Anti-Inflammatory Effect of Meriania hexamera Sprague by Targeting Syk Kinase in NF-κB Signaling
Abstract
1. Introduction
2. Results
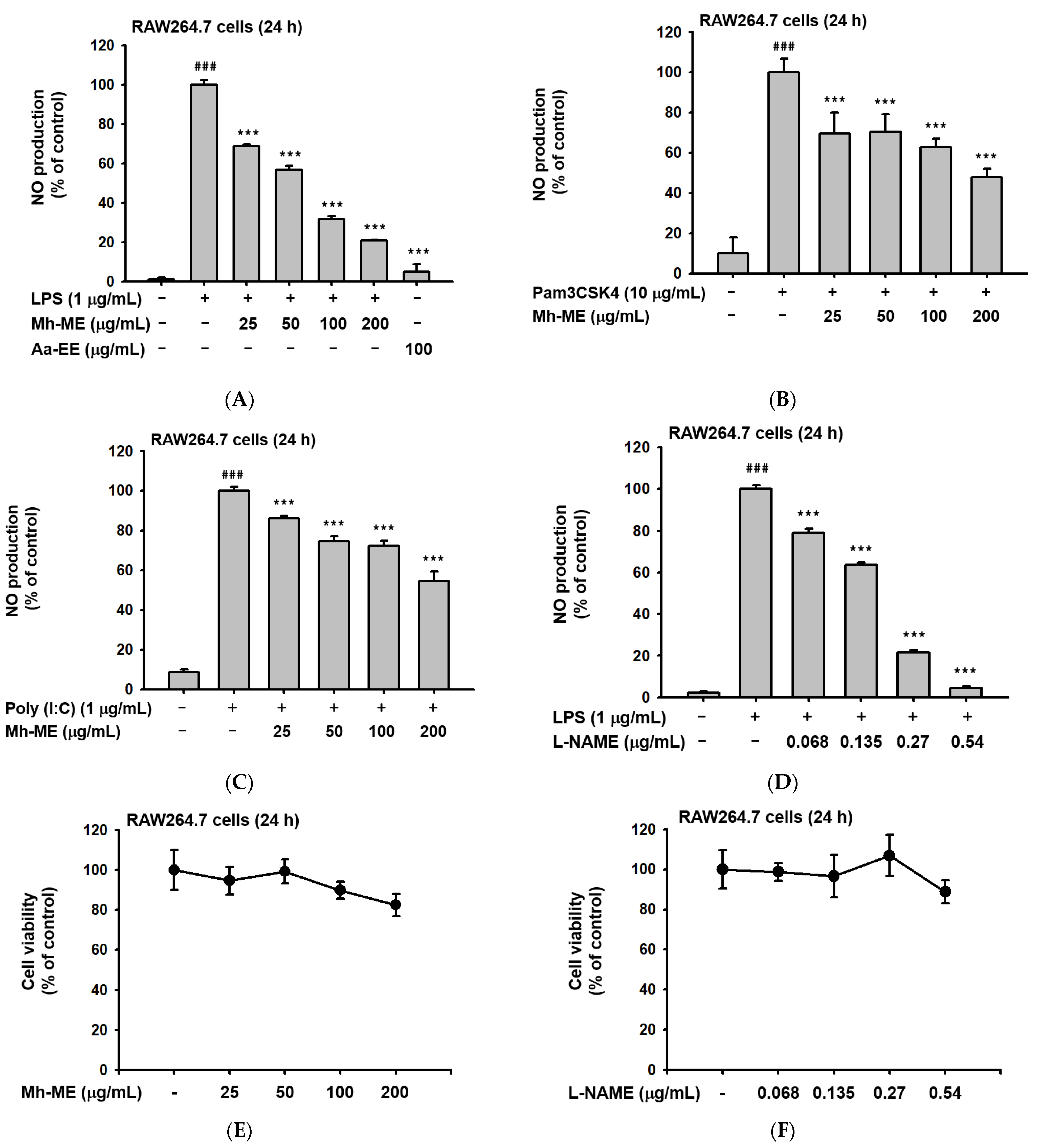
2.1. Mh-ME Ameliorated NO Production without Inducing Death of RAW264.7 Cells
2.2. Analysis of the Components of Mh-ME
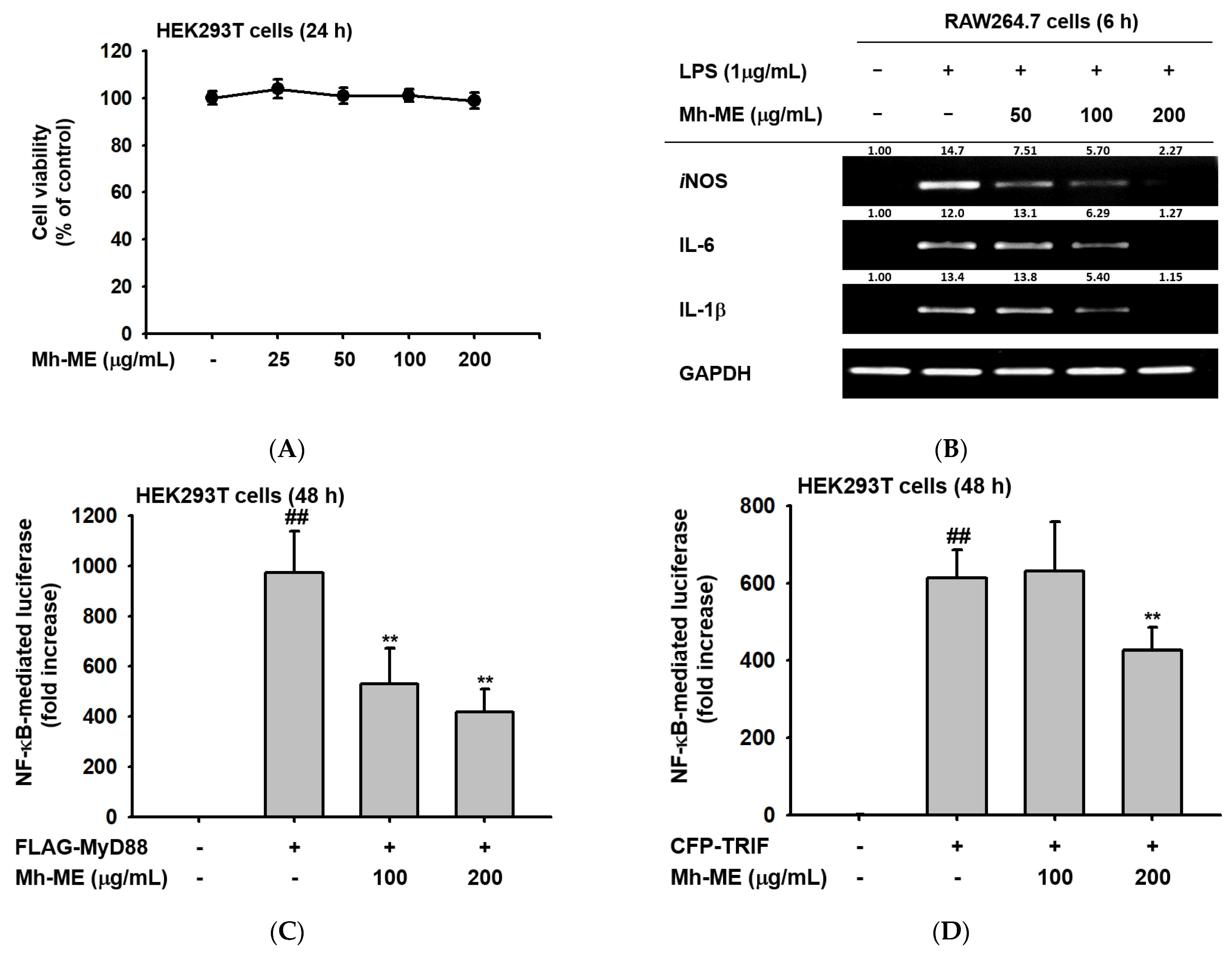
2.3. Effects of Mh-ME on the Transcriptional Activation of Pro-Inflammatory Proteins
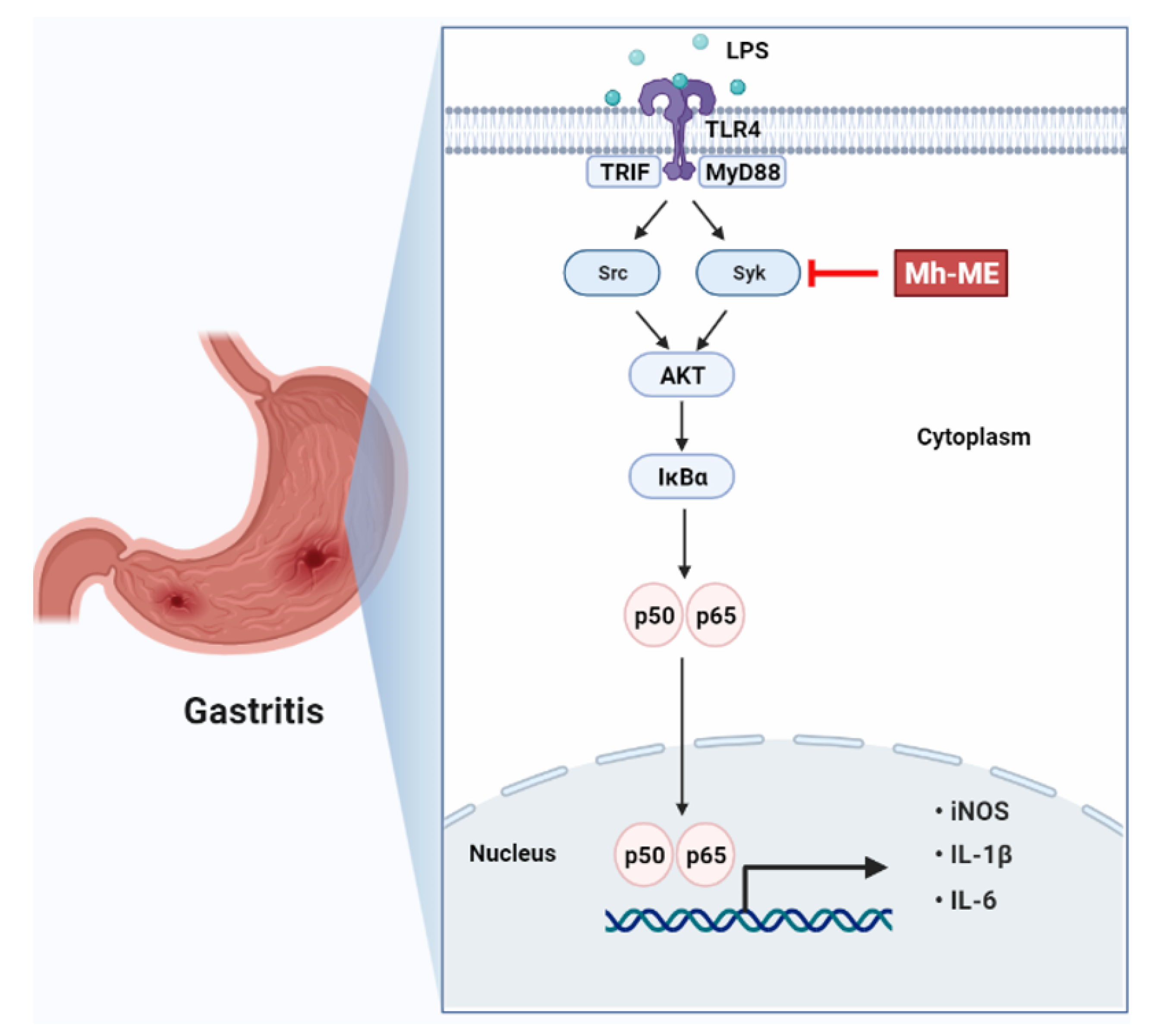
2.4. Mh-ME Lowered Phosphorylation of Proteins Inducing the NF-κB Acitvation
2.5. Mh-ME Showed Therapeutic Potential on Acute Gastritis
2.6. Acute Toxicity Test
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Mh-ME Preparation and GC-MS
4.3. Measurement of Total Phenolic and Flavonoid Contents
4.4. LC-MS/MS
4.5. Cell Culture
4.6. Nitric Oxide (NO) Assay
4.7. Cell Viability Assay
4.8. Semiquantitative RT-PCR and Quantitative Real-Time PCR
4.9. Luciferase Reproter Assay
4.10. Preparing Whole Lysates and Western Blotting
4.11. Plasmid Transfection for Exogeneous Expression of Syk and Src Kinase
4.12. Cellualr Thermal Shift Assay (CETSA)
4.13. Animal Experiments
4.14. Serum ALT and AST Activity Detection
4.15. Statistical Anaylsis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| PAMPs | pathogen-associated molecular patterns |
| PRRs | pattern recognition receptors |
| TLRs | toll-like receptors |
| LPS | lipopolysaccharide |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
| MyD88 | myeloid differentiation response gene 88 |
| DAMPs | damage-associated molecular patterns |
| NF-κB | nuclear factor-kappa B |
| AP-1 | activated protein-1 |
| Syk | spleen tyrosinase kinase |
| AKT | protein kinase B |
| IκBα | inhibitor of kappa B alpha |
| iNOS | nitric oxide synthase |
| IL-1β | interleukin-1β |
| TNF-α | tumor necrosis factor-alpha |
| COX-2 | cyclooxygenase-2 |
| Mh-ME | M. hexamera Sprague-Methanol Extract |
| Aa-EE | A. asiatica-Ethanol Extract |
| GC-MS | gas chromatography-mass spectrometry |
| RPMI 1640 | Roswell Park Memorial Institute 1640 |
| DMEM | Dulbecco’s modified Eagle’s medium |
| MTT | 3-(4-5-dimetyhlathiaol-2-yl)-2-5-diphenyltetrazolium bromide |
| DMSO | dimethyl sulfoxide |
| SDS | sodium dodecyl sulfate |
| PBS | phosphate-buffered saline |
| CMC | carboxymethylcellulose |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| PEI | polyethylenimine |
| TBST | tris-buffered saline with Tween 20 |
| CETSA | cellular thermal shift assay |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
References
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Rabbi Mashrur, F.; Runa, N.J.; Kwon, K.W.; Hosseinzadeh, H.; Cho, J.Y. Ginseng, a promising choice for SARS-COV-2: A mini review. J. Ginseng Res. 2022, 46, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997, 388, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Correia, J.; Soldau, K.; Christen, U.; Tobias, P.S.; Ulevitch, R.J. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. J. Biol. Chem. 2001, 276, 21129–21135. [Google Scholar] [CrossRef]
- Ullah, M.O.; Sweet, M.J.; Mansell, A.; Kellie, S.; Kobe, B. TRIF-dependent TLR signaling, its functions in host defense and inflammation, and its potential as a therapeutic target. J. Leuk. Biol. 2016, 100, 27–45. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Chain, B.M.; Cho, J.Y. Distinct role of spleen tyrosine kinase in the early phosphorylation of inhibitor of κBα via activation of the phosphoinositide-3-kinase and Akt pathways. Int. J. Biochem. Cell Biol. 2009, 41, 811–821. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Inflammation in atherosclerosis. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.d.M.; Kaplan, M. Medicinal uses of species from Myrtaceae and Melastomataceae families in Brazil. Floresta Ambiente 2014, 11, 47–52. [Google Scholar]
- Valverde Malaver, C.L.; Colmenares Dulcey, A.J.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Macías, F.A.; Isaza Martínez, J.H. Hydrolysable tannins and biological activities of Meriania hernandoi and Meriania nobilis (Melastomataceae). Molecules 2019, 24, 746. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Yi, Y.S.; Sung, G.H.; Yang, W.S.; Park, J.G.; Yoon, K.; Yoon, D.H.; Song, C.; Lee, Y.; Rhee, M.H.; et al. Anti-inflammatory activities and mechanisms of Artemisia asiatica ethanol extract. J. Ethnopharmacol. 2014, 152, 487–496. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Tang, Y.Z.; Liu, Y.H. Wogonoside displays anti-inflammatory effects through modulating inflammatory mediator expression using RAW264.7 cells. J. Ethnopharmacol. 2013, 148, 271–276. [Google Scholar] [CrossRef]
- You, L.; Cho, J.Y. The regulatory role of Korean ginseng in skin cells. J. Ginseng Res. 2021, 45, 363–370. [Google Scholar] [CrossRef]
- Deng, H.; Jiang, J.; Shu, J.; Huang, M.; Zhang, Q.L.; Wu, L.J.; Sun, W.K. Bavachinin ameliorates rheumatoid arthritis inflammation via PPARG/PI3K/AKT signaling pathway. Inflammation, 2023; in press. [Google Scholar] [CrossRef]
- Ichikawa, D.; Matsui, A.; Imai, M.; Sonoda, Y.; Kasahara, T. Effect of various catechins on the IL-12p40 production by murine peritoneal macrophages and a macrophage cell line, J774.1. Biol. Pharm. Bull. 2004, 27, 1353–1358. [Google Scholar] [CrossRef]
- Kresnamurti, A.; Rakhma, D.N.; Damayanti, A.; Santoso, S.D.; Restryarto, E.; Hadinata, W.; Hamid, I.S. AST/ALT levels, MDA, and liver histopathology of Echinometra mathaei ethanol extract on paracetamol-induced hepatotoxicity in rats. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 511–516. [Google Scholar] [CrossRef]
- Tahmasebi, M.; Sadeghi, H.; Nazem, H.; Kokhdan, E.P.; Omidifar, N. Hepatoprotective effects of Berberis vulgaris leaf extract on carbon tetrachloride-induced hepatotoxicity in rats. J. Educ. Health Promot. 2018, 7, 147. [Google Scholar]
- Barbagallo, M.; Sacerdote, P. Ibuprofen in the treatment of children’s inflammatory pain: A clinical and pharmacological overview. Minerva. Pediatr. 2019, 71, 82–99. [Google Scholar] [CrossRef]
- Vane, J.R.; Botting, R.M. Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 1998, 47 (Suppl. S2), S78–S87. [Google Scholar] [CrossRef]
- García-Rayado, G.; Navarro, M.; Lanas, A. NSAID induced gastrointestinal damage and designing GI-sparing NSAIDs. Expert Rev. Clin. Pharmacol. 2018, 11, 1031–1043. [Google Scholar] [CrossRef]
- Wang, L.; Jiao, X.F.; Wu, C.; Li, X.Q.; Sun, H.X.; Shen, X.Y.; Zhang, K.Z.; Zhao, C.; Liu, L.; Wang, M.; et al. Trimetazidine attenuates dexamethasone-induced muscle atrophy via inhibiting NLRP3/GSDMD pathway-mediated pyroptosis. Cell Death Discov. 2021, 7, 251. [Google Scholar] [CrossRef]
- White, C.M.; Hernandez, A.V. Ranitidine and Risk of N-Nitrosodimethylamine (NDMA) Formation. JAMA 2021, 326, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Kantor, E.D.; O’Connell, K.; Du, M.; Mendelsohn, R.B.; Liang, P.S.; Braunstein, L.Z. Ranitidine use and cancer risk: Results from UK biobank. Gastroenterology 2021, 160, 1856–1859.e5. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, J.G.S.; Bianchin, M.; Lopes, A.P.; Silva, E.; Ames, F.Q.; Pomini, A.M.; Carpes, S.T.; de Carvalho Rinaldi, J.; Cabral Melo, R.; Kioshima, E.S.; et al. Chemical profile, antioxidant and anti-inflammatory properties of Miconia albicans (Sw.) Triana (Melastomataceae) fruits extract. J. Ethnopharmacol. 2021, 273, 113979. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2007, 51, 443–452. [Google Scholar] [CrossRef]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef]
- Furuno, K.; Akasako, T.; Sugihara, N. The contribution of the pyrogallol moiety to the superoxide radical scavenging activity of flavonoids. Biol. Pharm. Bull. 2002, 25, 19–23. [Google Scholar] [CrossRef]
- Molloy, R.G.; Mannick, J.A.; Rodrick, M.L. Cytokines, sepsis and immunomodulation. Br. J. Surg. 2005, 80, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Stacey, K.J.; Sweet, M.J.; Hume, D.A. Macrophages ingest and are activated by bacterial DNA. J. Immunol. 1996, 157, 2116–2122. [Google Scholar] [CrossRef] [PubMed]
- Laird, M.H.W.; Rhee, S.H.; Perkins, D.J.; Medvedev, A.E.; Piao, W.; Fenton, M.J.; Vogel, S.N. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J. Leuk. Biol. 2009, 85, 966–977. [Google Scholar] [CrossRef]
- Christian, F.; Smith, E.; Carmody, R. The Regulation of NF-κB subunits by phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Gaynor, R.B. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J. Clin. Investig. 2001, 107, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-kB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Lu, P.; Chen, J.; Yan, L.; Yang, L.; Zhang, L.; Dai, J.; Hao, Z.; Bai, T.; Xi, Y.; Li, Y.; et al. RasGRF2 promotes migration and invasion of colorectal cancer cells by modulating expression of MMP9 through Src/Akt/NF-κB pathway. Cancer Biol. Ther. 2019, 20, 435–443. [Google Scholar] [CrossRef]
- Lee, H.S.; Moon, C.; Lee, H.W.; Park, E.-M.; Cho, M.-S.; Kang, J.L. Src Tyrosine kinases mediate activations of NF-κB and integrin signal during lipopolysaccharide-induced acute lung injury. J. Immunol. 2007, 179, 7001–7011. [Google Scholar] [CrossRef]
- Lowell, C.A. Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: Signaling cross talk. Cold Spring Harb. Perspect. Biol. 2011, 3, a002352. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Lee, H.P.; Kim, D.S.; Park, S.H.; Shin, C.Y.; Woo, J.J.; Kim, J.W.; An, R.B.; Lee, C.; Cho, J.Y. Antioxidant capacity of Potentilla paradoxa Nutt. and its beneficial effects related to anti-Aging in HaCaT and B16F10 Cells. Plants 2022, 11, 873. [Google Scholar] [CrossRef]
- Choi, W.; Kim, H.S.; Park, S.H.; Kim, D.; Hong, Y.D.; Kim, J.H.; Cho, J.Y. Syringaresinol derived from Panax ginseng berry attenuates oxidative stress-induced skin aging via autophagy. J. Ginseng Res. 2022, 46, 536–542. [Google Scholar] [CrossRef]
- Kim, J.K.; Shin, K.K.; Kim, H.; Hong, Y.H.; Choi, W.; Kwak, Y.S.; Han, C.K.; Hyun, S.H.; Cho, J.Y. Korean Red Ginseng exerts anti-inflammatory and autophagy-promoting activities in aged mice. J. Ginseng Res. 2021, 45, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Hwang, S.H.; Shen, T.; Kim, J.H.; You, L.; Hu, W.; Cho, J.Y. Enhancement of skin barrier and hydration-related molecules by protopanaxatriol in human keratinocytes. J. Ginseng Res. 2021, 45, 354–360. [Google Scholar] [CrossRef]
- Painsipp, E.; Wultsch, T.; Shahbazian, A.; Edelsbrunner, M.; Kreissl, M.C.; Schirbel, A.; Bock, E.; Pabst, M.A.; Thoeringer, C.K.; Huber, H.P.; et al. Experimental gastritis in mice enhances anxiety in a gender-related manner. Neuroscience 2007, 150, 522–536. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mitra, A.; Rahmawati, L.; Lee, H.P.; Kim, S.A.; Han, C.K.; Hyun, S.H.; Cho, J.Y. Korean Red Ginseng water extract inhibits cadmium-induced lung injury via suppressing MAPK/ERK1/2/AP-1 pathway. J. Ginseng Res. 2022, 46, 690–699. [Google Scholar] [CrossRef] [PubMed]






| Peak No. | RT (min) | Compound Name | Corrected Area | % of Total |
|---|---|---|---|---|
| 1 | 8.780 | 5-Hydroxymethylfurfural | 5,310,668 | 1.388 |
| 2 | 9.806 | 1,1-diethyl-2-(1-methyl propyl)-Hydrazine | 7,381,173 | 1.929 |
| 3 | 10.301 | 2,7-Oxepanedione | 4,908,751 | 1.283 |
| 4 | 10.810 | 1,2,3-Benzenetriol | 251,749,615 | 65.794 |
| 5 | 11.193 | 1,2,3-Benzenetriol | 5,064,771 | 1.324 |
| 6 | 12.231 | 1,6-anhydro-beta-D-Glucopyranose | 28,492,675 | 7.446 |
| 7 | 13.946 | 4-hydroxy-Benzenepropanoic acid | 7,835,372 | 2.048 |
| 8 | 14.221 | 4-hydroxy-3-methoxy- Benzenepropanol | 7,587,564 | 1.983 |
| 9 | 16.218 | Neophytadiene | 4,036,831 | 1.055 |
| 10 | 16.663 | Z-8-Methyl-9-tetradecen-1-ol formate | 2,380,805 | 0.622 |
| 11 | 17.410 | n-Hexadecanoic acid | 20,611,816 | 5.387 |
| 12 | 19.114 | (Z,Z,Z)-9,12,15-Octadecatrienoic acid | 9,550,118 | 2.496 |
| 13 | 19.299 | Octadecanoic acid | 2,647,456 | 0.692 |
| 14 | 29.383 | gamma-Sitosterol | 2,5075,899 | 6.554 |
| Extract | Total Phenolic Contents 1 | Total Flavonoid Contents 2 |
|---|---|---|
| Mh-ME | 301.33 ± 0.98 | 19.05 ± 1.19 |
| RT (min) | Component Name | Formula |
|---|---|---|
| 0.94 | Wogonoside | C22H20O11 |
| 0.98 | Kaempferol-7-O-α-L-rhamnoside | C21H20O10 |
| 2.65 | Bavachinin | C21H22O4 |
| 2.90 | Kaempferol-3-gentiobioside | C27H30O16 |
| Gene Name | Sequence (5′-3′) | |
|---|---|---|
| iNOS | Forward Reverse | TGCCAGGGTCACAACTTTACA ACCCCAAGCAAGACTTGGAC |
| IL-1β | Forward Reverse | CAGGATGAGGACATGAGCACC CTCTGCAGACTCAAACTCCAC |
| IL-6 | Forward Reverse | GGAAATCGTGGAAATGAG GCTTAGGCATAACGCACT |
| GAPDH | Forward Reverse | GAAGGTCGGTGTGAACGGAT AGTGATGGCATGGACTGTGG |
| Gene Name | Sequence (5′-3′) | |
|---|---|---|
| IL-1β | Forward Reverse | GTGAAATGCCACCTTTTACAGTG CCTGCCTGAAGCTCTTGTTG |
| TNF-α | Forward Reverse | TGCCTATGTCTCAGCCTCTT GAGGCCATTTGGGAACTTCT |
| GAPDH | Forward Reverse | GGAGAGTGTTTCCTCGTCCC ATGAAGGGGTCGTTGATGGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, K.W.; Jang, W.Y.; Kim, J.W.; Noh, J.K.; Yi, D.-K.; Cho, J.Y. Anti-Inflammatory Effect of Meriania hexamera Sprague by Targeting Syk Kinase in NF-κB Signaling. Plants 2023, 12, 3044. https://doi.org/10.3390/plants12173044
Kwon KW, Jang WY, Kim JW, Noh JK, Yi D-K, Cho JY. Anti-Inflammatory Effect of Meriania hexamera Sprague by Targeting Syk Kinase in NF-κB Signaling. Plants. 2023; 12(17):3044. https://doi.org/10.3390/plants12173044
Chicago/Turabian StyleKwon, Ki Woong, Won Young Jang, Ji Won Kim, Jin Kyoung Noh, Dong-Keun Yi, and Jae Youl Cho. 2023. "Anti-Inflammatory Effect of Meriania hexamera Sprague by Targeting Syk Kinase in NF-κB Signaling" Plants 12, no. 17: 3044. https://doi.org/10.3390/plants12173044
APA StyleKwon, K. W., Jang, W. Y., Kim, J. W., Noh, J. K., Yi, D.-K., & Cho, J. Y. (2023). Anti-Inflammatory Effect of Meriania hexamera Sprague by Targeting Syk Kinase in NF-κB Signaling. Plants, 12(17), 3044. https://doi.org/10.3390/plants12173044










