Antioxidant Effect of the Ethyl Acetate Extract of Potentilla indica on Kidney Mitochondria of Streptozotocin-Induced Diabetic Rats
Abstract
1. Introduction
2. Results
2.1. Determination of Phytochemical Compounds of the Potentilla indica Extract
2.2. In Vitro Antioxidant Activity
2.3. Effect of the Extract on Body Weight and Biochemical Parameters
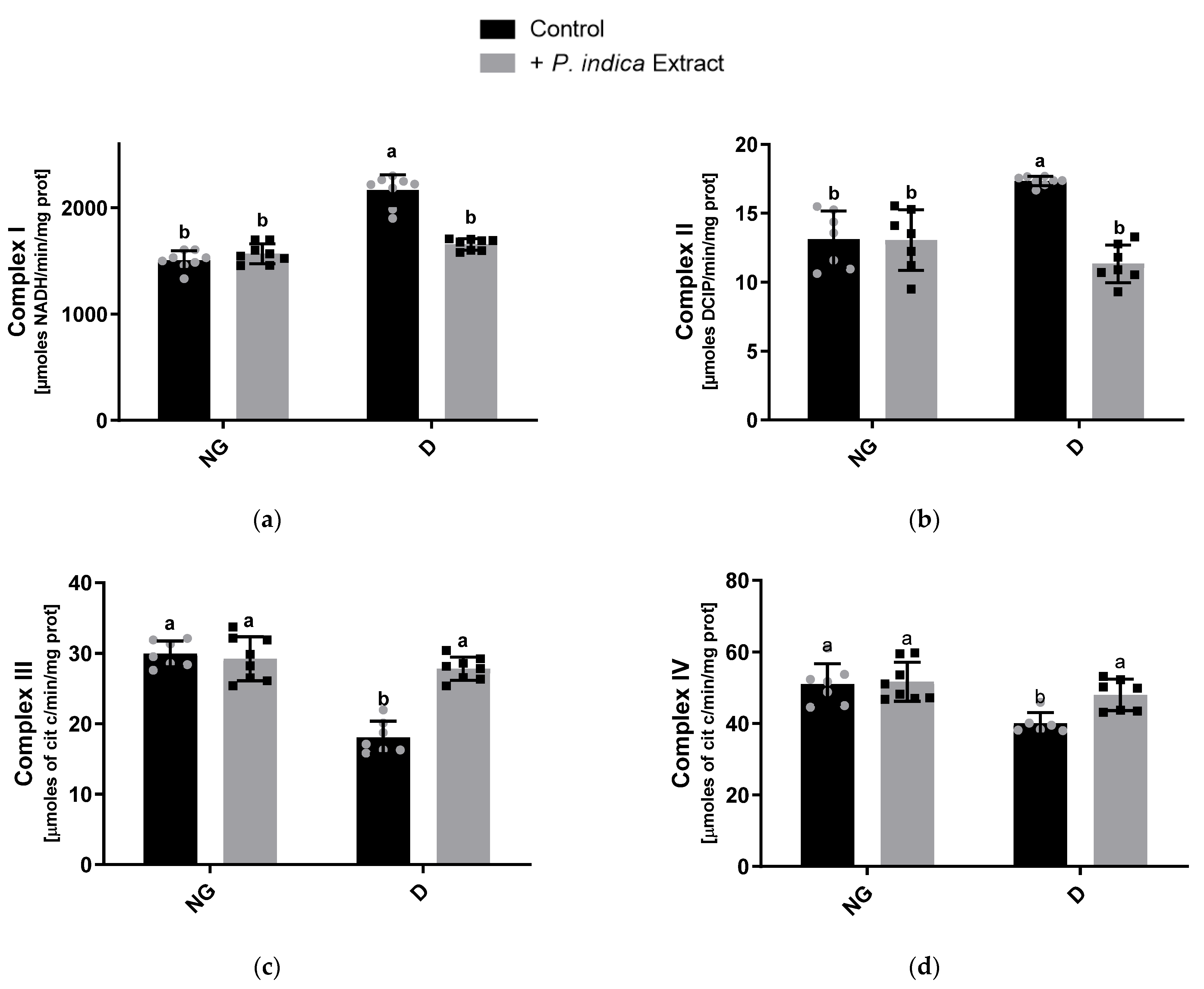
2.4. Effect of the Extract on Mitochondrial Respiratory Chain Complex Activities
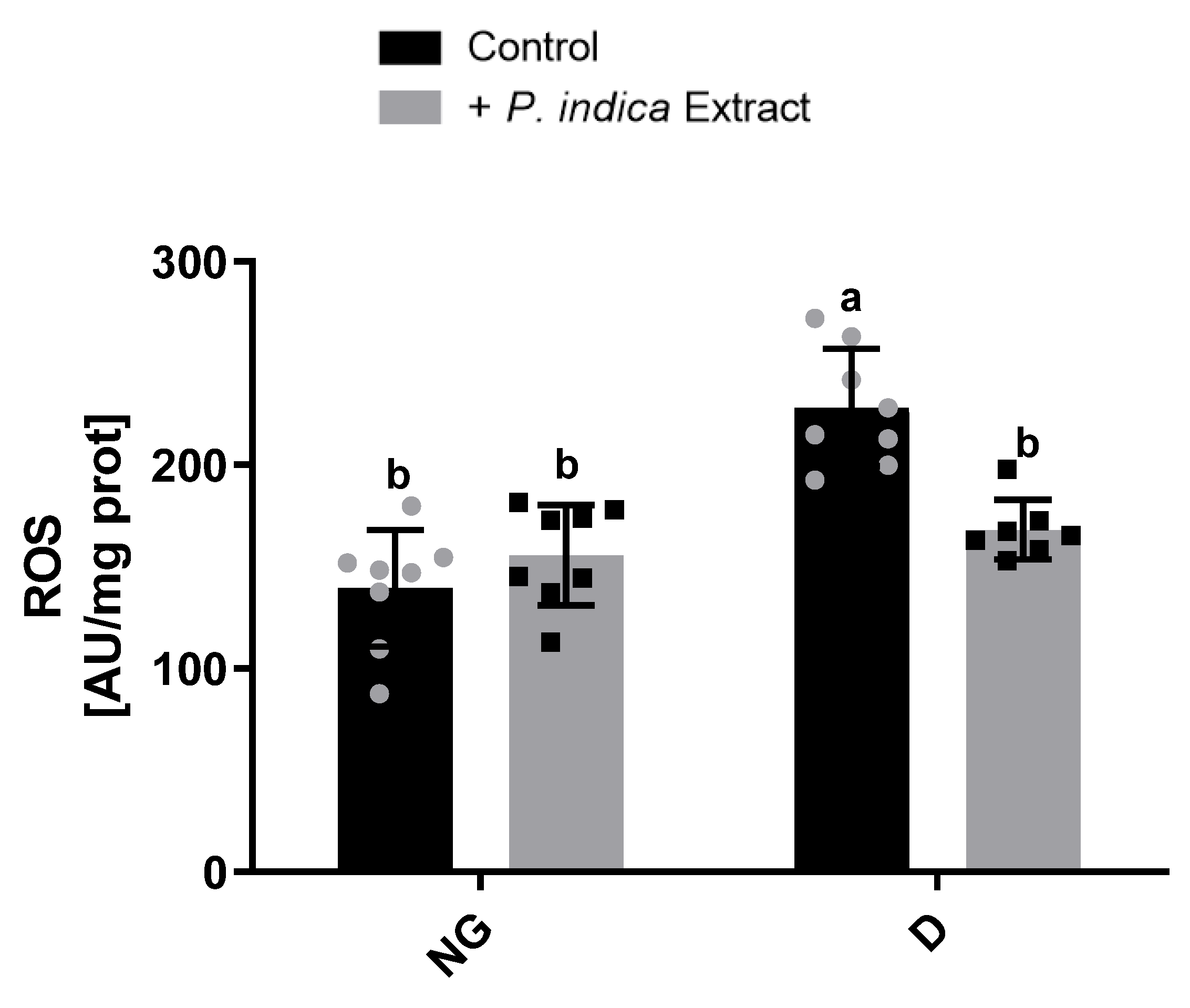
2.5. Effect of the Extract on ROS Production
2.6. Effect of the Ethyl Acetate Extract of P. indica on Mitochondrial Lipid Peroxidation
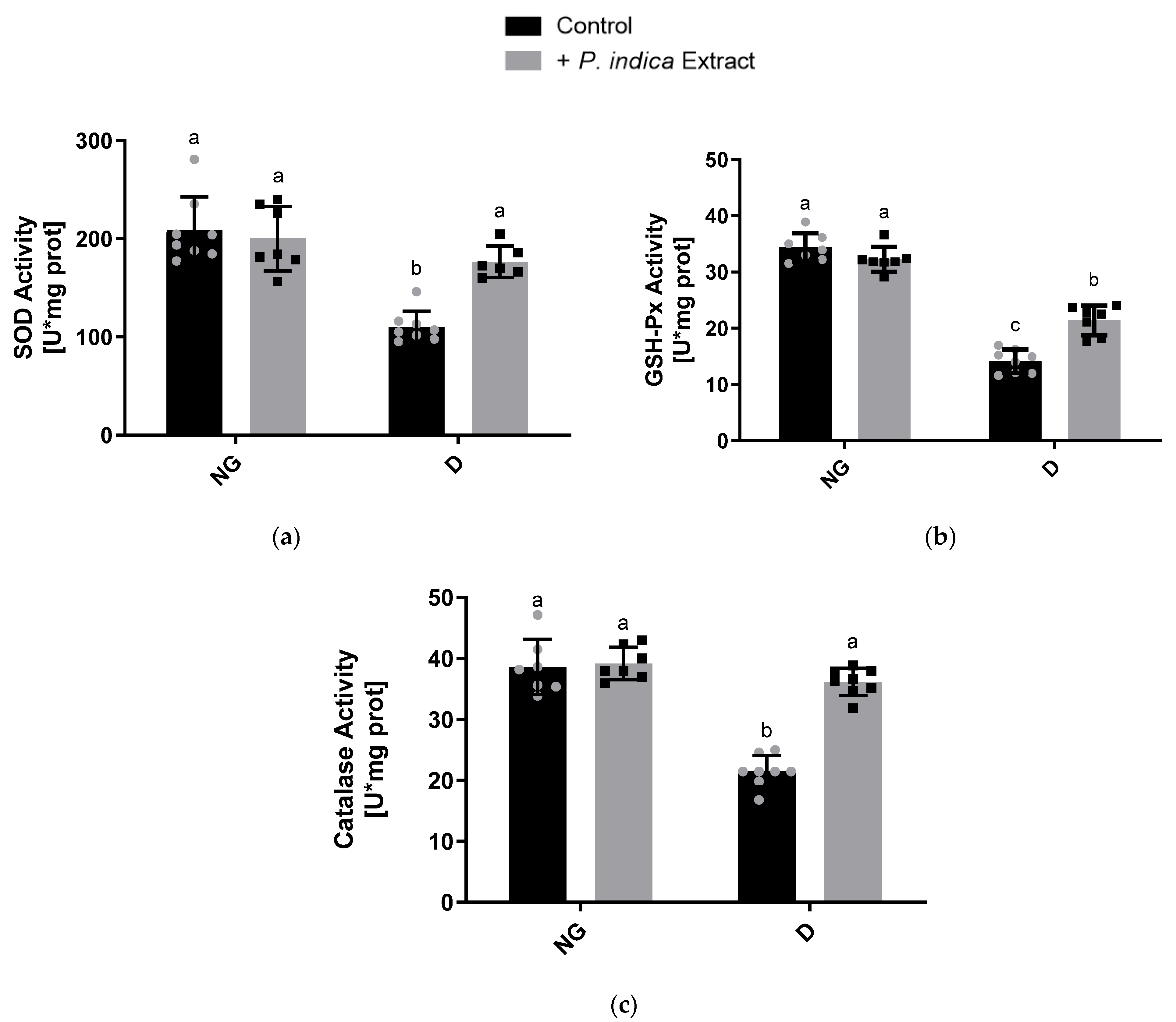
2.7. Effect of the Ethyl Acetate Extract of P. indica on the Antioxidant Enzyme Activities
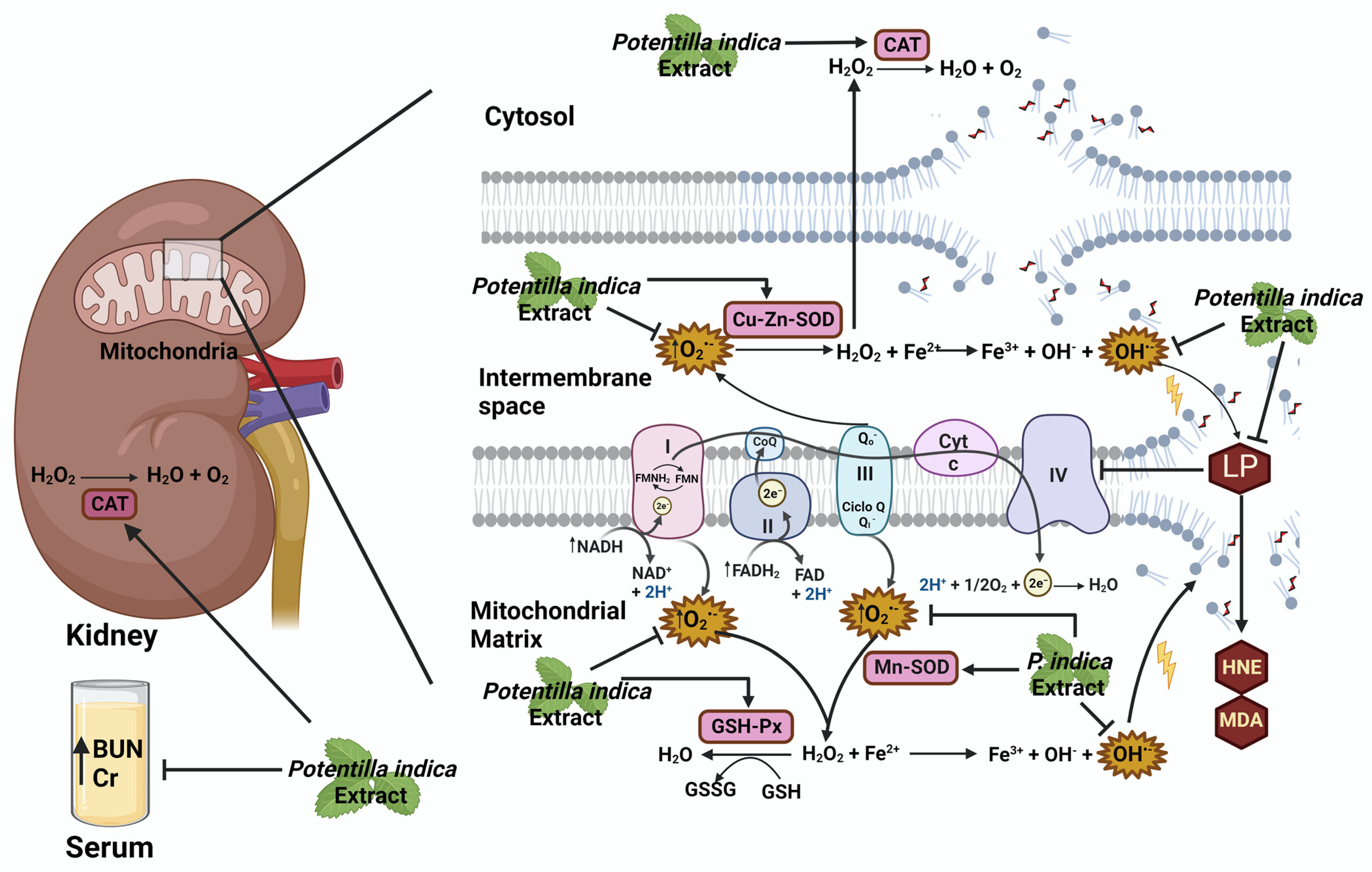
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction
4.2. Determination of Phytochemical Compounds of the Ethyl Acetate Extract of Potentilla indica
4.2.1. Total Phenolic Acids Determination
4.2.2. Total Flavonoids Determination
4.2.3. Determination of Total Terpenoids
4.2.4. Identification and Quantification of Phenolic Compounds
4.3. Evaluation of Antioxidant Activity of the Ethyl Acetate Extract of Potentilla indica In Vitro
4.3.1. DPPH Assay
4.3.2. Anti-Lipid Peroxidation
4.3.3. Ferric Reducing Antioxidant Power (FRAP) Determination
4.4. Animal Care and Use Statement
4.5. Evaluation of Antioxidant Activity of the Ethyl Acetate Extract of Potentilla indica In Vivo
4.5.1. Experimental Design
4.5.2. Measurement of Biochemical Serum Parameters
4.5.3. Tissue Preparation and Mitochondrial Isolation
4.5.4. Determination of Mitochondrial Respiratory Chain Complex Activity
4.5.5. Evaluation of ROS Production
4.5.6. Lipid Peroxidation Determination
4.5.7. Glutathione Peroxidase Activity Determination
4.5.8. Superoxide Dismutase Activity Determination
4.5.9. Evaluation of Catalase Activity
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Standards of Medical Care in Diabetes-2017. Abridged for Primary Care Providers. Clin. Diabetes 2017, 35, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Hernández, J.; Licea-Puig, M.; Hernández-García, P.; Abraham-Marcel, E.; Yanes-Quesada, M. Estrés oxidativo y diabetes mellitus. Rev. Mex. Patol. Clin. 2011, 58, 4–15. [Google Scholar]
- International Diabetes Federation. IDF Diabetes Atlas 2021, 10th ed. Available online: https://diabetesatlas.org/ (accessed on 15 October 2022).
- Correa-Freitas, P.A.; Rozales-Ehlert, L.; Camargo-Lins, J. Glycated albumin: A potential biomarker in diabetes. Arch. Endocrinol. Metab. 2017, 61, 3. [Google Scholar]
- Goutos, I.; Nicholas, R.S.; Pandya, A.A.; Ghosh, S.J. Diabetes mellitus and burns. Part I-basic science and implications for management. Int. J. Burns Trauma 2015, 1, 1. [Google Scholar]
- Eid, S.; Sas, K.M.; Abcouwer, S.F.; Feldman, E.L.; Gardner, T.W.; Pennathur, S.; Fort, P.E. New insights into the mechanisms of diabetic complications: Role of lipids and lipid metabolism. Diabetologia 2019, 62, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lee, K.; Ni, Z.; He, J. Diabetic Kidney Disease: Challenges, Advances, and Opportunities. Kidney Dis. 2020, 6, 215–225. [Google Scholar] [CrossRef]
- Ji, L.L.; Yeo, D. Oxidative stress: An evolving definition. Fac. Rev. 2021, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Jha, J.C.; Banal, C.; Chow, S.M.; Cooper, M.E.; Jandeleit-Dahm, K. Diabetes and Kidney Disease: Role of Oxidative Stress. Antioxid. Redox Signal. 2016, 25, 657–684. [Google Scholar] [CrossRef]
- Gnudi, L.; Coward, R.J.M.; Long, D.A. Diabetic Nephropathy: Perspective on Novel Molecular Mechanisms. Trends Endocrinol. Metab. 2016, 27, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.F.; Whaley, E.D.; Alexander, M.; Tantisattamo, E.; Ichii, H. Anti-Oxidative Therapy in Diabetic Nephropathy. Front. Biosci. 2022, 14, 14. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; Kumar, N.V.A.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.A.; Tsouh Fokou, V.; Kobarfard, F.; Amiruddin Zakaria, Z.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Ihahi, I.; Khuda, F.; Sahibzada, M.U.K.; Alghamdi, S.; Ullah, R.; Dablool, A.S.; Alam, M.; Khan, A.; Khalil, A.A.K. Synthesis of silver nanoparticles using root extract of Duchesnea indica and assessment of its biological activities. Arab. J. Chem. 2020, 14, 103110. [Google Scholar]
- Qiao, W.; Yao, Z.; Zhang, W.; Duan, H.Q. Two new triterpenes from Duchesnea indica. Chin. Chem. Lett. 2009, 20, 572–575. [Google Scholar] [CrossRef]
- Zhu, M.; Dong, X.; Guo, M. Phenolic Profiling of Duchesnea indica Combining Macroporous Resin Chromatography (MRC) with HPLC-ESI-MS/MS and ESI-IT- MS. Molecules 2015, 20, 22463–22475. [Google Scholar] [CrossRef] [PubMed]
- Arif Ullah, H.M.; Lee, Y.Y.; Kim, S.D.; Rhee, M.H. Duchesnea indica Extract Attenuates Coal Fly Ash-Induced Inflammation in Murine Alveolar Macrophages through the NF-Kappa B Pathway. eCAM 2021, 2021, 5546052. [Google Scholar]
- Liaqat, M.; Kakar, I.U.; Akram, M.; Hussain, S.; Kakar, M.U.; Ahmad, N.; Faryal, R. Antimicrobial and Phytochemical Exploration of Duchesnea indica plant. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 74–85. [Google Scholar]
- Mezil, S.; Ahmed, B. Complications of Diabetes Mellitus. Ann. Rom. Soc. Cell Biol. 2021, 25, 1546–1556. [Google Scholar]
- Siboto, A.; Sibiya, N.; Khathi, A.; Ngubane, P. The effects of Momordica balsamina methanolic extract on kidney function in STZ-induced diabetic rats: Effects on Selected Metabolic Markers. J. Diabetes Res. 2018, 2018, 7341242. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodnia, L.; Aghadavod, E.; Beigrezaei, S.; Rafieian-Kopaei, M. An update on diabetic kidney disease, oxidative stress and antioxidant agents. J. Ren. Inj. Prev. 2017, 6, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Wang, D.M.; Pu, W.J.; Li, D.W. Phytochemical profiles, antioxidant and antimicrobial activities of three Potentilla species. BMC Complement. Med. Ther. 2013, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Goli, A.H.; Barzegar; Sahari, M.A. Antioxidant activity and total phenolic compounds of pistachio (Pistachia vera) hull extracts. Food Chem. 2005, 92, 521–525. [Google Scholar] [CrossRef]
- Somayeh, A.; Mohammad, T.M.; Majid, A.S.; Zahra, L. Antioxidant potential and total phenolic compounds of extracts and fractions of Pistasia atlantica. Int. J. Pharm. Clin. Res. 2017, 9, 293–297. [Google Scholar]
- Gülcin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Xie, J.; Wang, W.; Dong, C.; Huang, L.; Wang, H.; Li, C.; Nie, S.; Xie, M. Protective effect of flavonoids from Cyclocarya paliurus leaves against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. 2018, 119, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Albasher, G.; Alwahaibi, M.; Abdel-Daim, M.; Alkahtani, S.; Almeer, R. Protective effects of Artemisia judaica extract compared to metformin against hepatorenal injury in high-fat diet/streptozotocine-induced diabetic rats. Environ. Sci. Pollut. Res. 2020, 27, 40525–40536. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Liang, B.; Li, Y. Antihyperglycemic effect of Ginkgo biloba extract in streptozotocin-induced diabetes in rats. Biomed. Res. Int. 2013, 2013, 162724. [Google Scholar] [CrossRef]
- Gnudi, L.; Thomas, S.M.; Viberti, G. Mechanical forces in diabetic kidney disease: A trigger for impaired glucose metabolism. JASN 2007, 18, 2226–2232. [Google Scholar] [CrossRef] [PubMed]
- Group, D.E.R.; de Boer, I.H.; Sun, W.; Cleary, P.A.; Lachin, J.M.; Molitch, M.E.; Steffes, M.W.; Zinman, B. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N. Engl. J. Med. 2011, 365, 2366–2376. [Google Scholar] [CrossRef]
- Manpreet, K. Sagoo and Luigi Gnudi, Diabetic nephropathy: Is there a role for oxidative stress? Free Radic. Biol. Med. 2017, 106, 50–63. [Google Scholar]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Luo, X.; Yan, L.J. Two dimensional blue native/SDS-PAGE to identify mitochondrial complex I subunits modified by 4-hydroxynonenal (HNE). Front. Physiol. 2015, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Luo, X.; Thangthaeng, N.; Sumien, N.; Chen, Z.; Rutledge, M.A.; Jing, S.; Forster, M.J.; Yan, L.J. Pancreatic mitochondrial complex I exhibits aberrant hyperactivity in diabetes. Biochem. Biophys. Rep. 2017, 11, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Peña-Montes, D.J.; Huerta-Cervantes, M.; Ríos-Silva, M.; Trujillo, X.; Cortés-Rojo, C.; Huerta, M.; Saavedra-Molina, A. Effects of dietary iron restriction on kidney mitochondria function and oxidative stress in streptozotocin-diabetic rats. Mitochondrion 2020, 54, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Bernadette, N.; Gallo, L.A.; Forbes, J.M. Mitochondrial Dysfunction and Signaling in Diabetic Kidney Disease: Oxidative Stress and Beyond. Semin. Nephrol. 2018, 38, 101–110. [Google Scholar]
- Raza, H.; Prabu, S.K.; John, A.; Avadhani, N.G. Impaired Mitochondrial Respiratory Functions and Oxidative Stress in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2011, 12, 3133–3147. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Han, W.; Huang, C. Protective effect of the methanolic extract from Duchesnea indica against oxidative stress in vitro and in vivo. Environ. Toxicol. Pharmacol. 2010, 31, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, H.B.; Hojati, V.; Shiravi, A.; Hosseinian, S.; Vaezi, G. The role of Artemisia turanica extract on renal oxidative and biochemical markers in STZ-induced diabetes in rat. Avicenna J. Phytomed. 2020, 10, 504–512. [Google Scholar]
- Mohebbati, R.; Abbasnezhad, A.; Havakhah, S.; Mousavi, M. The Effect of Nigella sativa on Renal Oxidative Injury in Diabetic Rats. Saudi J. Kidney Dis. Transpl. 2020, 31, 775–786. [Google Scholar]
- Jayaraman, R.; Subramani, S.; Abdullah, S.H.S.; Udaiyar, M. Antihyperglycemic effect of hesperetin, a citrus flavonoid, extenuates hyperglycemia and exploring the potential role in antioxidant and antihyperlipidemic in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2018, 97, 98–106. [Google Scholar]
- Fröde, T.S.; Medeiros, Y.S. Animal models to test drugs with potential antidiabetic activity. J. Ethnopharmacol. 2008, 115, 173–183. [Google Scholar] [PubMed]
- Khan, H.; Tundis, R.; Ullah, H.; Aschner, M.; Belwal, T.; Mirzaei, H.; Akkol, E.K. Flavonoids targeting NRF2 in neurodegenerative disorders. Food Chem. Toxicol. 2020, 146, 111817. [Google Scholar]
- Wang, C.X.; Lv, L.S.; Huang, H.; Guan, J.Q.; Ye, Z.; Li, S.M.; Wang, Y.; Lou, T.; Liu, X. Initiation time of renal replacement therapy on patients with acute kidney injury: A systematic review and meta-analysis of 8179 participants. Nephrology 2017, 22, 7–18. [Google Scholar]
- Madianov, I.V.; Balabolkin, M.I.; Markov, D.S.; Markova, T.N. Main causes of hyperuricemia in diabetes mellitus. Ter. Arkh. 2000, 72, 55–58. [Google Scholar] [PubMed]
- Schwarz, K.; Bertelsen, G.; Nissen, L.; Gardner, P.; Heinonem, M.; Hopia, A.; Huynh-Ba, T.; Lambelet, P.; McPhail, D.; Skibsted, L.; et al. Investigation of plants extracts for the protection of processed foods against lipid oxidation. Comparison of antioxidant assays based on radical scavenging, lipid oxidation and analysis of the principal antioxidant compounds. Eur. Food Res. Technol. 2001, 212, 319–328. [Google Scholar] [CrossRef]
- Kim, D.O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [PubMed]
- Ghorai, N.; Chakraborty, S.; Gucchait, S.; Saha, S.K.; Biswas, S. Estimation of total terpenoids concentration in plant tissues using a monoterpene, Linalool as standart reagent. Protoc. Exch. 2012, 5, 1038. [Google Scholar]
- Juárez-Trujillo, N.; Monribot-Villanueva, J.L.; Jiménez-Fernández, V.M.; Suárez-Montaño, R.; Aguilar-Colorado, A.S.; Guerrero-Analco, J.A.; Jiménez, M. Phytochemical characterization of Izote (Yucca elephantipes) flowers. J. Appl. Bot. Food Qual. 2018, 91, 202–210. [Google Scholar]
- Monribot-Villanueva, J.L.; Elizalde-Contreras, J.M.; Aluja, M.; Segura-Cabrera, A.; Birke, A.; Guerrero-Analco, J.A.; Ruiz-Maya, E. Endorsing and extending the repertory of nutraceutical and antioxidant sources in mangoes during postharvest shelf life. Food Chem. 2019, 285, 119–129. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. 1995, 28, 25–30. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Maruthamuthu, V.; Kandasamy, R. Ferric reducing antioxidant power assay in plant extract. Bangladesh J. Pharmacol. 2016, 11, 570–572. [Google Scholar]
- Noriega-Cisneros, R.; Ortiz-Avila, O.; Esquivel-Gutiérrez, E.; Clemente-Guerrero, M.; Manzo-Avalos, S.; Salgado-Garciglia, R.; Cortés-Rojo, C.; Boldogh, I.; Saavedra-Molina, A. Hypolipidemic activity of Eryngium carlinae on streptozotocin-induced diabetic rats. Biochem. Res. Int. 2012, 2012, 603501. [Google Scholar] [CrossRef] [PubMed]
- Saavedra-Molina, A.; Devlin, T.M. Effect of extra- and intra-mitochondrial calcium on citrulline synthesis. Amino Acids. 1997, 12, 293–298. [Google Scholar]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Spinazzi, M.; Casarin, A.; Pertegato, V.; Salviati, L.; Angelini, C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultures cells. Nat. Protoc. 2012, 7, 1235–1246. [Google Scholar] [CrossRef]
- Cortés-Rojo, C.; Calderón-Cortés, E.; Clemente-Guerrero, M.; Manzo-Avalos, S.; Uribe, S.; Boldogh, I.; Saavedra-Molina, A. Electron transport chain of Saccharomyces cerevisiae mitochondria is inhibited by H2O2 at succinate-cytochrome c oxidoreductase level without lipid peroxidation involvement. Free. Radic. Res. 2007, 41, 1212–1223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zentella de Piña, M.; Villalobos-Molina, R.; Saavedra-Molina, A.; Riveros-Rosas, H.; Piña, E. Effects of moderate chronic ethanol consumption on rat liver mitochondrial functions. Alcohol 1989, 6, 3–7. [Google Scholar]
- Ortiz-Avila, O.; Sámano-García, C.A.; Calderón-Cortés, E.; Pérez-Hernández, I.H.; Mejía-Zepeda, R.; Rodríguez-Orozco, A.R.; Saavedra-Molina, A.; Cortés-Rojo, C. Dietary avocado oil supplementation attenuates the alterations induced by type I diabetes and oxidative stress in electron transfer at the complex II-complex III segment of the electron transport chain in rat kidney mitochondria. J. Bioenerg. Biomembr. 2013, 45, 271–287. [Google Scholar]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Meth. Enzymol. 1978, 52, 302–310. [Google Scholar]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Jeulin, C.; Soufir, J.C.; Weber, P.; Laval-Martin, D.; Calvayrac, R. Catalase activity in human spermatozoa and seminal plasma. Gamete. Res. 1989, 24, 185–196. [Google Scholar] [CrossRef] [PubMed]

| Total Phenolic Acids (µg of Gallic Acid/mL of Extract) | Total Flavonoids (µg of Quercetin/mL of Extract) | Total Terpenoids (µg of Linalool/mL of Extract) |
|---|---|---|
| 2.84 ± 0.1 | 4251.7 ± 28.9 | 659.9 ± 65.5 |
| Compound | Retention Time (min) | Concentration (µg/g of Dried Extract) | Compound | Retention Time (min) | Concentration (µg/g of Dried Extract) |
|---|---|---|---|---|---|
| Gallic acid | 1.4 | 4.77 + 0.11 | Scopoletin | 8.4 | 3.43 + 0.02 |
| Protocatechuic acid | 2.5 | 18.46 + 0.15 | Ferulic acid | 8.6 | 42.09 + 0.74 |
| 4-Hydroxybenzoic acid | 3.76 | 25.68 + 0.10 | Salicylic acid | 9.15 | 161.29 + 3.18 |
| Vanillic acid | 5.12 | 33.23 + 0.57 | Ellagic acid | 9.98 | 7.24 + 0.43 |
| Chlorogenic acid | 5.34 | 2.46 + 0.01 | Quercetin-3-glucoside | 10.26 | 8.54 + 0.11 |
| Caffeic acid | 5.38 | 13.53 + 0.23 | p-Anisic acid | 10.45 | 11.22 + 0.06 |
| Vanillin | 6.52 | 20.49 + 0.42 | Kaempferol-3-O-glucoside | 11.91 | 8.44 + 0.25 |
| 4-Coumaric acid | 7.21 | 27.84 + 0.71 | t-Cinnamic acid | 14.08 | 27.92 + 0.67 |
| DPPH Scavenging (%) | Anti-Lipid Peroxidation (%) | FRAP (A) | |
|---|---|---|---|
| Trolox (control) | 93.7 ± 4.1 a | 82.1 ± 2.6 a | 0.1798 ± 0.04 a |
| Pi Extract (10 mg/mL) | 4.2 ± 1.03 c | 61.8 ± 0.3 c | 0.0342 ± 0.01 b |
| Pi Extract (25 mg/mL) | 23.8 ± 0.7 b | 75.7 ± 4.2 ab | 0.1406 ± 0.03 a |
| Pi Extract (35 mg/mL) | 24.8 ± 2.04 b | 72.2 ± 2.7 b | 0.1411 ± 0.01 a |
| Body Weight (g) | Glucose (mg/dL) | BUN (mg/dL) | Creatinine (mg/dL) | Uric Acid (mg/dL) | |||
|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | ||||
| NC | 342.3 ± 36.0 ns | 399.7 ± 26.4 a | 84.1 ± 7.0 b | 81.9 ± 11.1 b | 23.7 ± 5.9 c | 0.3 ± 0.02 b | 2.0 ± 0.6 ns |
| DC | 335.0 ± 17.2 ns | 271.5 ± 27.5 c | 468.9 ± 39.2 a | 489.6 ± 89.9 a | 49.2 ± 5.5 a | 0.6 ± 0.05 a | 3.4 ± 1.5 ns |
| N + Pi | 357.3 ± 13.0 ns | 418.6 ± 25.5 a | 81.8 ± 7.4 b | 87.5 ± 8.6 b | 19.1± 6.7 c | 0.3 ± 0.04 b | 1.8 ± 0.8 ns |
| D + Pi | 322.4 ± 41.3 ns | 315.3 ± 32.7 b | 440.8 ± 46.5 a | 381.6 ± 86.7 a | 34.8 ± 4.9 b | 0.3 ± 0.09 b | 2.2 ± 0.6 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landa-Moreno, C.I.; Trejo-Hurtado, C.M.; Lemus-de la Cruz, J.; Peña-Montes, D.J.; Murillo-Villicaña, M.; Huerta-Cervantes, M.; Montoya-Pérez, R.; Salgado-Garciglia, R.; Manzo-Avalos, S.; Cortés-Rojo, C.; et al. Antioxidant Effect of the Ethyl Acetate Extract of Potentilla indica on Kidney Mitochondria of Streptozotocin-Induced Diabetic Rats. Plants 2023, 12, 3196. https://doi.org/10.3390/plants12183196
Landa-Moreno CI, Trejo-Hurtado CM, Lemus-de la Cruz J, Peña-Montes DJ, Murillo-Villicaña M, Huerta-Cervantes M, Montoya-Pérez R, Salgado-Garciglia R, Manzo-Avalos S, Cortés-Rojo C, et al. Antioxidant Effect of the Ethyl Acetate Extract of Potentilla indica on Kidney Mitochondria of Streptozotocin-Induced Diabetic Rats. Plants. 2023; 12(18):3196. https://doi.org/10.3390/plants12183196
Chicago/Turabian StyleLanda-Moreno, Cinthia I., Cristian M. Trejo-Hurtado, Jenaro Lemus-de la Cruz, Donovan J. Peña-Montes, Marina Murillo-Villicaña, Maribel Huerta-Cervantes, Rocío Montoya-Pérez, Rafael Salgado-Garciglia, Salvador Manzo-Avalos, Christian Cortés-Rojo, and et al. 2023. "Antioxidant Effect of the Ethyl Acetate Extract of Potentilla indica on Kidney Mitochondria of Streptozotocin-Induced Diabetic Rats" Plants 12, no. 18: 3196. https://doi.org/10.3390/plants12183196
APA StyleLanda-Moreno, C. I., Trejo-Hurtado, C. M., Lemus-de la Cruz, J., Peña-Montes, D. J., Murillo-Villicaña, M., Huerta-Cervantes, M., Montoya-Pérez, R., Salgado-Garciglia, R., Manzo-Avalos, S., Cortés-Rojo, C., Monribot-Villanueva, J. L., Guerrero-Analco, J. A., & Saavedra-Molina, A. (2023). Antioxidant Effect of the Ethyl Acetate Extract of Potentilla indica on Kidney Mitochondria of Streptozotocin-Induced Diabetic Rats. Plants, 12(18), 3196. https://doi.org/10.3390/plants12183196









