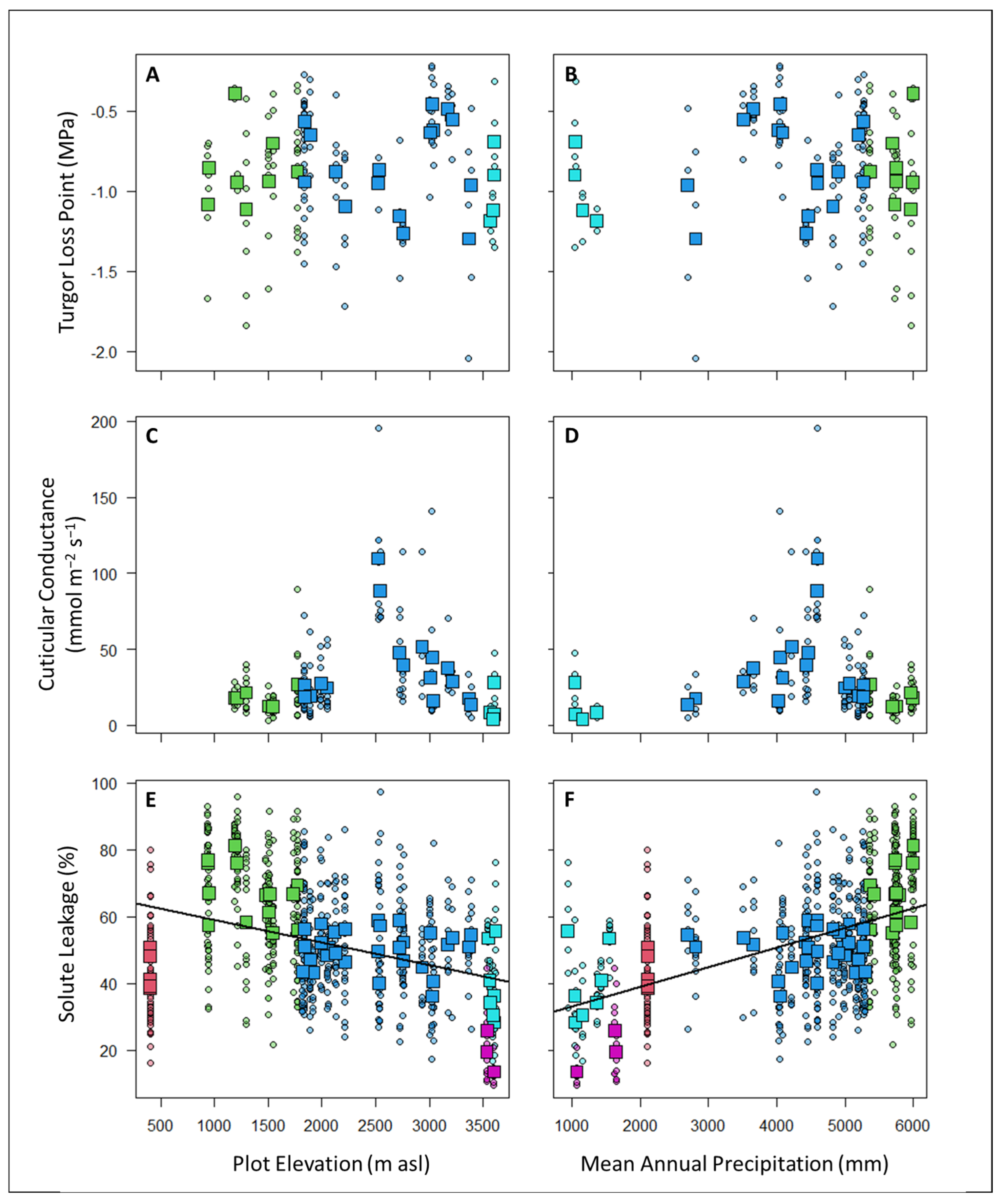
| Plot | Latitude (°) | Longitude (°) | Elevation (m asl) | MAT (°C) | MAP (mm/Year) | Habitat | No. of Individuals | No. of Shrubs | No. of Saplings | No. of Families | No. of Genera | No. of (Morpho) Species |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | −13.096 | −71.630 | 3604 | 8.2 | 1617 | gallery forest | 5 | 3 | 2 | 3 | 4 | 4 |
| 2 | −13.097 | −71.630 | 3569 | 8.2 | 1437 | gallery forest | 13 | 5 | 8 | 3 | 3 | 4 |
| 3 | −13.103 | −71.630 | 3603 | 8.0 | 1054 | gallery forest | 4 | 3 | 1 | 2 | 2 | 4 |
| 4 | −13.103 | −71.629 | 3592 | 18.9 | 1157 | gallery forest | 9 | 0 | 9 | 1 | 2 | 2 |
| 5 | −13.114 | −71.625 | 3548 | 18.9 | 1549 | gallery forest | 9 | 5 | 4 | 2 | 1 | 7 |
| 6 | −13.113 | −71.626 | 3562 | 10.1 | 1515 | gallery forest | 9 | 3 | 6 | 3 | 2 | 5 |
| 7 | −13.103 | −71.628 | 3615 | 12.5 | 937 | gallery forest | 7 | 1 | 6 | 3 | 2 | 3 |
| 8 | −13.110 | −71.603 | 3172 | 18.7 | 3654 | cloud forest | 12 | 7 | 5 | 4 | 5 | 9 |
| 9 | −13.110 | −71.604 | 3214 | 18.7 | 3507 | cloud forest | 3 | 3 | 0 | 2 | 3 | 3 |
| 10 | −13.114 | −71.607 | 3366 | 17.7 | 2812 | cloud forest | 13 | 9 | 4 | 2 | 2 | 2 |
| 11 | −13.112 | −71.607 | 3387 | 17.7 | 2692 | cloud forest | 9 | 1 | 8 | 4 | 4 | 5 |
| 12 | −13.033 | −71.526 | 1189 | 10.1 | 5992 | sub-montane forest | 29 | 21 | 8 | 5 | 5 | 6 |
| 13 | −13.035 | −71.526 | 1212 | 17.7 | 5990 | sub-montane forest | 31 | 16 | 15 | 5 | 5 | 19 |
| 14 | −13.045 | −71.532 | 1296 | 20.2 | 5989 | sub-montane forest | 19 | 7 | 12 | 10 | 9 | 15 |
| 15 | −13.047 | −71.544 | 1771 | 19.7 | 5363 | sub-montane forest | 27 | 9 | 18 | 6 | 8 | 18 |
| 16 | −13.047 | −71.542 | 1837 | 17.7 | 5266 | cloud forest | 29 | 6 | 23 | 5 | 6 | 12 |
| 17 | −13.049 | −71.536 | 1503 | 20.1 | 5744 | sub-montane forest | 40 | 8 | 32 | 6 | 9 | 16 |
| 18 | −13.048 | −71.537 | 1545 | 8.0 | 5694 | sub-montane forest | 31 | 19 | 12 | 7 | 8 | 20 |
| 19 | −13.119 | −71.609 | 3601 | 10.1 | 1082 | puna shrubs | 8 | 8 | 0 | 0 | 0 | 3 |
| 20 | −13.116 | −71.608 | 3539 | 12.5 | 1082 | puna shrubs | 7 | 7 | 0 | 0 | 0 | 4 |
| 21 | −13.118 | −71.611 | 3536 | 10.1 | 1082 | puna shrubs | 11 | 11 | 0 | 0 | 0 | 4 |
| 22 | −13.101 | −71.590 | 2754 | 13.3 | 4428 | cloud forest | 12 | 5 | 7 | 4 | 3 | 7 |
| 23 | −13.101 | −71.590 | 2762 | 13.6 | 4421 | cloud forest | 14 | 5 | 9 | 7 | 6 | 7 |
| 24 | −13.100 | −71.589 | 2721 | 11.2 | 4457 | cloud forest | 10 | 5 | 5 | 5 | 5 | 6 |
| 25 | −13.100 | −71.589 | 2724 | 12.5 | 4455 | cloud forest | 8 | 4 | 4 | 5 | 4 | 7 |
| 26 | −13.089 | −71.575 | 2525 | 14.4 | 4595 | cloud forest | 9 | 6 | 3 | 4 | 4 | 7 |
| 27 | −13.089 | −71.575 | 2526 | 14.4 | 4594 | cloud forest | 8 | 4 | 4 | 2 | 1 | 5 |
| 28 | −13.088 | −71.574 | 2542 | 13.6 | 4584 | cloud forest | 8 | 1 | 7 | 4 | 1 | 5 |
| 29 | −13.094 | −71.580 | 2532 | 17.7 | 4590 | cloud forest | 15 | 12 | 3 | 3 | 4 | 5 |
| 30 | −13.104 | −71.599 | 3036 | 22.1 | 4021 | cloud forest | 10 | 7 | 3 | 3 | 2 | 7 |
| 31 | −13.104 | −71.599 | 3025 | 14.5 | 4045 | cloud forest | 12 | 8 | 4 | 3 | 4 | 6 |
| 32 | −13.104 | −71.599 | 3006 | 21.8 | 4084 | cloud forest | 12 | 1 | 11 | 3 | 3 | 6 |
| 33 | −13.098 | −71.597 | 2933 | 17.7 | 4213 | cloud forest | 10 | 4 | 6 | 3 | 3 | 4 |
| 34 | −13.070 | −71.560 | 2064 | 8.7 | 4973 | cloud forest | 12 | 3 | 9 | 6 | 7 | 8 |
| 35 | −13.070 | −71.560 | 2048 | 9.2 | 4991 | cloud forest | 14 | 4 | 10 | 7 | 7 | 8 |
| 36 | −13.068 | −71.559 | 1994 | 8.2 | 5056 | cloud forest | 11 | 6 | 5 | 6 | 6 | 7 |
| 37 | −13.067 | −71.559 | 1992 | 8.2 | 5059 | cloud forest | 15 | 3 | 12 | 6 | 8 | 10 |
| 38 | −13.065 | −71.556 | 1891 | 10.1 | 5191 | cloud forest | 22 | 8 | 14 | 8 | 9 | 13 |
| 39 | −13.066 | −71.556 | 1920 | 8.2 | 5152 | cloud forest | 12 | 7 | 5 | 6 | 6 | 8 |
| 40 | −13.065 | −71.555 | 1839 | 13.3 | 5265 | cloud forest | 15 | 1 | 14 | 7 | 9 | 9 |
| 41 | −13.064 | −71.555 | 1820 | 14.4 | 5292 | cloud forest | 17 | 8 | 9 | 4 | 6 | 11 |
| 42 | −13.074 | −71.565 | 2217 | 17.7 | 4789 | cloud forest | 13 | 6 | 7 | 7 | 6 | 10 |
| 43 | −13.075 | −71.565 | 2121 | 17.7 | 4789 | cloud forest | 11 | 1 | 10 | 5 | 5 | 7 |
| 44 | −13.074 | −71.565 | 2217 | 18.7 | 4789 | cloud forest | 13 | 10 | 3 | 4 | 5 | 11 |
| 45 | −13.073 | −71.564 | 2130 | 18.7 | 4789 | cloud forest | 9 | 3 | 6 | 7 | 7 | 8 |
| 46 | −13.044 | −71.536 | 1474 | 17.7 | 5754 | sub-montane forest | 15 | 2 | 13 | 5 | 6 | 8 |
| 47 | −13.043 | −71.537 | 1514 | 17.7 | 5754 | sub-montane forest | 24 | 8 | 16 | 10 | 10 | 13 |
| 48 | −13.042 | −71.543 | 1772 | 18.9 | 5395 | sub-montane forest | 19 | 11 | 8 | 13 | 12 | 16 |
| 49 | −13.042 | −71.541 | 1732 | 18.9 | 5395 | sub-montane forest | 20 | 2 | 18 | 7 | 11 | 12 |
| 50 | −12.954 | −71.565 | 937 | 17.7 | 5849 | sub-montane | 18 | 4 | 14 | 9 | 10 | 14 |
| 51 | −12.954 | −71.566 | 938 | 18.9 | 5849 | sub-montane forest | 10 | 6 | 4 | 6 | 6 | 9 |
| 52 | −12.954 | −71.567 | 946 | 22.1 | 5849 | sub-montane forest | 22 | 13 | 9 | 7 | 8 | 14 |
| 53 | −12.954 | −71.567 | 944 | 21.8 | 5849 | sub-montane forest | 23 | 13 | 10 | 8 | 11 | 17 |
| 54 | −11.900 | −71.370 | 400 | 25.0 | 1366 | lowland forest | 9 | 0 | 9 | 0 | 0 | 9 |
| 55 | −11.900 | −71.370 | 400 | 25.0 | 1366 | lowland forest | 16 | 0 | 16 | 11 | 11 | 11 |
| 56 | −11.900 | −71.370 | 400 | 25.0 | 1366 | lowland forest | 15 | 0 | 15 | 0 | 0 | 15 |
| 57 | −11.900 | −71.370 | 400 | 25.0 | 1366 | lowland forest | 14 | 0 | 14 | 0 | 0 | 14 |
| 58 | −11.900 | −71.370 | 400 | 25.0 | 1366 | lowland forest | 12 | 0 | 12 | 11 | 12 | 12 |
| Plot | SL (%) | SL_se | TLP (MPa) | TLP_se | Gmin (mmol m−2 s−1) | Gmin_se |
|---|---|---|---|---|---|---|
| 1 | 36.40 | 6.74 | −0.95 | 0.15 | 26.43 | 5.00 |
| 2 | 34.26 | 2.30 | −1.20 | 0.02 | 8.87 | 2.04 |
| 3 | 28.37 | 4.07 | −0.69 | 0.12 | 7.06 | 0.22 |
| 4 | 30.47 | 3.77 | −1.04 | 0.04 | 4.18 | 0.00 |
| 5 | 53.59 | 1.90 | NA | NA | NA | NA |
| 6 | 41.06 | 1.28 | NA | NA | NA | NA |
| 7 | 55.77 | 6.78 | NA | NA | NA | NA |
| 8 | 51.76 | 4.23 | −0.44 | 0.04 | 33.88 | 6.53 |
| 9 | 53.79 | 20.95 | −0.55 | 0.10 | 28.86 | 3.21 |
| 10 | 50.96 | 2.07 | −1.42 | 0.31 | 21.11 | 4.92 |
| 11 | 54.59 | 4.64 | −0.96 | 0.22 | 12.73 | 1.65 |
| 12 | 81.36 | 1.17 | −0.36 | 0.01 | 23.20 | 2.61 |
| 13 | 76.05 | 2.06 | −0.94 | 0.03 | 22.48 | 1.99 |
| 14 | 58.32 | 4.62 | −0.96 | 0.15 | 26.02 | 3.02 |
| 15 | 56.19 | 3.21 | −0.93 | 0.09 | 20.46 | 4.26 |
| 16 | 56.33 | 2.65 | −0.51 | 0.03 | 17.98 | 3.37 |
| 17 | 61.42 | 2.02 | −0.89 | 0.06 | 11.60 | 0.84 |
| 18 | 55.12 | 2.16 | −0.65 | 0.07 | 11.97 | 1.10 |
| 19 | 13.62 | 1.24 | NA | NA | NA | NA |
| 20 | 26.02 | 4.38 | NA | NA | NA | NA |
| 21 | 19.50 | 2.62 | NA | NA | NA | NA |
| 22 | 46.82 | 2.68 | −1.28 | 0.02 | 35.85 | 7.35 |
| 23 | 52.47 | 3.55 | NA | NA | NA | NA |
| 24 | 50.92 | 5.19 | −1.16 | 0.07 | NA | NA |
| 25 | 59.04 | 5.72 | NA | NA | 47.17 | 8.20 |
| 26 | 58.89 | 6.56 | −0.96 | 0.04 | 99.11 | 14.28 |
| 27 | 49.76 | 6.76 | NA | NA | NA | NA |
| 28 | 57.37 | 8.18 | NA | NA | 96.76 | 8.34 |
| 29 | 40.11 | 2.95 | −0.87 | 0.00 | NA | NA |
| 30 | 40.69 | 5.38 | −0.62 | 0.06 | 15.63 | 2.25 |
| 31 | 36.21 | 3.47 | −0.37 | 0.07 | 74.28 | 22.50 |
| 32 | 55.23 | 3.52 | −0.60 | 0.06 | 31.21 | 0.00 |
| 33 | 44.89 | 2.93 | NA | NA | 57.21 | 19.77 |
| 34 | 50.80 | 4.98 | NA | NA | NA | NA |
| 35 | 48.40 | 2.65 | NA | NA | 21.30 | 5.44 |
| 36 | 52.36 | 3.51 | NA | NA | NA | NA |
| 37 | 57.97 | 3.21 | NA | NA | 23.95 | 3.87 |
| 38 | 47.25 | 2.53 | −0.70 | 0.06 | 17.71 | 3.38 |
| 39 | 43.25 | 2.94 | NA | NA | NA | NA |
| 40 | 51.18 | 4.49 | −0.96 | 0.09 | 17.76 | 2.39 |
| 41 | 43.68 | 2.71 | NA | NA | NA | NA |
| 42 | 46.59 | 4.25 | −1.02 | 0.09 | NA | NA |
| 43 | 55.56 | 3.60 | NA | NA | NA | NA |
| 44 | 56.27 | 4.06 | NA | NA | NA | NA |
| 45 | 49.21 | 4.06 | −0.81 | 0.11 | NA | NA |
| 46 | 66.56 | 3.82 | NA | NA | NA | NA |
| 47 | 66.67 | 3.39 | NA | NA | NA | NA |
| 48 | 69.45 | 3.56 | NA | NA | NA | NA |
| 49 | 66.79 | 4.22 | NA | NA | NA | NA |
| 50 | 75.95 | 3.47 | −1.01 | 0.11 | NA | NA |
| 51 | 76.98 | 3.78 | NA | NA | NA | NA |
| 52 | 66.99 | 4.14 | −0.84 | 0.03 | NA | NA |
| 53 | 57.47 | 3.80 | NA | NA | NA | NA |
| 54 | 46.46 | 5.40 | NA | NA | NA | NA |
| 55 | 38.61 | 3.47 | NA | NA | NA | NA |
| 56 | 48.30 | 3.35 | NA | NA | NA | NA |
| 57 | 41.27 | 3.77 | NA | NA | NA | NA |
| 58 | 39.19 | 3.52 | NA | NA | NA | NA |
| TLP | Gmin | SL | |
|---|---|---|---|
| TLP | 0.067 | 0.092 | |
| Gmin | 0.130 | 0.174 | |
| SL | −0.043 | 0.139 |
| Habitat | N. Plots | TLP | Gmin | SL |
|---|---|---|---|---|
| Lowland Forest | 4 | NA | NA | 42.77 (4.37) |
| Sub-Montane Forest | 5 | −0.82 (0.22) | 19.29 (6.08) | 66.81 (8.45) |
| Cloud Forest | 29 | −0.83 (0.31) | 38.38 (27.64) | 50.43 (5.93) |
| High-Elevation Gallery Forest | 7 | −0.97 (0.21) | 11.64 (10.05) | 39.99 (10.85) |
| High-Elevation Shrubs | 3 | NA | NA | 19.71 (6.21) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo-Avila, C.H.; Feeley, K.J. Variation in the Drought Tolerance of Tropical Understory Plant Communities across an Extreme Elevation and Precipitation Gradient. Plants 2023, 12, 2957. https://doi.org/10.3390/plants12162957
Bravo-Avila CH, Feeley KJ. Variation in the Drought Tolerance of Tropical Understory Plant Communities across an Extreme Elevation and Precipitation Gradient. Plants. 2023; 12(16):2957. https://doi.org/10.3390/plants12162957
Chicago/Turabian StyleBravo-Avila, Catherine H., and Kenneth J. Feeley. 2023. "Variation in the Drought Tolerance of Tropical Understory Plant Communities across an Extreme Elevation and Precipitation Gradient" Plants 12, no. 16: 2957. https://doi.org/10.3390/plants12162957
APA StyleBravo-Avila, C. H., & Feeley, K. J. (2023). Variation in the Drought Tolerance of Tropical Understory Plant Communities across an Extreme Elevation and Precipitation Gradient. Plants, 12(16), 2957. https://doi.org/10.3390/plants12162957







