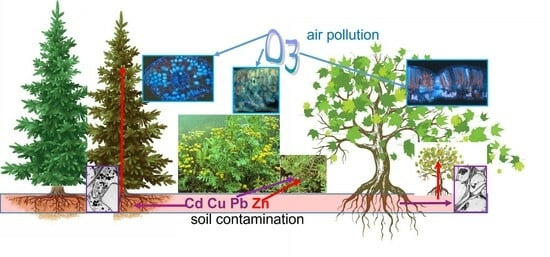
Responses to Airborne Ozone and Soilborne Metal Pollution in Afforestation Plants with Different Life Forms
Abstract
Highlights
- Ozone and metal stress caused injuries that were partly similar but differed in their tissue and cell location irrespective of the species.
- Combined ozone and metal stress showed few interactions.
- The conifer efficiently blocked the metals in its roots and was more tolerant of ozone stress, resulting in a biomass reduction rating in response to ozone and metal stress: conifer < ruderal forb < deciduous tree.
- With the current increases in environmental stress, our findings outline the relevance of “slow-return” species strategies, with low productivity but enhanced stress tolerance.
Abstract
1. Introduction
2. Results
2.1. Visible Injury in Foliage
2.2. Structural Injury within Foliar and Root Tissues
2.3. Morphological Changes within Foliage
2.4. Physiological Effects on Leaf Gas Exchange
2.5. Growth Reduction by O3 and Metal Stress
2.6. Effects of Ozone Stress and Metal Contamination on the Mineral Nutrition
3. Discussion
3.1. Stress Responses to Ozone and Metal Contaminants
3.2. Changes in Functional Traits within Foliage in the Context of Species-Specific Ecological Strategies
3.3. Contrasting Effects of Ozone and Metal Stress on Biomass and Mineral Nutrition
3.4. Multiple Airborne and Soilborne Stress Factors
4. Materials and Methods
4.1. Experimental Design and Treatments
4.2. Visible, Morpho-Anatomical, Biochemical, and Physiological Assessments
4.3. Biomass and Chemical Assessments
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A




| CO | O3 | MC | O3MC | |||||
|---|---|---|---|---|---|---|---|---|
| Plant Pots | Picea | Acer | Picea | Acer | Picea | Acer | Picea | Acer |
| C (%) | 47.86 ± 0.69 | 48.00 ± 0.63 | 48.01 ± 0.73 | 48.46 ± 0.77 | 47.76 ± 0.050 | 47.88 ± 0.05 | 46.52 ± 0.23 | 47.34 ± 0.25 |
| N (%) | 1.03 ± 0.03 | 1.13 ± 0.08 | 1.04 ± 0.02 | 1.13 ± 0.11 | 1.08 ± 0.05 | 1.20 ± 0.06 | 0.98 ± 0.03 | 1.39 ± 0.18 |
| P (mg kg−1) | 355.33 ± 56.28 | 649.75 ± 263.52 | 348.17 ± 22.58 | 401.67 ± 64.10 | 504.67 ± 30.46 | 614.50 ± 17.02 | 442.33 ± 40.13 | 1012.50 ± 441.34 |
| K (mg kg−1) | 1508.33 ± 150.06 | 1266.10 ± 163.05 | 1463.33 ± 99.39 | 1579.96 ± 623.14 | 1508.33 ± 132.96 | 1554.76 ± 233.61 | 1291.67 ± 156.96 | 2229.69 ± 1068.26 |
| Ca (mg kg−1) | 2638.33 ± 331.47 | 7672.50 ± 255.16 P | 2473.33 ± 122.83 | 5775.00 ± 958.13 P | 2136.67 ± 78.81 | 4230.00 ± 1248.86 P | 2925.00 ± 775.19 | 5110.00 ± 1336.45 P |
| Mg (mg kg−1) | 414.67 ± 62.02 | 1423.47 ± 26.55 P | 389.67 ± 28.88 | 1031.35 ± 216.84 | 399.17 ± 24.83 | 662.27 ± 240.24 | 527.17 ± 162.86 | 711.27 ± 222.20 P |
| Cd (mg kg−1) | 0.17 ± 0.02 | 0.21 ± 0.01 | 0.17 ± 0.004 | 0.21 ± 0.02 | 21.55 ± 0.53 #o | 28.64 ± 2.05 #oP | 20.78± 0.81 #o | 31.38 ± 3.21 #oP |
| Cu (mg kg−1) | 21.03 ± 4.39 | 16.54 ± 1.18 | 22.83 ± 2.51 | 18.95 ± 6.61 | 1131.67 ± 38.12 #o | 1124.16 ± 6.52 #o | 1106.67 ± 41.67 #o | 1181.67 ± 31.03 #o |
| Pb (mg kg−1) | 6.05 ± 0.67 | 6.79 ± 1.36 | 4.85 ± 0.18 | 6.18 ± 0.62 | 2600.00 ± 62.45 #o | 2561.43 ± 11.85 #o | 2526.67 ± 89.46 #o | 2564.03 ± 96.76 #o |
| Zn (mg kg−1) | 34.97 ± 6.20 | 27.14 ± 0.50 | 27.82 ± 1.94 | 28.17 ± 4.14 | 1311.67 ± 30.60 #o | 1785.49 ± 214.24 #o | 1340.00 ± 66.58 #o | 1934.77 ± 279.76 #o |
| pH | 3.45 ± 0.10 | 5.14 ± 0.36 P | 3.25 ± 0.04 | 4.66 ± 0.66 | 3.33 ± 0.04 | 4.00 ± 0.45 | 3.22 ± 0.02 | 3.57 ± 0.07 |
| Picea | Acer | |||||
|---|---|---|---|---|---|---|
| O3 | MC | O3 × MC | O3 | MC | O3 × MC | |
| Foliage morphological traits | ||||||
| colour cy | 14.97 *** | 1.78 | 4.88 * | 0.62 | 42.06 *** | 0.16 |
| colour py | 0.21 | 0.02 | 0.58 | |||
| shed foliage mass cy | no shed foliage in the cy | 1.23 | 0.20 | 0.56 | ||
| shed foliage mass py | 18.01 *** | 50.81 *** | 22.16 *** | |||
| LDMC cy | 1.89 | 1.12 | 0.00 | 1.01 | 29.88 *** | 0.03 |
| LDMC py | 2.16 | 1.61 | 2.62 | |||
| LMA cy | 5.28 * | 0.37 | 0.00 | 4.47 * | 3.88 | 15.26 *** |
| LMA py | 1.74 | 1.73 | 1.82 | |||
| Foliage tannin traits | ||||||
| OPC cy | 20.12 *** | 2.89 | 1.47 | 15.51 *** | 40.06 *** | 3.14 |
| PPC cy | 9.54 ** | 1.18 | 0.82 | 11.40 ** | 65.01 *** | 2.00 |
| PPCcw cy | 5.57 * | 4.45* | 0.00 | 2.59 | 22.32 *** | 0.03 |
| phen. ox. int. cy | 1.65 | 1.85 | 0.26 | 9.45 ** | 2.71 | 0.01 |
| Root tannin traits | ||||||
| OPC | 5.92 * | 31.11 *** | 8.78 ** | |||
| PPC | 0.04 | 29.19 *** | 0.04 | |||
| PPCcw | 28.79 *** | 90.90 *** | 44.61 *** | |||
| phen. ox. int. | 14.93 ** | 36.53 *** | 14.29 ** | |||
| Picea | Acer | Tanacetum | |||||||
|---|---|---|---|---|---|---|---|---|---|
| O3 | MC | O3 × MC | O3 | MC | O3 × MC | O3 | MC | O3 × MC | |
| Biomass | |||||||||
| roots | 1.02 | 7.67 * | 0.22 | 9.24 ** | 274.76 *** | 14.41 ** | 9.79 * | 76.34 *** | 1.95 |
| wood | 0.28 | 11.99 ** | 0.62 | 30.95 *** | 215.35 *** | 20.98 *** | |||
| foliage | 1.94 | 0.53 | 1.12 | 30.78 *** | 220.11 *** | 26.70 *** | 1.94 | 174.55 *** | 9.65 * |
| root/shoot-ratio | 0.02 | 1.51 | 0.48 | 3.13 | 27.17 *** | 0.23 | 9.59 * | 0.92 | 0.95 |
| Picea | Acer | Tanacetum | |||||||
|---|---|---|---|---|---|---|---|---|---|
| O3 | MC | O3 × MC | O3 | MC | O3 × MC | O3 | MC | O3 × MC | |
| Roots | |||||||||
| Cd | 5.28 | 970.20 *** | 0.51 | NA | NA | NA | NA | ||
| Cu | 0.14 | 1112.69 *** | 1.20 | 0.18 | 887.51 *** | 0.09 | 0.24 | 1256.10 *** | 0.35 |
| Pb | 0.94 | 2026.70 *** | 0.00 | 0.77 | 521.62 *** | 0.28 | 0.42 | 981.00 *** | 0.05 |
| Zn | 0.05 | 810.08 *** | 0.80 | 6.28 * | 227.15 *** | 3.08 | 3.54 | 941.62 *** | 0.07 |
| C | 2.06 | 50.42 *** | 2.02 | 3.42 | 0.00 | 0.20 | 0.34 | 30.35 *** | 5.10 |
| N | 1.51 | 27.79 *** | 0.41 | 0.05 | 10.88 ** | 2.82 | 4.23 | 71.34 *** | 0.01 |
| P | 0.14 | 82.81 *** | 0.42 | 0.08 | 0.01 | 6.46 * | 2.18 | 39.35 *** | 0.43 |
| K | 3.13 | 3.16 | 1.84 | 0.83 | 5.39 * | 0.48 | 2.85 | 191.15 *** | 1.17 |
| Ca | 0.36 | 13.17 ** | 0.28 | 1.57 | 3.21 | 0.69 | 0.06 | 0.34 | 6.53 * |
| Mg | 0.61 | 312.36 *** | 0.21 | 0.99 | 9.78 ** | 0.20 | 0.38 | 9.19 * | 5.18 |
| Wood | |||||||||
| Cd | NA | NA | NA | NA | |||||
| Cu | NA | NA | NA | NA | |||||
| Pb | NA | NA | NA | NA | |||||
| Zn | 0.70 | 197.93 *** | 1.45 | 0.44 | 356.34 *** | 0.83 | |||
| C | 8.43 * | 0.52 | 0.05 | 0.00 | 20.82 *** | 2.09 | |||
| N | 10.77 ** | 27.91 ** | 0.02 | 17.01 ** | 12.49 ** | 5.34 * | |||
| P | 0.55 | 9.67 ** | 0.04 | 6.23 * | 29.28 *** | 6.06 * | |||
| K | 4.25 | 0.22 | 1.57 | 9.12 ** | 0.38 | 0.08 | |||
| Ca | 6.34 * | 111.64 *** | 0.07 | 0.03 | 1.24 | 0.27 | |||
| Mg | 0.00 | 0.61 | 0.75 | 4.20 | 17.71 *** | 0.07 | |||
| Leaves | |||||||||
| Cd | NA | NA | NA | NA | NA | NA | |||
| Cu | NA | NA | NA | NA | 1.82 | 203.48 *** | 0.04 | ||
| Pb | NA | NA | NA | NA | 0.27 | 12.17 ** | 0.05 | ||
| Zn | 23.63 ** | 268.73 *** | 26.8 *** | 2.24 | 106.90 *** | 0.07 | 2.55 | 2263.97 *** | 2.44 |
| C | 0.45 | 11.71 ** | 1.52 | 1.02 | 11.33 ** | 0.02 | 0.44 | 516.36 *** | 5.27 * |
| N | 0.66 | 0.03 | 1.08 | 0.23 | 5.08 * | 0.87 | 0.04 | 38.75 *** | 0.83 |
| P | 6.19 * | 0.34 | 0.41 | 2.37 | 2.48 | 0.14 | 0.02 | 55.48 *** | 0.00 |
| K | 4.81 | 1.80 | 1.90 | 3.14 | 16.47 *** | 0.66 | 0.87 | 47.53 *** | 1.60 |
| Ca | 0.00 | 141.02 *** | 0.47 | 2.41 | 29.40 *** | 0.35 | 0.75 | 3.47 | 0.00 |
| Mg | 3.52 | 0.02 | 3.68 | 0.34 | 43.03 *** | 3.14 | 2.01 | 14.41 *** | 2.04 |
| Picea | Acer | |||||
|---|---|---|---|---|---|---|
| O3 | MC | O3 × MC | O3 | MC | O3 × MC | |
| Biomass | ||||||
| roots | 2.42 | 32.77 *** | 0.19 | 2.63 | 112.14 *** | 3.24 |
| wood | 0.68 | 88.79 *** | 0.43 | 7.56 * | 230.30 *** | 10.54 ** |
| foliage | 0.00 | 41.76 *** | 0.56 | 0.01 | 100.66 *** | 0.13 |
| root/shoot-ratio | 2.65 | 0.00 | 1.28 | 0.35 | 1.35 | 0.02 |
References
- Moura, B.B.; de Souza, S.R.; Alves, E.S. Response of Brazilian native trees to acute ozone dose. Environ. Sci. Pollut. Res. 2014, 21, 4220–4227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wei, Y.; Fang, Z. Ozone Pollution: A major health hazard worldwide. Front. Immunol. 2019, 10, 2518. [Google Scholar] [CrossRef] [PubMed]
- Leung, F.; Pang, J.Y.S.; Tai, A.P.K.; Lam, T.; Tao, D.K.C.; Sharps, K. Evidence of ozone-induced visible foliar injury in Hong Kong using Phaseolus vulgaris as a bioindicator. Atmosphere 2020, 11, 266. [Google Scholar] [CrossRef]
- Andreev, V.V.; Arshinov, M.Y.; Belan, B.D.; Belan, S.B.; Davydov, D.K.; Demin, V.I.; Elansky, N.F.; Zhamsueva, G.S.; Zayakhanov, A.S.; Ivlev, G.A.; et al. Surface ozone concentration in Russia in the second half of 2020. Atmos. Ocean. Opt. 2021, 34, 347–356. [Google Scholar] [CrossRef]
- Leung, K.H.Y.; Arnillas, C.A.; Cheng, V.Y.S.; Gough, W.A.; Arhonditsis, G.B. Seasonality patterns and distinctive signature of latitude and population on ozone concentrations in Southern Ontario, Canada. Atmos. Environ. 2021, 246, 118077. [Google Scholar] [CrossRef]
- Mills, G.; Pleijel, H.; Malley, C.S.; Sinha, B.; Cooper, O.R.; Schultz, M.G.; Neufeld, H.S.; Simpson, D.; Sharps, K.; Feng, Z.Z.; et al. Tropospheric Ozone Assessment Report: Present-day tropospheric ozone distribution and trends relevant to vegetation. Elem. Sci. Anthrop. 2018, 6, 898–908. [Google Scholar] [CrossRef]
- Sharma, S.; Chatani, S.; Mahtta, R.; Goel, A.; Kumar, A. Sensitivity analysis of ground level ozone in India using WRF-CMAQ models. Atmos. Environ. 2016, 131, 29–40. [Google Scholar] [CrossRef]
- Li, K.; Jacob, D.J.; Liao, H.; Shen, L.; Zhang, Q.; Bates, K.H. Anthropogenic drivers of 2013-2017 trends in summer surface ozone in China. Proc. Natl. Acad. Sci. USA 2019, 116, 422–427. [Google Scholar] [CrossRef]
- De Marco, A.; Anav, A.; Sicard, P.; Feng, Z.; Paoletti, E. High spatial resolution ozone risk-assessment for Asian forests. Environ. Res. Lett. 2020, 15, 104095. [Google Scholar] [CrossRef]
- Da Silveira, V.R.; de Oliveira Junior, J.F.; da Silva, M.S.; Silva, C.; Alves, A.R.; Pontes, A.d.S.; Gomes Pimentel, L.C.; Rotunno Filho, O.C. Analysis of urban—Industrial expansion and increasing level of ozone concentration as subsiding an environmental management plan for the east of Rio de Janeiro metropolitan area—Brazil. Land Use Policy 2021, 101, 105148. [Google Scholar] [CrossRef]
- Yu, R.; Lin, Y.; Zou, J.; Dan, Y.; Cheng, C. Review on Atmospheric Ozone Pollution in China: Formation, spatiotemporal distribution, precursors and affecting factors. Atmosphere 2021, 12, 1675. [Google Scholar] [CrossRef]
- Schaub, M.; Häni, M.; Calatayud, V.; Ferretti, M.; Gottardini, E.; Michel, A.K.; Seidling, W. Ozone concentrations are decreasing but exposure remains high in European forests. In ICP Forests Brief; Michel, A., Seidling, W., Eds.; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2018; Volume 3. [Google Scholar]
- Seltzer, K.M.; Shindell, D.T.; Kasibhatla, P.; Malley, C.S. Magnitude, trends, and impacts of ambient long-term ozone exposure in the United States from 2000 to 2015. Atmos. Chem. Phys. 2020, 20, 1757–1775. [Google Scholar] [CrossRef]
- Ito, A.; Wakamatsu, S.; Morikawa, T.; Kobayashi, S. 30 years of air quality trends in Japan. Atmosphere 2021, 12, 1072. [Google Scholar] [CrossRef]
- Proietti, C.; Fornasier, M.F.; Sicard, P.; Anav, A.; Paoletti, E.; De Marco, A. Trends in tropospheric ozone concentrations and forest impact metrics in Europe over the time period 2000–2014. J. For. Res. 2021, 32, 543–551. [Google Scholar] [CrossRef]
- Miyazaki, K.; Bowman, K.; Sekiya, T.; Takigawa, M.; Neu, J.L.; Sudo, K.; Osterman, G.; Eskes, H. Global tropospheric ozone responses to reduced NOx emissions linked to the COVID-19 worldwide lockdowns. Sci. Adv. 2021, 7, eabf7460. [Google Scholar] [CrossRef]
- Huang, S.S.; Tu, J.; Liu, H.Y.; Hua, M.; Liao, Q.L.; Feng, J.S.; Weng, Z.H.; Huang, G.M. Multivariate analysis of trace element concentrations in atmospheric deposition in the Yangtze River Delta, East China. Atmos. Environ. 2009, 43, 5781–5790. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Toth, G.; Hermann, T.; Szatmari, G.; Pasztor, L. Maps of heavy metals in the soils of the European Union and proposed priority areas for detailed assessment. Sci. Total Environ. 2016, 565, 1054–1062. [Google Scholar] [CrossRef]
- Schraudner, M.; Moeder, W.; Wiese, C.; Van Camp, W.; Inze, D.; Langebartels, C.; Sandermann, H. Ozone-induced oxidative burst in the ozone biomonitor plant, tobacco Bel W3. Plant J. 1998, 16, 235–245. [Google Scholar] [CrossRef]
- Pell, E.J.; Schlagnhaufer, C.D.; Arteca, R.N. Ozone-induced oxidative stress: Mechanisms of action and reaction. Physiol. Plant. 1997, 100, 264–273. [Google Scholar] [CrossRef]
- Rao, M.V.; Davis, K.R. The physiology of ozone induced cell death. Planta 2001, 213, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Langebartels, C.; Wohlgemuth, H.; Kschieschan, S.; Grun, S.; Sandermann, H. Oxidative burst and cell death in ozone-exposed plants. Plant Physiol. Biochem. 2002, 40, 567–575. [Google Scholar] [CrossRef]
- Vainonen, J.P.; Kangasjarvi, J. Plant signalling in acute ozone exposure. Plant Cell Environ. 2015, 38, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, I.J.; Rathore, D. Assessment of ozone toxicity on cotton (Gossypium hirsutum L.) cultivars: Its defensive system and intraspecific sensitivity. Plant Physiol. Biochem. 2021, 166, 912–927. [Google Scholar] [CrossRef]
- Pell, E.J.; Sinn, J.P.; Brendley, B.W.; Samuelson, L.; Vinten Johansen, C.; Tien, M.; Skillman, J. Differential response of four tree species to ozone-induced acceleration of foliar senescence. Plant Cell Environ. 1999, 22, 779–790. [Google Scholar] [CrossRef]
- Günthardt-Goerg, M.S.; Vollenweider, P. Linking stress with macroscopic and microscopic leaf response in trees: New diagnostic perspectives. Environ. Pollut. 2007, 147, 467–488. [Google Scholar] [CrossRef]
- Vollenweider, P.; Günthardt-Goerg, M.S.; Menard, T.; Baumgarten, M.; Matyssek, R.; Schaub, M. Macro- and microscopic leaf injury triggered by ozone stress in beech foliage (Fagus sylvatica L.). Ann. For. Sci. 2019, 76, 71. [Google Scholar] [CrossRef]
- Vollenweider, P.; Ottiger, M.; Günthardt-Goerg, M.S. Validation of leaf ozone symptoms in natural vegetation using microscopical methods. Environ. Pollut. 2003, 124, 101–118. [Google Scholar] [CrossRef]
- Moura, B.B.; Alves, E.S.; Marabesi, M.A.; de Souza, S.R.; Schaub, M.; Vollenweider, P. Ozone affects leaf physiology and causes injury to foliage of native tree species from the tropical Atlantic Forest of southern Brazil. Sci. Total Environ. 2018, 610, 912–925. [Google Scholar] [CrossRef]
- Turc, B.; Vollenweider, P.; Le Thiec, D.; Gandin, A.; Schaub, M.; Cabane, M.; Jolivet, Y. Dynamics of foliar responses to O-3 stress as a function of phytotoxic O-3 dose in hybrid poplar. Front. Plant Sci. 2021, 12, 679852. [Google Scholar] [CrossRef]
- Siedlecka, A. Some aspects of interactions between heavy-metals and plant mineral nutrients. Acta Soc. Bot. Pol. 1995, 64, 265–272. [Google Scholar] [CrossRef]
- Günthardt-Goerg, M.S.; Vollenweider, P.; Hermle, S.; Schulin, R. Growth and metal accumulation of young forest trees and understorey plants on contaminated topsoil: Influence of subsoil and time. Plant Soil 2019, 437, 375–395. [Google Scholar] [CrossRef]
- Buckley, T.N. The contributions of apoplastic, symplastic and gas phase pathways for water transport outside the bundle sheath in leaves. Plant Cell Environ. 2015, 38, 7–22. [Google Scholar] [CrossRef]
- Vollenweider, P.; Cosio, C.; Günthardt-Goerg, M.S.; Keller, C. Localization and effects of cadmium in leaves of a cadmium-tolerant willow (Salix viminalis L.). Part II Microlocalization and cellular effects of cadmium. Environ. Exp. Bot. 2006, 58, 25–40. [Google Scholar] [CrossRef]
- André, O.; Vollenweider, P.; Günthardt-Goerg, M.S. Foliage response to heavy metal contamination in Sycamore Maple (Acer pseudoplatanus L.). For. Snow Landsc. Res. 2006, 80, 275–288. [Google Scholar]
- Hermle, S.; Vollenweider, P.; Günthardt-Goerg, M.S.; McQuattie, C.J.; Matyssek, R. Leaf responsiveness of Populus tremula and Salix viminalis to soil contaminated with heavy metals and acidic rainwater. Tree Physiol. 2007, 27, 1517–1531. [Google Scholar] [CrossRef] [PubMed]
- Babst-Kostecka, A.A.; Waldmann, P.; Frerot, H.; Vollenweider, P. Plant adaptation to metal polluted environments. Physiological, morphological, and evolutionary insights from Biscutella laevigata. Environ. Exp. Bot. 2016, 127, 1–13. [Google Scholar] [CrossRef]
- Martin, D.M.; Vollenweider, P.; Buttler, A.; Günthardt-Goerg, M.S. Bioindication of heavy metal contamination in vegetable gardens. For. Snow Landsc. Res. 2006, 80, 169–180. [Google Scholar]
- Vollenweider, P.; Günthardt-Goerg, M.S. Diagnosis of abiotic and biotic stress factors using the visible symptoms in foliage. Environ. Pollut. 2006, 140, 562–571. [Google Scholar] [CrossRef]
- Maurer, S.; Matyssek, R.; GünthardtGoerg, M.S.; Landolt, W.; Einig, W. Nutrition and the ozone sensitivity of birch (Betula pendula). 1. Responses at the leaf level. Trees 1997, 12, 1–10. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Robinson, B.H.; Günthardt-Goerg, M.S.; Schulin, R. Metal uptake and allocation in trees grown on contaminated land: Implications for biomass production. Int. J. Phytoremediat. 2013, 15, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hoshika, Y.; Carrari, E.; Badea, O.; Paoletti, E. Ozone risk assessment is affected by nutrient availability: Evidence from a simulation experiment under free air controlled exposure (FACE). Environ. Pollut. 2018, 238, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhou, H.M.; Xu, Y.S.; Shang, B.; Feng, Z.Z. The effects of elevated ozone on the accumulation and allocation of poplar biomass depend strongly on water and nitrogen availability. Sci. Total Environ. 2019, 665, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Maurer, S.; Matyssek, R. Nutrition and the ozone sensitivity of birch (Betula pendula). 2. Carbon balance, water-use efficiency and nutritional statue of the whole plant. Trees 1997, 12, 11–20. [Google Scholar] [CrossRef]
- Marzban, L.; Akhzari, D.; Ariapour, A.; Mohammadparast, B.; Pessarakli, M. Effects of cadmium stress on seedlings of various rangeland plant species (Avena fatua L., Lathyrus sativus L., and Lolium temulentum L.): Growth, physiological traits, and cadmium accumulation. J. Plant Nutr. 2017, 40, 2127–2137. [Google Scholar] [CrossRef]
- Günthardt-Goerg, M.S.; Vollenweider, P.; Schulin, R. Metal Accumulation and Biomass Production in Young Afforestations Established on Soil Contaminated by Heavy Metals. Plants 2022, 11, 523. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Ernst, W.H.O. Evolution of metal tolerance in higher plants. For. Snow Landsc. Res. 2006, 80, 251–274. [Google Scholar]
- Marschner, P.; Rengel, Z. Nutrient availability in soils. In Marschner’s Mineral Nutrition of Plants, 4th ed.; Rengel, Z., Cakmak, I., White, P.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 499–522. [Google Scholar]
- Vollenweider, P.; Bernasconi, P.; Gautschi, H.-P.; Menard, T.; Frey, B.; Günthardt-Goerg, M.S. Compartmentation of metals in foliage of Populus tremula grown on soils with mixed contamination. II. Zinc binding inside leaf cell organelles. Environ. Pollut. 2011, 159, 337–347. [Google Scholar] [CrossRef]
- Alves, E.S.; Moura, B.B.; Vaz Pedroso, A.N.; Tresmondi, F.; Machado, S.R. Cellular markers indicative of ozone stress on bioindicator plants growing in a tropical environment. Ecol. Indic. 2016, 67, 417–424. [Google Scholar] [CrossRef]
- Estiarte, M.; Penuelas, J. Alteration of the phenology of leaf senescence and fall in winter deciduous species by climate change: Effects on nutrient proficiency. Glob. Chang. Biol. 2015, 21, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Long-distance transport in the xylem and phloem and its regulation. In Marschner’s Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: Cambridge, MA, USA, 2002; pp. 79–115. [Google Scholar]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.C.; Falster, D.S.; Garnier, E.; Hikosaka, K.; Lamont, B.B.; Lee, W.; Oleksyn, J.; Osada, N.; et al. Assessing the generality of global leaf trait relationships. New Phytol. 2005, 166, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B. The world-wide ‘fast-slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Füzy, A.; Kovacs, R.; Cseresnyes, I.; Paradi, I.; Szili-Kovacs, T.; Kelemen, B.; Rajkai, K.; Takacs, T. Selection of plant physiological parameters to detect stress effects in pot experiments using principal component analysis. Acta Physiol. Plant. 2019, 41, 41–56. [Google Scholar] [CrossRef]
- Zhang, J.; Ferdinand, J.A.; Vanderheyden, D.J.; Skelly, J.M.; Innes, J.L. Variation of gas exchange within native plant species of Switzerland and relationships with ozone injury: An open-top experiment. Environ. Pollut. 2001, 113, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Hoshika, Y.; Carrari, E.; Zhang, L.; Carriero, G.; Pignatelli, S.; Fasano, G.; Materassi, A.; Paoletti, E. Testing a ratio of photosynthesis to O3 uptake as an index for assessing O3-induced foliar visible injury in poplar trees. Environ. Sci. Pollut. Res. 2018, 25, 8113–8124. [Google Scholar] [CrossRef]
- Shafi, A.; Zahoor, I. Abiotic and biotic stress-induced alterations in the micronutrient status of plants. In Plant Micronutrients; Aftab, T., Hakeen, K.R., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 285–296. [Google Scholar]
- Jolivet, Y.; Bagard, M.; Cabane, M.; Vaultier, M.N.; Gandin, A.; Afif, D.; Dizengremel, P.; Le Thiec, D. Deciphering the ozone-induced changes in cellular processes: A prerequisite for ozone risk assessment at the tree and forest levels. Ann. For. Sci. 2016, 73, 923–943. [Google Scholar] [CrossRef]
- Dinakar, C.; Abhaypratap, V.; Yearla, S.R.; Raghavendra, A.S.; Padmasree, K. Importance of ROS and antioxidant system during the beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Planta 2010, 231, 461–474. [Google Scholar] [CrossRef]
- Brehelin, C.; Kessler, F.; van Wijk, K.J. Plastoglobules: Versatile lipoprotein particles in plastids. Trends Plant Sci. 2007, 12, 260–266. [Google Scholar] [CrossRef]
- Turc, B.; Jolivet, Y.; Cabane, M.; Schaub, M.; Vollenweider, P. Ante- and post-mortem cellular injury dynamics in hybrid poplar foliage as a function of phytotoxic O-3 dose. PLoS ONE 2023, 18, e0282006. [Google Scholar] [CrossRef]
- Seigler, D.S. Plant Secondary Metabolism; Springer: Berlin, Germany, 1998; p. 759. [Google Scholar]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and Regulation of Plants Phenolics in Abiotic Stress Tolerance: An Overview. In Plant Signaling Molecules: Role and Regulation under Stressful Environments; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 157–168. [Google Scholar]
- Gutmann, M. Localization of proanthocyanidins using insitu-hydrolysis with sulfuric-acid. Biotech. Histochem. 1993, 68, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Leigh, R.A.; Tomos, A.D. Ion distribution in cereal leaves—Pathways and mechanisms. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 1993, 341, 75–86. [Google Scholar] [CrossRef]
- Yang, L.Y.; Xian, D.H.; Xiong, X.; Lai, R.; Song, J.; Zhong, J.Q. Proanthocyanidins against oxidative stress: From molecular mechanisms to clinical applications. BioMed Res. Int. 2018, 2018, 8584136. [Google Scholar] [CrossRef]
- Günthardt-Goerg, M.S.; Vollenweider, P. Responses of beech and spruce foliage to elevated carbon dioxide, increased nitrogen deposition and soil type. AoB PLANTS 2015, 7, plv067. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ossipova, S.; Ossipov, V.; Haukioja, E.; Loponen, J.; Pihlaja, K. Proanthocyanidins of mountain birch leaves: Quantification and properties. Phytochem. Anal. 2001, 12, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Cosio, C.; Vollenweider, P.; Keller, C. Localization and effects of cadmium in leaves of a cadmium-tolerant willow (Salix viminalis L.) I. Macrolocalization and phytotoxic effects of cadmium. Environ. Exp. Bot. 2006, 58, 64–74. [Google Scholar] [CrossRef][Green Version]
- Nowack, B.; Schulin, R.; Luster, J. Metal fractionation in a contaminated soil after reforestation: Temporal changes versus spatial variability. Environ. Pollut. 2010, 158, 3272–3278. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.-S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Shiklomanov, A.N.; Cowdery, E.M.; Bahn, M.; Byun, C.; Jansen, S.; Kramer, K.; Minden, V.; Niinemets, U.; Onoda, Y.; Soudzilovskaia, N.A.; et al. Does the leaf economic spectrum hold within plant functional types? A Bayesian multivariate trait meta-analysis. Ecol. Appl. 2020, 30, e02064. [Google Scholar] [CrossRef]
- El-Khatib, A.A.; Youssef, N.A.; Barakat, N.A.; Samir, N.A. Responses of Eucalyptus globulus and Ficus nitida to different potential of heavy metal air pollution. Int. J. Phytoremediat. 2020, 22, 986–999. [Google Scholar] [CrossRef]
- Abdelgawad, H.; Zinta, G.; Hamed, B.A.; Selim, S.; Beemster, G.; Hozzein, W.N.; Wadaan, M.A.M.; Asard, H.; Abuelsoud, W. Maize roots and shoots show distinct profiles of oxidative stress and antioxidant defense under heavy metal toxicity. Environ. Pollut. 2020, 258, 113705. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, V.; Cervero, J.; Sanz, M.J. Foliar, physiologial and growth responses of four maple species exposed to ozone. Water Air Soil Pollut. 2007, 185, 239–254. [Google Scholar] [CrossRef]
- Paoletti, E.; Contran, N.; Bernasconi, P.; Günthardt-Goerg, M.S.; Vollenweider, P. Structural and physiological responses to ozone in Manna ash (Fraxinus ornus L.) leaves of seedlings and mature trees under controlled and ambient conditions. Sci. Total Environ. 2010, 408, 2013–2024. [Google Scholar] [CrossRef]
- Marzuoli, R.; Gerosa, G.; Bussotti, F.; Pollastrini, M. Assessing the impact of ozone on forest trees in an integrative perspective: Are foliar visible symptoms suitable predictors for growth reduction? A critical review. Forests 2019, 10, 1144. [Google Scholar] [CrossRef]
- Grulke, N.E.; Heath, R.L. Ozone effects on plants in natural ecosystems. Plant Biol. 2020, 22, 12–37. [Google Scholar] [CrossRef]
- Langebartels, C.; Heller, W.; Fuhrer, G.; Lippert, M.; Simons, S.; Sandermann, H. Memory effects in the action of ozone on conifers. Ecotoxicol. Environ. Saf. 1998, 41, 62–72. [Google Scholar] [CrossRef]
- Wittig, V.E.; Ainsworth, E.A.; Naidu, S.L.; Karnosky, D.F.; Long, S.P. Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: A quantitative meta-analysis. Glob. Chang. Biol. 2009, 15, 396–424. [Google Scholar] [CrossRef]
- Yamaji, K.; Julkunen-Tiitto, R.; Rousi, M.; Freiwald, V.; Oksanen, E. Ozone exposure over two growing seasons alters root-to-shoot ratio and chemical composition of birch (Betula pendula Roth). Glob. Chang. Biol. 2003, 9, 1363–1377. [Google Scholar] [CrossRef]
- Jatav, H.S.; Sharma, D.; Sandhukhan, R.; Singh, S.K.; Singh, S.; Rajput, V.D.; Parihar, M.; Jatav, S.S.; Jinger, D.; Kumar, S.; et al. An overview of micronutrients: Prospects and implications in crop production. In Plant Nutrients; Aftab, T., Hakeen, K.R., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 1–39. [Google Scholar]
- Shri, P.U.; Pillay, V. Excess of soil zinc interferes with uptake of other micro and macro nutrients in Sorghum bicolor (L.) plants. Indian J. Plant Physiol. 2017, 22, 304–308. [Google Scholar] [CrossRef]
- Hahner, J.L.; Robinson, B.H.; Zhong, H.-T.; Dickinson, N.M. The phytoremediation potential of native plants on New Zealand diary farms. Int. J. Phytoremediat. 2014, 16, 719–734. [Google Scholar] [CrossRef]
- Di Cagno, R.; Guidi, L.; De Gara, L.; Soldatini, G.F. Combined cadmium and ozone treatments affect photosynthesis and ascorbate-dependent defences in sunflower. New Phytol. 2001, 151, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Castagna, A.; Di Baccio, D.; Ranieri, A.M.; Sebastiani, L.; Tognetti, R. Effects of combined ozone and cadmium stresses on leaf traits in two poplar clones. Environ. Sci. Pollut. Res. 2015, 22, 2064–2075. [Google Scholar] [CrossRef] [PubMed]
- Degl’Innocenti, E.; Castagna, A.; Ranieri, A.; Guidi, L. Combined effects of cadmium and ozone on photosynthesis of Lycopersicon esculentum. Photosynthetica 2014, 52, 179–185. [Google Scholar] [CrossRef]
- Xu, S.; He, X.Y.; Du, Z.; Chen, W.; Li, B.; Li, Y.; Li, M.H.; Schaub, M. Tropospheric ozone and cadmium do not have interactive effects on growth, photosynthesis and mineral nutrients of Catalpa ovata seedlings in the urban areas of Northeast China. Sci. Total Environ. 2020, 704, 135307. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, B.; Li, P.; He, X.; Chen, W.; Yan, K.; Li, Y.; Wang, Y. Soil high Cd exacerbates the adverse impact of elevated O3 on Populus alba ‘Berolinensis’ L. Ecotoxicol. Environ. Saf. 2019, 174, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Kaciene, G.; Juknys, R.; Januskaitiene, I. The role of oxidative stress in spring barley cross-adaptation to different heavy metals. Arc. Agron. Soil Sci. 2017, 63, 1037–1048. [Google Scholar] [CrossRef]
- Swiss Federal Council. Ordinance on Pollution of the Soil; Swiss Federal Council: Bern, Switzerland, 2016.
- Biesalski, E. Pflanzenfarbenatlas mit Farbzeichen nach DIN 6164; Musterschmidt-Verlag: Göttingen, Germany; Berlin, Germany; Frankfurt, Germany, 1957. [Google Scholar]
- Sarkar, S.K.; Howarth, R.E. Specificity of the vanilin test for flavonoids. J. Agric. Food Chem. 1976, 24, 317–320. [Google Scholar] [CrossRef]
- Günthardt-Goerg, M.S.; McQuattie, C.J.; Scheidegger, C.; Rhiner, C.; Matyssek, R. Ozone-induced cytochemical and ultrastructural changes in leaf mesophyll cell walls. Can. J. For. Res. Rev. Can. Rech. For. 1997, 27, 453–463. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Amiot, M.J.; Tacchini, M.; Aubert, S.; Nicolas, J. Phenolic composition and browning susceptibiliy of various apple cultivars at maturity. J. Food Sci. 1992, 57, 958–962. [Google Scholar] [CrossRef]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites; Wiley: London, UK, 1994; p. 248. [Google Scholar]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphidins to cyanidin and delphidin. Phytochemistry 1986, 25, 223–230. [Google Scholar] [CrossRef]
- Egli, P.; Maurer, S.; Günthardt-Goerg, M.S.; Körner, C. Effects of elevated CO2 and soil quality on leaf gas exchange and above-ground growth in beech-spruce model ecosystems. New Phytol. 1998, 140, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Antweiler, R.C.; Taylor, H.E. Evaluation of statistical treatments of left-censored environmental data using coincident uncensored data sets: I. Summary statistics. Environ. Sci. Technol. 2008, 42, 3732–3738. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Günthardt-Goerg, M.S.; Schläpfer, R.; Vollenweider, P. Responses to Airborne Ozone and Soilborne Metal Pollution in Afforestation Plants with Different Life Forms. Plants 2023, 12, 3011. https://doi.org/10.3390/plants12163011
Günthardt-Goerg MS, Schläpfer R, Vollenweider P. Responses to Airborne Ozone and Soilborne Metal Pollution in Afforestation Plants with Different Life Forms. Plants. 2023; 12(16):3011. https://doi.org/10.3390/plants12163011
Chicago/Turabian StyleGünthardt-Goerg, Madeleine S., Rodolphe Schläpfer, and Pierre Vollenweider. 2023. "Responses to Airborne Ozone and Soilborne Metal Pollution in Afforestation Plants with Different Life Forms" Plants 12, no. 16: 3011. https://doi.org/10.3390/plants12163011
APA StyleGünthardt-Goerg, M. S., Schläpfer, R., & Vollenweider, P. (2023). Responses to Airborne Ozone and Soilborne Metal Pollution in Afforestation Plants with Different Life Forms. Plants, 12(16), 3011. https://doi.org/10.3390/plants12163011