Genome-Wide Identification and Analysis of Collar Region-Preferential Genes in Rice
Abstract
:1. Introduction
2. Results
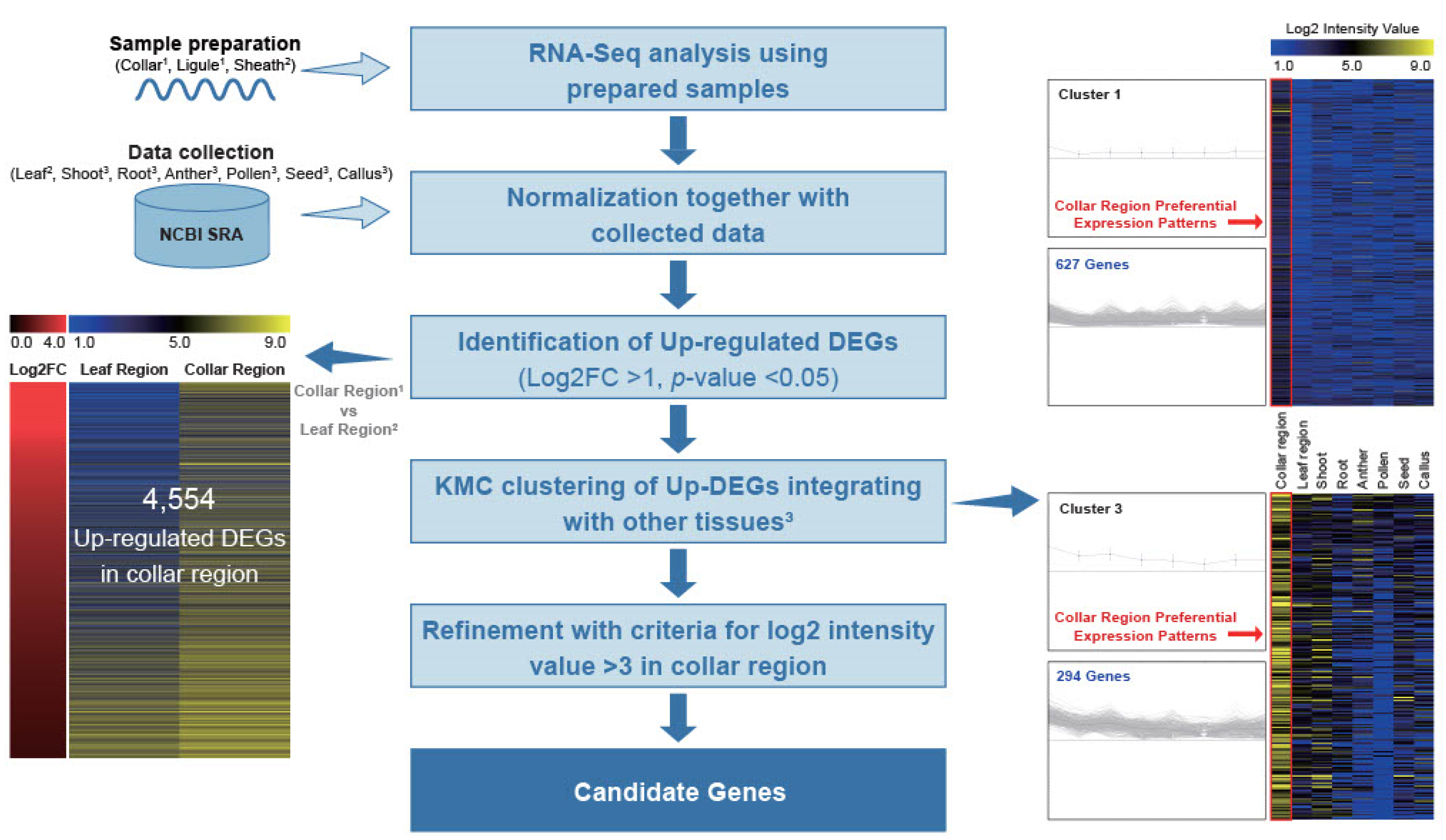
2.1. Genome-Wide Identification of Collar Region-Preferential Genes
2.2. Authentication of Tissue-Preferential Expression Patterns of Six CRPGs
2.3. Literature Analysis of Functionally Characterized CRPGs
2.4. Functional Enrichment Analysis and Classification of CRPGs
2.5. Case Study Using the lg1 Mutant Revealed Downstream Regulatory Elements
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. RNA-Seq Analysis and Data Collection
4.3. Identification of Candidates Coupled with Heatmap Analysis
4.4. Literature Analysis
4.5. Enrichment Analysis Via GO, KEGG, and MapMan
4.6. RNA Extraction and qRT-PCR
4.7. Motif Scanning
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812. [Google Scholar] [CrossRef]
- Roychowdhury, R. Crop Improvement in the Era of Climate Change, 1st ed.; I.K. International Publishing: New Delhi, India, 2014; ISBN 978-93-82332-61-9. [Google Scholar]
- Hasanuzzaman, M.; Roychowdhury, R.; Karmakar, J.; Dey, N.; Nahar, K.; Fujita, M. Recent advances in biotechnology and genomic approaches for abiotic stress tolerance in crop plants. In Genomics and Proteomics; Apple Academic Press: New York, NY, USA, 2015; pp. 333–366. ISBN 978-1-77188-114-2. [Google Scholar]
- Sakamoto, T.; Morinaka, Y.; Ohnishi, T.; Sunohara, H.; Fujioka, S.; Ueguchi-Tanaka, M.; Mizutani, M.; Sakata, K.; Takatsuto, S.; Yoshida, S.; et al. Erect Leaves Caused by Brassinosteroid Deficiency Increase Biomass Production and Grain Yield in Rice. Nat. Biotechnol. 2006, 24, 105–109. [Google Scholar] [CrossRef]
- Hoshikawa, K. The Growing Rice Plant. An Anatomical Monograph; Nosan Gyoson Bunka: Tokyo, Japan, 1989; pp. 199–205. [Google Scholar]
- Donald, C.M. The Breeding of Crop Ideotypes. Euphytica 1968, 17, 385–403. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Sheehy, J.E. Erect Leaves and Photosynthesis in Rice. Science 1999, 283, 1455. [Google Scholar] [CrossRef]
- Zhang, S.-W.; Li, C.-H.; Cao, J.; Zhang, Y.-C.; Zhang, S.-Q.; Xia, Y.-F.; Sun, D.-Y.; Sun, Y. Altered Architecture and Enhanced Drought Tolerance in Rice via the Down-Regulation of Indole-3-Acetic Acid by TLD1/OsGH3.13 Activation. Plant Physiol. 2009, 151, 1889–1901. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Ou, X.; Tang, H.; Wang, R.; Wu, P.; Jia, Y.; Wei, X.; Xu, X.; Kang, S.-H.; Kim, S.-K.; et al. Rice MicroRNA Osa-MiR1848 Targets the Obtusifoliol 14α-Demethylase Gene OsCYP51G3 and Mediates the Biosynthesis of Phytosterols and Brassinosteroids during Development and in Response to Stress. New Phytol. 2015, 208, 790–802. [Google Scholar] [CrossRef]
- Zhou, L.-J.; Xiao, L.-T.; Xue, H.-W. Dynamic Cytology and Transcriptional Regulation of Rice Lamina Joint Development. Plant Physiol. 2017, 174, 1728–1746. [Google Scholar] [CrossRef]
- Wang, R.; Liu, C.; Li, Q.; Chen, Z.; Sun, S.; Wang, X. Spatiotemporal Resolved Leaf Angle Establishment Improves Rice Grain Yield via Controlling Population Density. iScience 2020, 23, 101489. [Google Scholar] [CrossRef]
- Zhao, S.-Q.; Hu, J.; Guo, L.-B.; Qian, Q.; Xue, H.-W. Rice Leaf Inclination2, a VIN3-like Protein, Regulates Leaf Angle through Modulating Cell Division of the Collar. Cell Res. 2010, 20, 935–947. [Google Scholar] [CrossRef]
- Ning, J.; Zhang, B.; Wang, N.; Zhou, Y.; Xiong, L. Increased Leaf Angle1, a Raf-like MAPKKK That Interacts with a Nuclear Protein Family, Regulates Mechanical Tissue Formation in the Lamina Joint of Rice. Plant Cell 2011, 23, 4334–4347. [Google Scholar] [CrossRef]
- Sun, S.; Chen, D.; Li, X.; Qiao, S.; Shi, C.; Li, C.; Shen, H.; Wang, X. Brassinosteroid Signaling Regulates Leaf Erectness in Oryza Sativa via the Control of a Specific U-Type Cyclin and Cell Proliferation. Dev. Cell 2015, 34, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-Y.; Bai, M.-Y.; Wu, J.; Zhu, J.-Y.; Wang, H.; Zhang, Z.; Wang, W.; Sun, Y.; Zhao, J.; Sun, X.; et al. Antagonistic HLH/BHLH Transcription Factors Mediate Brassinosteroid Regulation of Cell Elongation and Plant Development in Rice and Arabidopsis. Plant Cell 2009, 21, 3767–3780. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Ito, M.; Sumikura, T.; Nakayama, A.; Nishimura, T.; Kitano, H.; Yamaguchi, I.; Koshiba, T.; Hibara, K.-I.; Nagato, Y.; et al. The Rice FISH BONE Gene Encodes a Tryptophan Aminotransferase, Which Affects Pleiotropic Auxin-Related Processes. Plant J. 2014, 78, 927–936. [Google Scholar] [CrossRef]
- Du, H.; Wu, N.; Fu, J.; Wang, S.; Li, X.; Xiao, J.; Xiong, L. A GH3 Family Member, OsGH3-2, Modulates Auxin and Abscisic Acid Levels and Differentially Affects Drought and Cold Tolerance in Rice. J. Exp. Bot. 2012, 63, 6467–6480. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; You, J.; Xiong, L. Characterization of OsIAA1 Gene, a Member of Rice Aux/IAA Family Involved in Auxin and Brassinosteroid Hormone Responses and Plant Morphogenesis. Plant Mol. Biol. 2009, 70, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, S.; Xu, Y.; Yu, C.; Shen, C.; Qian, Q.; Geisler, M.; Jiang, D.A.; Qi, Y. The Auxin Response Factor, OsARF19, Controls Rice Leaf Angles through Positively Regulating OsGH3-5 and OsBRI1. Plant Cell Environ. 2015, 38, 638–654. [Google Scholar] [CrossRef]
- Yamamuro, C.; Ihara, Y.; Wu, X.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Ashikari, M.; Kitano, H.; Matsuoka, M. Loss of Function of a Rice Brassinosteroid Insensitive1 Homolog Prevents Internode Elongation and Bending of the Lamina Joint. Plant Cell 2000, 12, 1591–1606. [Google Scholar] [CrossRef]
- Li, H.; Jiang, L.; Youn, J.-H.; Sun, W.; Cheng, Z.; Jin, T.; Ma, X.; Guo, X.; Wang, J.; Zhang, X.; et al. A Comprehensive Genetic Study Reveals a Crucial Role of CYP90D2/D2 in Regulating Plant Architecture in Rice (Oryza Sativa). New Phytol. 2013, 200, 1076–1088. [Google Scholar] [CrossRef]
- Liu, X.; Yang, C.Y.; Miao, R.; Zhou, C.L.; Cao, P.H.; Lan, J.; Zhu, X.J.; Mou, C.L.; Huang, Y.S.; Liu, S.J.; et al. DS1/OsEMF1 Interacts with OsARF11 to Control Rice Architecture by Regulation of Brassinosteroid Signaling. Rice 2018, 11, 46. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Xu, Y.; Joo, S.-H.; Kim, S.-K.; Xue, Z.; Xu, Z.; Wang, Z.; Chong, K. OsGSR1 Is Involved in Crosstalk between Gibberellins and Brassinosteroids in Rice. Plant J. 2009, 57, 498–510. [Google Scholar] [CrossRef]
- Han, K.-S.; Ko, K.-W.; Nam, S.-J.; Park, S.-H.; Kim, S.-K. Optimization of a Rice Lamina Inclination Assay for Detection of Brassinosteroids: I. Effect of Phytohormones on the Inclination Activity. J. Plant Biol. 1997, 40, 240–244. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.-J.; Kim, S.L.; Yim, J.; An, G. Mutations in the Rice Liguleless Gene Result in a Complete Loss of the Auricle, Ligule, and Laminar Joint. Plant Mol. Biol. 2007, 65, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, C.; Chen, Z.; Sun, S.; Wang, X. Oryza Sativa LIGULELESS 2s Determine Lamina Joint Positioning and Differentiation by Inhibiting Auxin Signaling. New Phytol. 2021, 229, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; An, G.; Li, H.-Y. Rice Leaf Angle and Grain Size Are Affected by the OsBUL1 Transcriptional Activator Complex. Plant Physiol. 2017, 173, 688–702. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.-J.; Kim, Y.-J.; Kim, E.-J.; Kumar Nalini Chandran, A.; Moon, S.; Gho, Y.-S.; Yoou, M.-H.; Kim, S.T.; Jung, K.-H. CAFRI-Rice: CRISPR Applicable Functional Redundancy Inspector to Accelerate Functional Genomics in Rice. Plant J. 2020, 104, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Jiang, Y.; Chen, T.; Li, H.; Fu, M.; Wang, Y.; Xu, Y.; Li, Y.; Zhou, Z.; Jia, L.; et al. New Data and New Features of the FunRiceGenes (Functionally Characterized Rice Genes) Database: 2021 Update. Rice 2022, 15, 23. [Google Scholar] [CrossRef]
- Yamamoto, E.; Yonemaru, J.-I.; Yamamoto, T.; Yano, M. OGRO: The Overview of Functionally Characterized Genes in Rice Online Database. Rice 2012, 5, 26. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Cao, X.; Song, X. Epigenetic Mutation of RAV6 Affects Leaf Angle and Seed Size in Rice. Plant Physiol. 2015, 169, 2118–2128. [Google Scholar] [CrossRef]
- Huang, J.; Tang, D.; Shen, Y.; Qin, B.; Hong, L.; You, A.; Li, M.; Wang, X.; Yu, H.; Gu, M.; et al. Activation of Gibberellin 2-Oxidase 6 Decreases Active Gibberellin Levels and Creates a Dominant Semi-Dwarf Phenotype in Rice (Oryza Sativa L.). J. Genet. Genom. 2010, 37, 23–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, C.; Lin, J.; Liu, J.; Liu, B.; Wang, J.; Huang, A.; Li, H.; Zhao, T. OsMPH1 Regulates Plant Height and Improves Grain Yield in Rice. PLoS ONE 2017, 12, e0180825. [Google Scholar] [CrossRef]
- Tseng, I.-C.; Hong, C.-Y.; Yu, S.-M.; Ho, T.-H.D. Abscisic Acid- and Stress-Induced Highly Proline-Rich Glycoproteins Regulate Root Growth in Rice. Plant Physiol. 2013, 163, 118–134. [Google Scholar] [CrossRef]
- Wu, X.; Shiroto, Y.; Kishitani, S.; Ito, Y.; Toriyama, K. Enhanced Heat and Drought Tolerance in Transgenic Rice Seedlings Overexpressing OsWRKY11 under the Control of HSP101 Promoter. Plant Cell Rep. 2009, 28, 21–30. [Google Scholar] [CrossRef]
- Lee, H.; Cha, J.; Choi, C.; Choi, N.; Ji, H.-S.; Park, S.R.; Lee, S.; Hwang, D.-J. Rice WRKY11 Plays a Role in Pathogen Defense and Drought Tolerance. Rice 2018, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, X.; Yin, D.; Yuan, H.; Xie, Q.; Zhao, X.; Li, X.; Zhu, L.; Li, S.; Li, D. Constitutive Expression of OsDof4, Encoding a C2-C2 Zinc Finger Transcription Factor, Confesses Its Distinct Flowering Effects under Long- and Short-Day Photoperiods in Rice (Oryza Sativa L.). BMC Plant Biol. 2017, 17, 166. [Google Scholar] [CrossRef]
- Taniguchi, S.; Hosokawa-Shinonaga, Y.; Tamaoki, D.; Yamada, S.; Akimitsu, K.; Gomi, K. Jasmonate Induction of the Monoterpene Linalool Confers Resistance to Rice Bacterial Blight and Its Biosynthesis Is Regulated by JAZ Protein in Rice. Plant Cell Environ. 2014, 37, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Qin, F.; Huang, L.; Sun, Q.; Li, C.; Zhao, Y.; Zhou, D.-X. Rice Histone Deacetylase Genes Display Specific Expression Patterns and Developmental Functions. Biochem. Biophys. Res. Commun. 2009, 388, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Gomi, K.; Satoh, M.; Ozawa, R.; Shinonaga, Y.; Sanada, S.; Sasaki, K.; Matsumura, M.; Ohashi, Y.; Kanno, H.; Akimitsu, K.; et al. Role of Hydroperoxide Lyase in White-Backed Planthopper (Sogatella Furcifera Horváth)-Induced Resistance to Bacterial Blight in Rice, Oryza Sativa L. Plant J. 2010, 61, 46–57. [Google Scholar] [CrossRef]
- Han, M.; Kim, C.-Y.; Lee, J.; Lee, S.-K.; Jeon, J.-S. OsWRKY42 Represses OsMT1d and Induces Reactive Oxygen Species and Leaf Senescence in Rice. Mol. Cells 2014, 37, 532–539. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, H.; Deng, Y.; Xiao, J.; Li, X.; Wang, S. The WRKY45-2 WRKY13 WRKY42 Transcriptional Regulatory Cascade Is Required for Rice Resistance to Fungal Pathogen. Plant Physiol. 2015, 167, 1087–1099. [Google Scholar] [CrossRef]
- Haga, K.; Takano, M.; Neumann, R.; Iino, M. The Rice COLEOPTILE PHOTOTROPISM1 Gene Encoding an Ortholog of Arabidopsis NPH3 Is Required for Phototropism of Coleoptiles and Lateral Translocation of Auxin. Plant Cell 2005, 17, 103–115. [Google Scholar] [CrossRef]
- Lo, S.-F.; Yang, S.-Y.; Chen, K.-T.; Hsing, Y.-I.; Zeevaart, J.A.D.; Chen, L.-J.; Yu, S.-M. A Novel Class of Gibberellin 2-Oxidases Control Semidwarfism, Tillering, and Root Development in Rice. Plant Cell 2008, 20, 2603–2618. [Google Scholar] [CrossRef]
- Huang, X.; Duan, M.; Liao, J.; Yuan, X.; Chen, H.; Feng, J.; Huang, J.; Zhang, H.-S. OsSLI1, a Homeodomain Containing Transcription Activator, Involves Abscisic Acid Related Stress Response in Rice (Oryza sativa L.). Sci. World J. 2014, 2014, 809353. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Sharma, R.; Jain, M. Over-Expression of OsHOX24 Confers Enhanced Susceptibility to Abiotic Stresses in Transgenic Rice via Modulating Stress-Responsive Gene Expression. Front. Plant Sci. 2017, 8, 628. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.; Qi, M.; Sheng, G.; Yang, Y. Inducible Overexpression of a Rice Allene Oxide Synthase Gene Increases the Endogenous Jasmonic Acid Level, PR Gene Expression, and Host Resistance to Fungal Infection. MPMI 2006, 19, 1127–1137. [Google Scholar] [CrossRef]
- Lin, Z.; Li, X.; Shannon, L.M.; Yeh, C.-T.; Wang, M.L.; Bai, G.; Peng, Z.; Li, J.; Trick, H.N.; Clemente, T.E.; et al. Parallel Domestication of the Shattering1 Genes in Cereals. Nat. Genet. 2012, 44, 720–724. [Google Scholar] [CrossRef]
- Ray, S.; Kapoor, S.; Tyagi, A.K. Analysis of Transcriptional and Upstream Regulatory Sequence Activity of Two Environmental Stress-Inducible Genes, NBS-Str1 and BLEC-Str8, of Rice. Transgenic Res. 2012, 21, 351–366. [Google Scholar] [CrossRef]
- Shang, X.-L.; Xie, R.-R.; Tian, H.; Wang, Q.-L.; Guo, F.-Q. Putative Zeatin O-Glucosyltransferase OscZOG1 Regulates Root and Shoot Development and Formation of Agronomic Traits in Rice. J. Integr. Plant Biol. 2016, 58, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Q.; Meng, X.-P.; Zhang, Y.; Xia, M.; Wang, X.-P. Over-Expression of OsDREB Genes Lead to Enhanced Drought Tolerance in Rice. Biotechnol. Lett. 2008, 30, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Yamaji, N.; Mitani-Ueno, N.; Kashino, M.; Ma, J.F. A Node-Localized Transporter OsZIP3 Is Responsible for the Preferential Distribution of Zn to Developing Tissues in Rice. Plant J. 2015, 84, 374–384. [Google Scholar] [CrossRef]
- Ishii, T.; Numaguchi, K.; Miura, K.; Yoshida, K.; Thanh, P.T.; Htun, T.M.; Yamasaki, M.; Komeda, N.; Matsumoto, T.; Terauchi, R.; et al. OsLG1 Regulates a Closed Panicle Trait in Domesticated Rice. Nat. Genet. 2013, 45, 462–465. [Google Scholar] [CrossRef]
- Qi, J.; Li, J.; Han, X.; Li, R.; Wu, J.; Yu, H.; Hu, L.; Xiao, Y.; Lu, J.; Lou, Y. Jasmonic Acid Carboxyl Methyltransferase Regulates Development and Herbivory-Induced Defense Response in Rice. J. Integr. Plant Biol. 2016, 58, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Katsura, K.; Maruyama, K.; Taji, T.; Kobayashi, M.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional Analysis of Rice DREB1/CBF-Type Transcription Factors Involved in Cold-Responsive Gene Expression in Transgenic Rice. Plant Cell Physiol. 2006, 47, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Yu, Y.; Chen, Q.; Mu, G.; Shen, Z.; Zheng, L. OsMYB45 Plays an Important Role in Rice Resistance to Cadmium Stress. Plant Sci. 2017, 264, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-G.; Lee, C.-Y.; Tseng, C.-S. Heterologous Expression of Rice 9-Cis-Epoxycarotenoid Dioxygenase 4 (OsNCED4) in Arabidopsis Confers Sugar Oversensitivity and Drought Tolerance. Bot. Stud. 2018, 59, 2. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qian, Q.; Liu, Z.; Sun, H.; He, S.; Luo, D.; Xia, G.; Chu, C.; Li, J.; Fu, X. Natural Variation at the DEP1 Locus Enhances Grain Yield in Rice. Nat. Genet. 2009, 41, 494–497. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, J.; Li, Z.; Yi, C.; Liu, J.; Zhang, H.; Tang, S.; Gu, M.; Liang, G. Deletion in a Quantitative Trait Gene QPE9-1 Associated With Panicle Erectness Improves Plant Architecture During Rice Domestication. Genetics 2009, 183, 315–324. [Google Scholar] [CrossRef]
- Qi, W.; Sun, F.; Wang, Q.; Chen, M.; Huang, Y.; Feng, Y.-Q.; Luo, X.; Yang, J. Rice Ethylene-Response AP2/ERF Factor OsEATB Restricts Internode Elongation by Down-Regulating a Gibberellin Biosynthetic Gene. Plant Physiol. 2011, 157, 216–228. [Google Scholar] [CrossRef]
- Xu, Y.-X.; Xiao, M.-Z.; Liu, Y.; Fu, J.-L.; He, Y.; Jiang, D.-A. The Small Auxin-up RNA OsSAUR45 Affects Auxin Synthesis and Transport in Rice. Plant Mol. Biol. 2017, 94, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Nakamura, H.; Ichikawa, H.; Miyao, A.; Hirochika, H.; Kobayashi, K.; Yamaoka, N.; Nishiguchi, M. Response of an Aspartic Protease Gene OsAP77 to Fungal, Bacterial and Viral Infections in Rice. Rice 2014, 7, 9. [Google Scholar] [CrossRef]
- Wang, H.; Hao, J.; Chen, X.; Hao, Z.; Wang, X.; Lou, Y.; Peng, Y.; Guo, Z. Overexpression of Rice WRKY89 Enhances Ultraviolet B Tolerance and Disease Resistance in Rice Plants. Plant Mol. Biol. 2007, 65, 799–815. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Qian, Q.; Fu, Z.; Wang, M.; Zeng, D.; Li, B.; Wang, X.; Li, J. LAZY1 Controls Rice Shoot Gravitropism through Regulating Polar Auxin Transport. Cell Res. 2007, 17, 402–410. [Google Scholar] [CrossRef]
- Kusano, H.; Asano, T.; Shimada, H.; Kadowaki, K. Molecular Characterization of ONAC300, a Novel NAC Gene Specifically Expressed at Early Stages in Various Developing Tissues of Rice. Mol. Genet. Genom. 2005, 272, 616–626. [Google Scholar] [CrossRef]
- Cao, P.; Jung, K.-H.; Choi, D.; Hwang, D.; Zhu, J.; Ronald, P.C. The Rice Oligonucleotide Array Database: An Atlas of Rice Gene Expression. Rice 2012, 5, 17. [Google Scholar] [CrossRef]
- Wasternack, C.; Feussner, I. The Oxylipin Pathways: Biochemistry and Function. Annu. Rev. Plant Biol. 2018, 69, 363–386. [Google Scholar] [CrossRef]
- Farmer, E.E.; Goossens, A. Jasmonates: What ALLENE OXIDE SYNTHASE Does for Plants. J. Exp. Bot. 2019, 70, 3373–3378. [Google Scholar] [CrossRef]
- Kropat, J.; Tottey, S.; Birkenbihl, R.P.; Depège, N.; Huijser, P.; Merchant, S. A Regulator of Nutritional Copper Signaling in Chlamydomonas Is an SBP Domain Protein That Recognizes the GTAC Core of Copper Response Element. Proc. Natl. Acad. Sci. USA 2005, 102, 18730–18735. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Cao, J.; Yu, K.; Liu, X.; Gao, Y.; Chen, Q.; Zhang, W.; Peng, H.; Du, J.; Xin, M.; et al. Wheat TaSPL8 Modulates Leaf Angle Through Auxin and Brassinosteroid Signaling. Plant Physiol. 2019, 181, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.A.; Harper, L.C.; Krueger, R.W.; Dellaporta, S.L.; Freeling, M. Liguleless1 Encodes a Nuclear-Localized Protein Required for Induction of Ligules and Auricles during Maize Leaf Organogenesis. Genes Dev. 1997, 11, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, J.-J.; Xue, H.-W.; Zhang, G.-H. Leaf Direction: Lamina Joint Development and Environmental Responses. Plant Cell Environ. 2021, 44, 2441–2454. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Wu, H.; Wu, D.; Zhang, Z.; Guo, Z.; Yang, N.; Xia, K.; Zhou, X.; Oh, K.; Matsuoka, M.; et al. Methyl Jasmonate Inhibits Lamina Joint Inclination by Repressing Brassinosteroid Biosynthesis and Signaling in Rice. Plant Sci. 2015, 241, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Yoon, J.; Kim, H.; Lee, S.-J.; Kim, T.; Kang, K.; Paek, N.-C. OsMYB7 Determines Leaf Angle at the Late Developmental Stage of Lamina Joints in Rice. Front. Plant Sci. 2023, 14, 1167202. [Google Scholar] [CrossRef]
- Senapati, M.; Tiwari, A.; Sharma, N.; Chandra, P.; Bashyal, B.M.; Ellur, R.K.; Bhowmick, P.K.; Bollinedi, H.; Vinod, K.K.; Singh, A.K.; et al. Rhizoctonia Solani Kühn Pathophysiology: Status and Prospects of Sheath Blight Disease Management in Rice. Front. Plant Sci. 2022, 13, 881116. [Google Scholar] [CrossRef]
- Hong, W.-J.; Lee, S.K.; Kim, S.-H.; Kim, Y.-J.; Moon, S.; Kim, E.-J.; Silva, J.; Jung, K.-H. Comparative Transcriptome Analysis of Pollen and Anther Wall Reveals Novel Insights into the Regulatory Mechanisms Underlying Anther Wall Development and Its Dehiscence in Rice. Plant Cell Rep. 2022, 41, 1229–1242. [Google Scholar] [CrossRef]
- Howe, E.; Holton, K.; Nair, S.; Schlauch, D.; Sinha, R.; Quackenbush, J. MeV: MultiExperiment Viewer. In Biomedical Informatics for Cancer Research; Ochs, M.F., Casagrande, J.T., Davuluri, R.V., Eds.; Springer: Boston, MA, USA, 2010; pp. 267–277. ISBN 978-1-4419-5714-6. [Google Scholar]
- Hong, W.-J.; Jiang, X.; Choi, S.-H.; Kim, Y.-J.; Kim, S.-T.; Jeon, J.-S.; Jung, K.-H. A systemic view of carbohydrate metabolism in rice to facilitate productivity. Plants 2021, 10, 1690. [Google Scholar] [CrossRef]
- Hong, W.-J.; Jiang, X.; Ahn, H.R.; Choi, J.; Kim, S.-R.; Jung, K.-H. Systematic analysis of cold stress response and diurnal rhythm using transcriptome data in rice reveals the molecular networks related to various biological processes. Int. J. Mol. Sci. 2020, 21, 6872. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Thimm, O.; Bläsing, O.; Gibon, Y.; Nagel, A.; Meyer, S.; Krüger, P.; Selbig, J.; Müller, L.A.; Rhee, S.Y.; Stitt, M. MAPMAN: A User-Driven Tool to Display Genomics Data Sets onto Diagrams of Metabolic Pathways and Other Biological Processes. Plant J. 2004, 37, 914–939. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.S.; Kim, J.K.; Baek, S.-A.; Lee, J.-Y.; Lee, D.; Ha, S.-H. Reciprocal Crosses Between Astaxanthin and Capsanthin Rice Unravel Effects of Metabolic Gene Efficacy in Rice Endosperm. J. Plant Biol. 2021, 64, 371–377. [Google Scholar] [CrossRef]
- Gong, H.; You, J.; Zhang, X.; Liu, Y.; Zhao, F.; Cui, X.; Zhang, Y. Genome-Wide Identification and Functional Analysis of Long Non-Coding RNAs in Sesame Response to Salt Stress. J. Plant Biol. 2021, 64, 555–565. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, M.-H.; Hong, W.-J.; Moon, S.; Kim, E.-J.; Silva, J.; Lee, J.; Lee, S.; Kim, S.T.; Park, S.K.; et al. GORI, Encoding the WD40 Domain Protein, Is Required for Pollen Tube Germination and Elongation in Rice. Plant J. 2021, 105, 1645–1664. [Google Scholar] [CrossRef]
- Castro-Mondragon, J.A.; Riudavets-Puig, R.; Rauluseviciute, I.; Berhanu Lemma, R.; Turchi, L.; Blanc-Mathieu, R.; Lucas, J.; Boddie, P.; Khan, A.; Manosalva Pérez, N.; et al. JASPAR 2022: The 9th Release of the Open-Access Database of Transcription Factor Binding Profiles. Nucleic Acids Res. 2022, 50, D165–D173. [Google Scholar] [CrossRef]
- Rahman, M.; Rahman, M.M.; Eom, J.-S.; Jeon, J.-S. Genome-Wide Identification, Expression Profiling and Promoter Analysis of Trehalose-6-Phosphate Phosphatase Gene Family in Rice. J. Plant Biol. 2020, 64, 55–71. [Google Scholar] [CrossRef]
- Grant, C.E.; Bailey, T.L.; Noble, W.S. FIMO: Scanning for Occurrences of a given Motif. Bioinformatics 2011, 27, 1017–1018. [Google Scholar] [CrossRef] [PubMed]





| Locus | Symbol | Category | Keyword | References |
|---|---|---|---|---|
| LOC_Os01g43650 | OsWRKY11 | R, T | Heat and drought tolerance, Disease resistance | [35,36] |
| LOC_Os01g48290 | OsDof4 | P | Flowering time | [37] |
| LOC_Os02g02930 | OsLIS | R, T | Blast resistance, JA | [38] |
| LOC_Os02g12350 | HDA703 | M | Panicle development, Fertility | [39] |
| LOC_Os02g12680 | OsAOS3|OsHPL2 | R, T | Blight resistance | [40] |
| LOC_Os02g26430 | OsWRKY42 | P, R, T | Leaf senescence, Blast susceptibility, JA | [41,42] |
| LOC_Os02g35970 | CPT1 | M | Root phototropism, Auxin | [43] |
| LOC_Os02g41954 | GA2ox9 | M | Dwarfism, GA | [44] |
| LOC_Os02g43330 | OsHOX24|OsSLI1 | R, T | Abiotic stress response, ABA | [45,46] |
| LOC_Os02g45850 | RAV6 | M | Leaf angle, Seed size, BR | [31] |
| LOC_Os03g12500 | OsAOS2 | R, T | Defense response, JA | [47] |
| LOC_Os03g44710 | OsSh1 | M | Seed shattering | [48] |
| LOC_Os04g01950 | BLEC-Str8 | R, T | Salt stress | [49] |
| LOC_Os04g20330 | OscZOG1 | M | Grain-yielding traits, Cytokinin | [50] |
| LOC_Os04g44150 | OsGA2ox6 | M | Dwarf, GA | [32] |
| LOC_Os04g48350 | OsDREB1E | R, T | Drought tolerance | [51] |
| LOC_Os04g52310 | OsZIP3 | P | Zn distribution | [52] |
| LOC_Os04g56170 | OsLG1 | M | Liguleless, Closed panicle | [25,53] |
| LOC_Os05g01140 | OsJMT1 | R, T | Defense response, JA | [54] |
| LOC_Os06g03670 | OsDREB1C | M, R, T | Abiotic stress tolerance, Growth retardation | [55] |
| LOC_Os06g45890 | OsMPH1|OsMYB45 | M, R, T | Grain yield, Plant height, Cadmium tolerance | [33,56] |
| LOC_Os07g05940 | OsNCED4 | R, T | Drought tolerance, ABA | [57] |
| LOC_Os07g23660 | RePRP2.1 | M | Root cell elongation, ABA | [34] |
| LOC_Os09g26999 | OsDEP1|DN1|qPE9-1 | M | Erect panicle, Dwarf, Grain yield, GA | [58,59] |
| LOC_Os09g28440 | OsEATB | M | Dwarf, Tillering, Panicle branching, Ethylene, GA | [60] |
| LOC_Os09g33580 | OsBC1 | M | Grain size, Leaf angle | [27] |
| LOC_Os09g37400 | OsSAUR45 | M | Plant growth, Auxin | [61] |
| LOC_Os10g39260 | OsAP77 | R, T | Defense response, ABA, SA | [62] |
| LOC_Os11g02520 | OsWRKY89 | R, T | Abiotic and biotic stress, JA | [63] |
| LOC_Os11g29840 | LA1|LAZY1 | M | Tiller angle, Auxin | [64] |
| LOC_Os12g03050 | ONAC300 | M | Shoot apical meristem | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Hong, W.-J.; Lee, S.-K.; Jung, K.-H. Genome-Wide Identification and Analysis of Collar Region-Preferential Genes in Rice. Plants 2023, 12, 2959. https://doi.org/10.3390/plants12162959
Jiang X, Hong W-J, Lee S-K, Jung K-H. Genome-Wide Identification and Analysis of Collar Region-Preferential Genes in Rice. Plants. 2023; 12(16):2959. https://doi.org/10.3390/plants12162959
Chicago/Turabian StyleJiang, Xu, Woo-Jong Hong, Su-Kyoung Lee, and Ki-Hong Jung. 2023. "Genome-Wide Identification and Analysis of Collar Region-Preferential Genes in Rice" Plants 12, no. 16: 2959. https://doi.org/10.3390/plants12162959







