Reconstructed Global Invasion and Spatio-Temporal Distribution Pattern Dynamics of Sorghum halepense under Climate and Land-Use Change
Abstract
:1. Introduction
2. Results
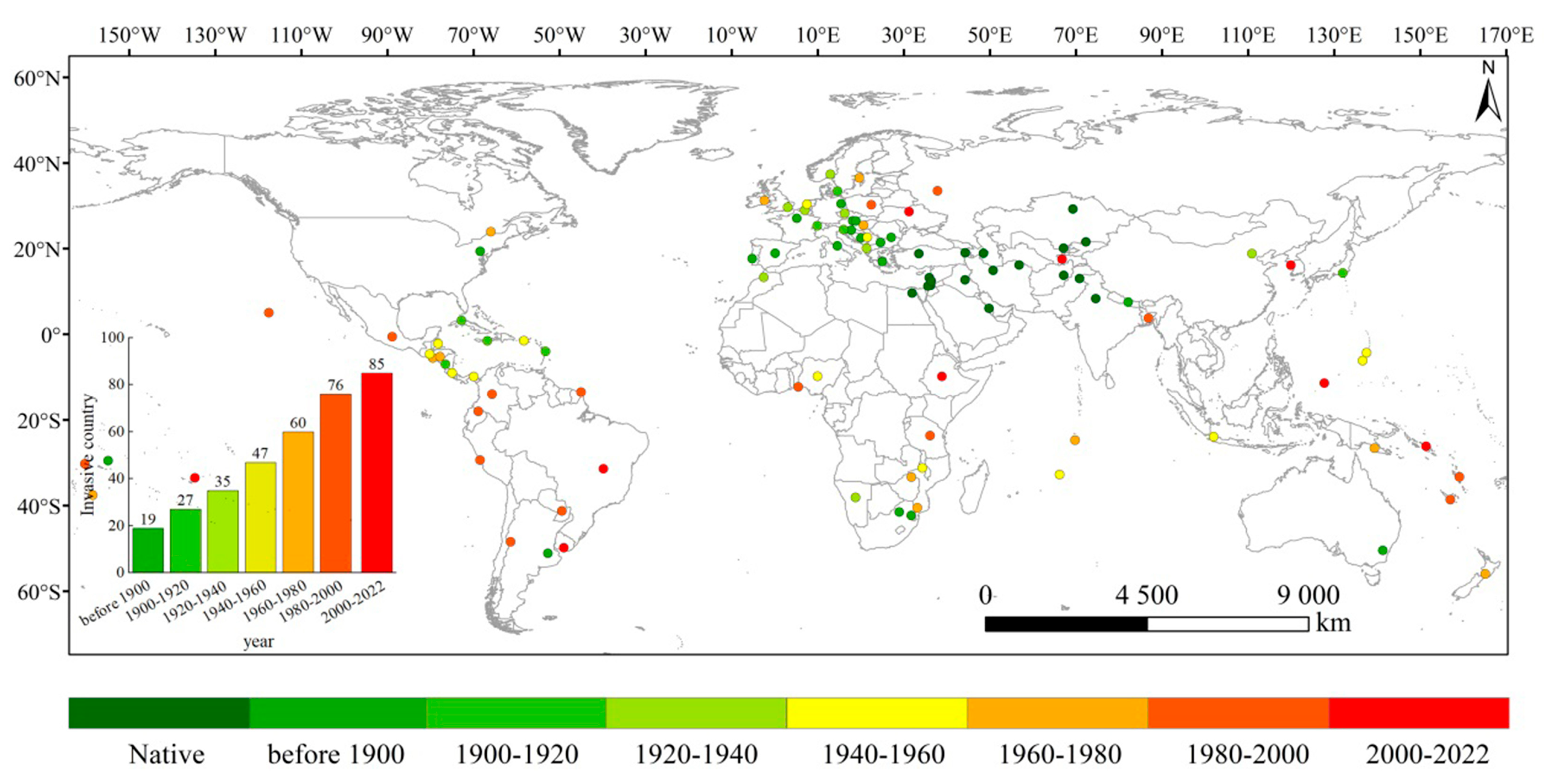
2.1. Reconstruction of Historically Invaded Countries
2.2. Model Performance and Significant Environmental Variables
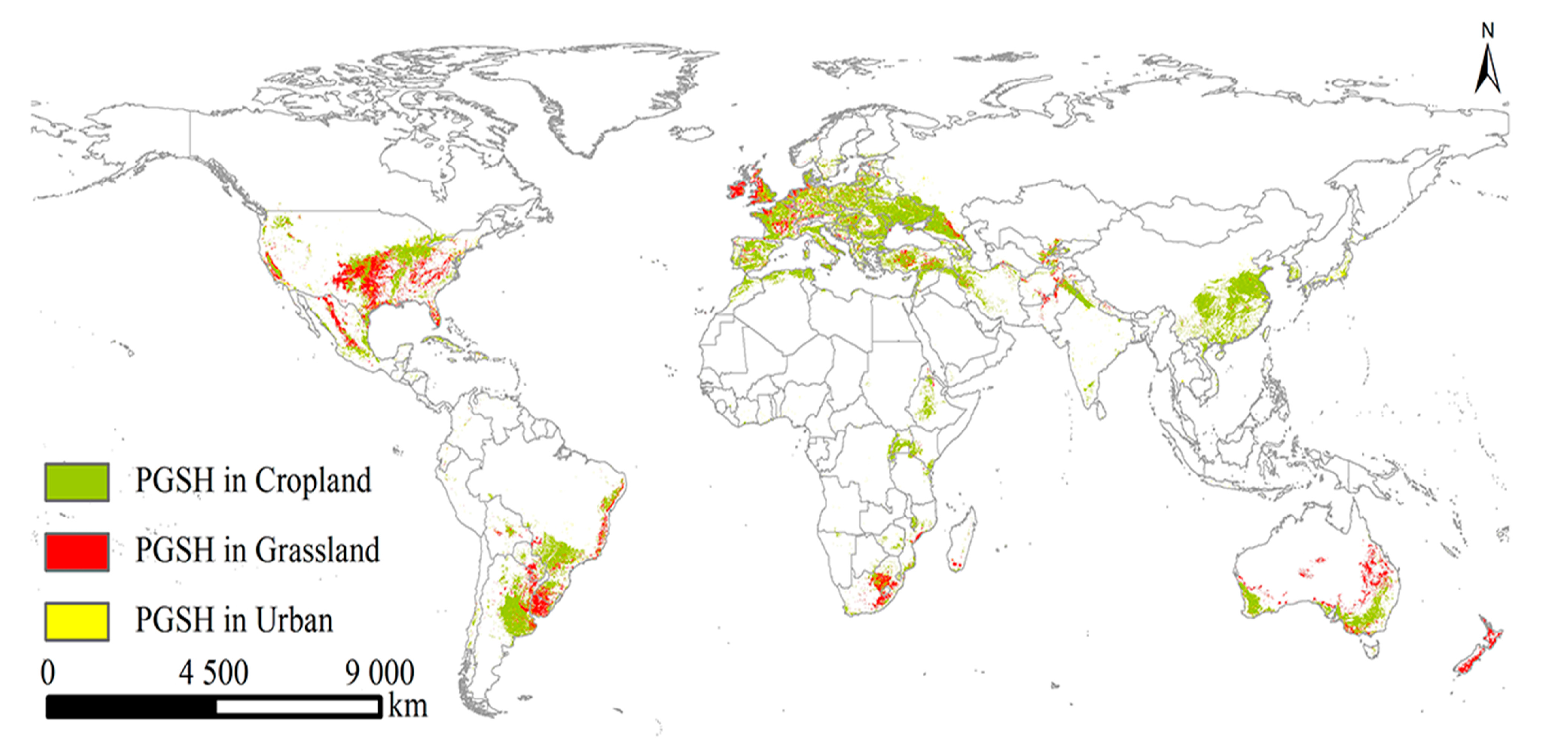
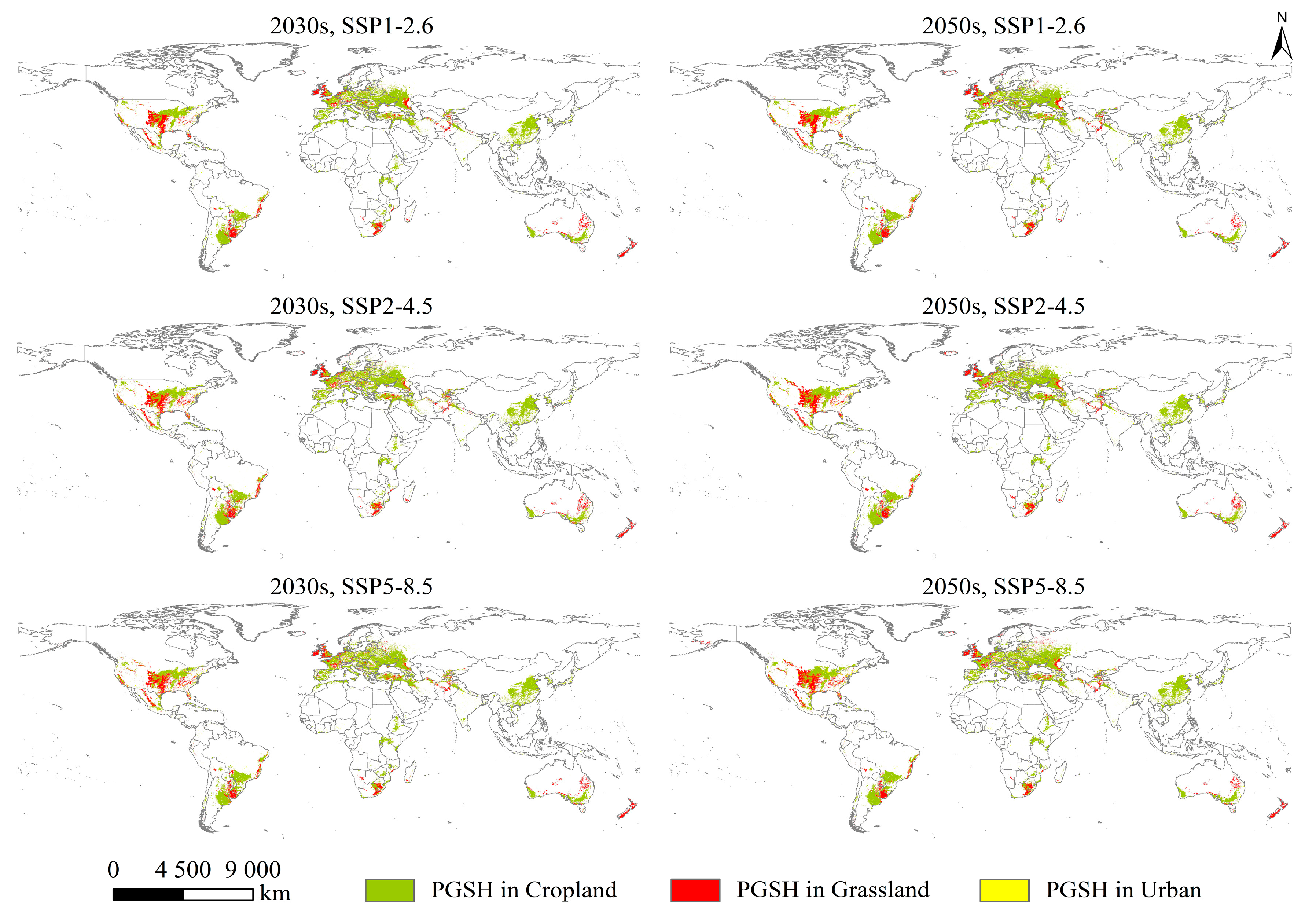
2.3. The PGSHs of S. halepense under Current and Future Climate Scenarios
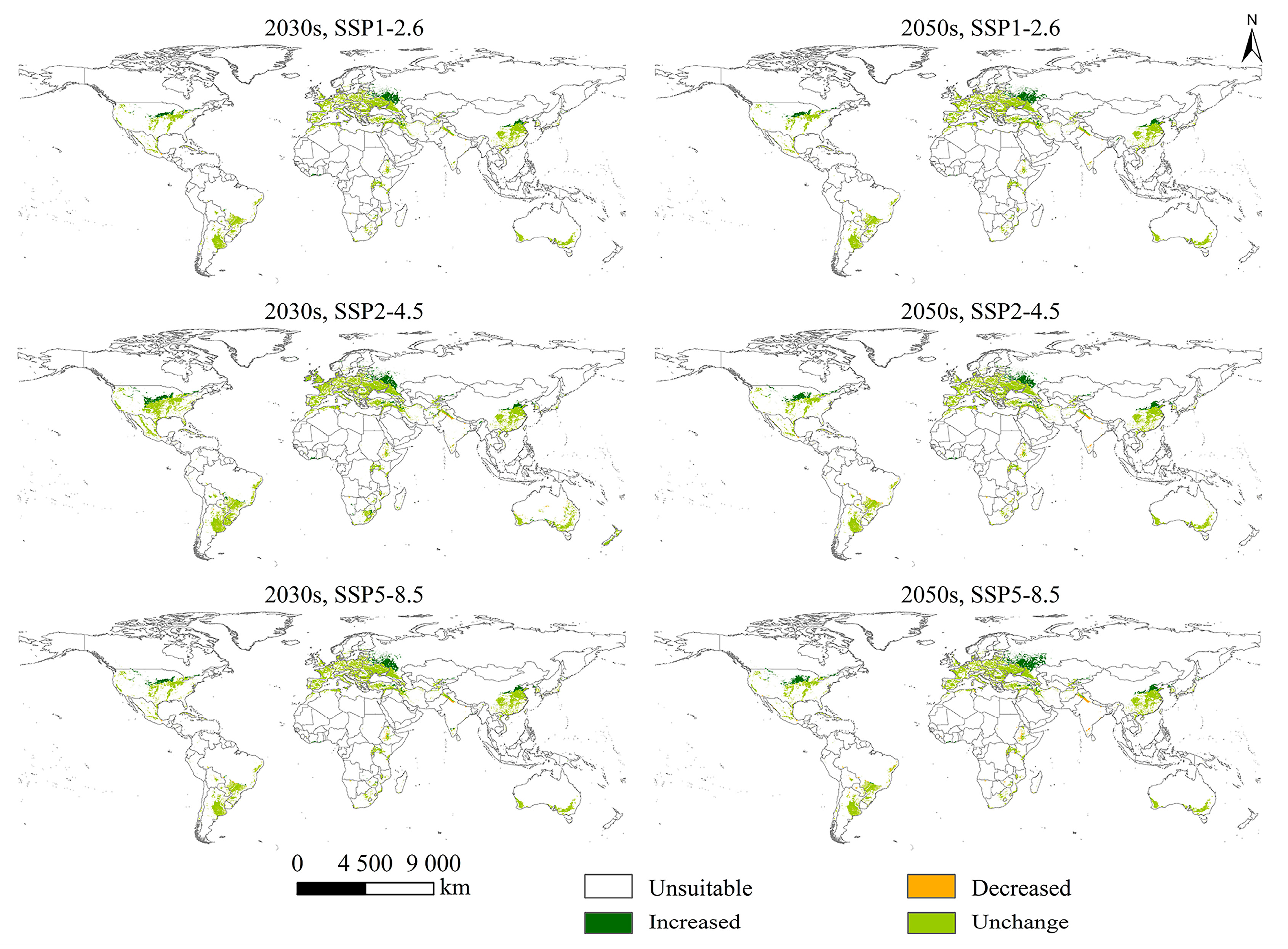
2.4. Changes in PGSHs of S. halepense
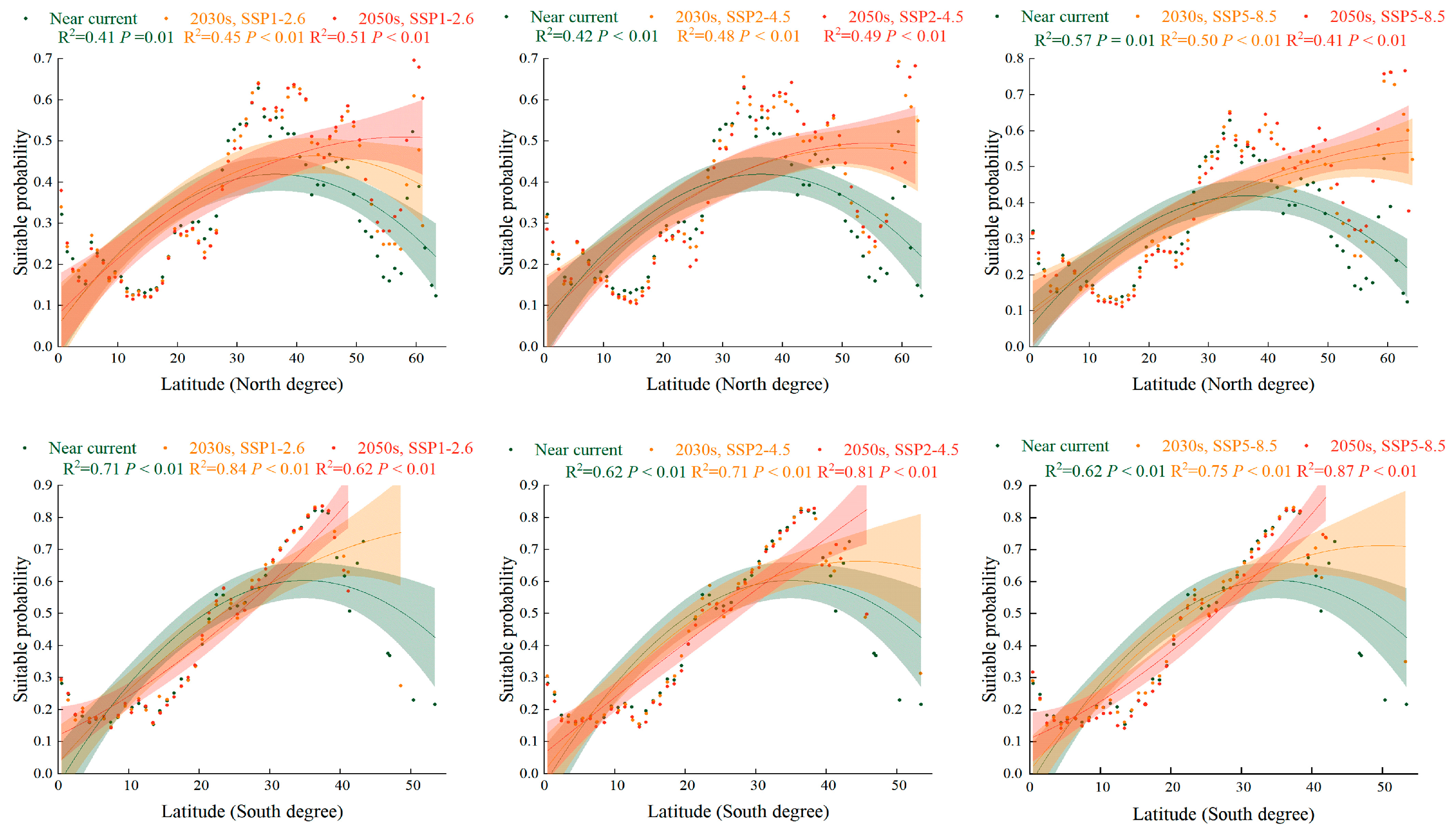
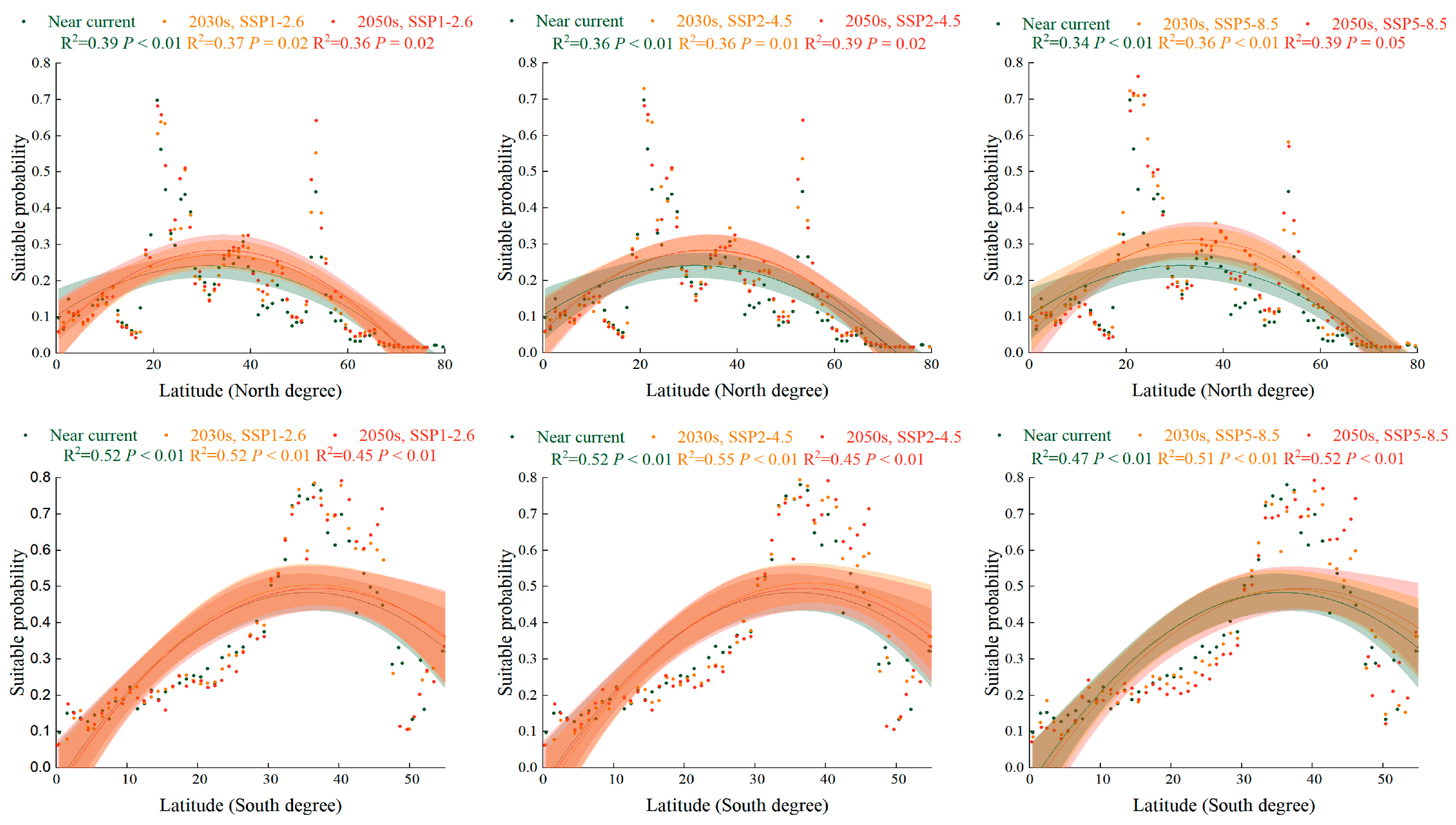
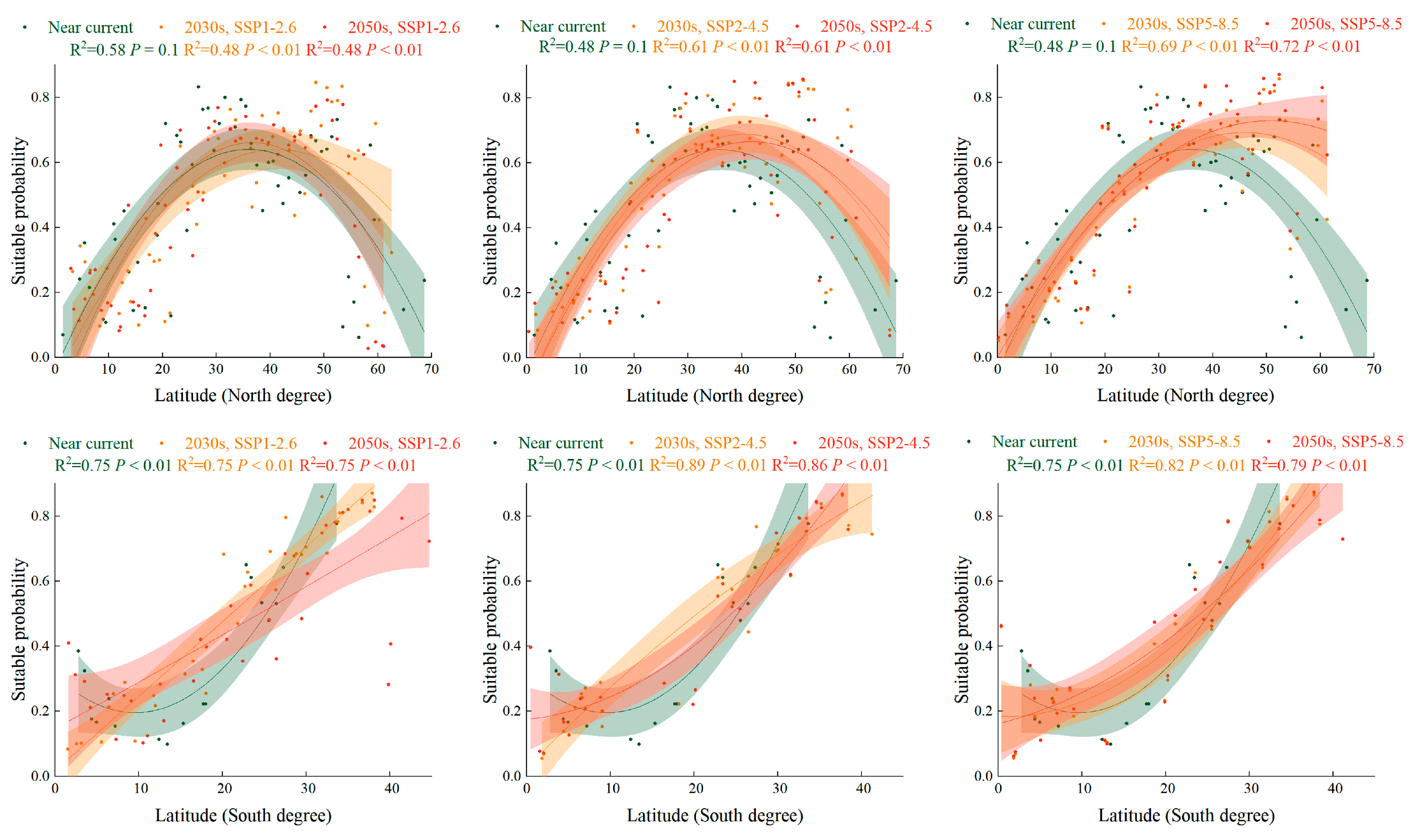
2.5. Trend of Suitabillity Probability for S. halepense according to Latitudinal Gradient
3. Discussion
3.1. Historical Invasion Reconstruction
3.2. Impact of Climate Change and LUC on Suitable Habitats
3.3. Prevention and Control
4. Materials and Methods
4.1. Occurrence Data and Reconstructed Historically Invaded Countries
4.2. Land-Use Harmonization Data
4.3. Climate Data
4.4. Model Construction and Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Walther, G.-R.; Roques, A.; Hulme, P.; Sykes, M.; Pyšek, P.; Kühn, I.; Zobel, M.; Bacher, S.; Botta-Dukat, Z.; Bugmann, H.; et al. Alien species in a warmer world: Risks and opportunities. Trends Ecol. Evol. 2009, 24, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.; Bingre, P.; Strubbe, D.; Santana, J.; Capinha, C.; Araújo, M.B.; Reino, L. Exploring the effects of geopolitical shifts on global wildlife trade. Bioscience 2022, 72, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-Y.; Milne, R.I.; Liu, J.; Nathan, R.; Corlett, R.T.; Li, D.-Z. The establishment of plants following long-distance dispersal. Trends Ecol. Evol. 2023, 38, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.R.U.; Dormontt, E.E.; Prentis, P.J.; Lowe, A.J.; Richardson, D.M. Something in the way you move: Dispersal pathways affect invasion success. Trends Ecol. Evol. 2009, 24, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Humair, F.; Humair, L.; Kuhn, F.; Kueffer, C. E-commerce trade in invasive plants. Conserv. Biol. 2015, 29, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Beaury, E.M.; Patrick, M.; Bradley, B.A. Invaders for sale: The ongoing spread of invasive species by the plant trade industry. Front. Ecol. Environ. 2021, 19, 550–556. [Google Scholar] [CrossRef]
- Freeman, B.G.; Lee-Yaw, J.A.; Sunday, J.M.; Hargreaves, A.L. Expanding, shifting and shrinking: The impact of global warming on species’ elevational distributions. Glob. Ecol. Biogeogr. 2018, 27, 1268–1276. [Google Scholar] [CrossRef]
- Elsen, P.R.; Monahan, W.B.; Merenlender, A.M. Topography and human pressure in mountain ranges alter expected species responses to climate change. Nat. Commun. 2020, 11, 1974. [Google Scholar] [CrossRef]
- Nogués-Bravo, D.; Araújo, M.B.; Romdal, T.; Rahbek, C. Scale effects and human impact on the elevational species richness gradients. Nature 2008, 453, 216–219. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.J.; McComb, S.; O’Neill, C.; Padgett, K.A.; Larsen, A.E. Projected climate and land use change alter western blacklegged tick phenology, seasonal host-seeking suitability and human encounter risk in California. Glob. Chang. Biol. 2020, 26, 5459–5474. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; García-Callejas, D.; Araújo, M.B. Discriminating climate, land-cover and random effects on species range dynamics. Glob. Chang. Biol. 2021, 27, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.; Smith, C. Invasive Johnsongrass, a threat to native grasslands and agriculture. Biologia 2020, 76, 413–420. [Google Scholar] [CrossRef]
- Nouri, H.R.; Talab, Z.A.; Tavassoli, A. Effect of weed allelopathic of sorghum (Sorghum halepense) on germination and seedling growth of wheat, Alvand cultivar. Ann. Biol. Res. 2012, 3, 1283–1293. [Google Scholar]
- Bridges, D.C.; Chandler, J.M. Influence of johnsongrass (Sorghum halepense) density and period of competition on cotton yield. Weed Sci. 1987, 35, 63–67. [Google Scholar] [CrossRef]
- Williams, C.S.; Hayes, R.M. Johnsongrass (Sorghum halepense) competition in soybeans (Glycine max). Weed Sci. 1984, 32, 498–501. [Google Scholar] [CrossRef]
- Mitskas, M.B.; Tsolis, C.E.; Eleftherohorinos, I.G.; Damalas, C.A. Interference between corn and johnsongrass (Sorghum halepense) from seed or rhizomes. Weed Sci. 2003, 51, 540–545. [Google Scholar] [CrossRef]
- Holm, L.R.G.; Plucknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weeds. Distribution and Biology; University Press of Hawaii: Honolulu, HI, USA, 1977; p. 621. [Google Scholar]
- Warwick, S.I.W.I.; Black, L.D. The biology of Canadian weeds.: 61. Sorghum halepense (L.) PERS. Can. J. Plant Sci. 1983, 63, 997–1014. [Google Scholar] [CrossRef]
- Asgharipour, M. Inhibitory effects of Sorghum Halepens root and leaf extracts on germination and early seedling growth of widely used medicinal plants. Adv. Environ. Biol. 2010, 4, 316–324. [Google Scholar]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J.; Gordon, A.; Kujala, H.; Lentini, P.E.; McCarthy, M.A.; Tingley, R.; Wintle, B.A. Is my species distribution model fit for purpose? Matching data and models to applications. Glob. Ecol. Biogeogr. 2015, 24, 276–292. [Google Scholar] [CrossRef]
- Lopatin, J.; Dolos, K.; Hernández, H.J.; Galleguillos, M.; Fassnacht, F.E. Comparing generalized linear models and random forest to model vascular plant species richness using LiDAR data in a natural forest in central Chile. Remote Sens. Environ. 2016, 173, 200–210. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Favretti, M. Remarks on the maximum entropy principle with application to the maximum entropy theory of ecology. Entropy 2018, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Kriticos, D.; Maywald, G.; Yonow, T.; Zurcher, E.; Herrmann, N.; Sutherst, R. CLIMEX. Version 4. Exploring the Effects of Climate on Plants, Animals and Diseases; CSIRO: Canberra, Australia, 2015. [Google Scholar]
- Thuiller, W. BIOMOD—Optimizing predictions of species distributions and projecting potential future shifts under global change. Glob. Chang. Biol. 2003, 9, 1353–1362. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J. A review of evidence about use and performance of species distribution modelling ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Stohlgren, T.J.; Ma, P.; Kumar, S.; Rocca, M.; Morisette, J.T.; Jarnevich, C.S.; Benson, N. Ensemble habitat mapping of invasive plant species. Risk Anal. 2010, 30, 224–235. [Google Scholar] [CrossRef]
- Xian, X.; Zhao, H.; Wang, R.; Huang, H.; Chen, B.; Zhang, G.; Liu, W.; Wan, F. Climate change has increased the global threats posed by three ragweeds (Ambrosia L.) in the Anthropocene. Sci. Total Environ. 2023, 859, 160252. [Google Scholar] [CrossRef]
- Shrestha, U.B.; Sharma, K.P.; Devkota, A.; Siwakoti, M.; Shrestha, B.B. Potential impact of climate change on the distribution of six invasive alien plants in Nepal. Ecol. Indic. 2018, 95, 99–107. [Google Scholar] [CrossRef]
- De Almeida, J.; Freitas, H. Exotic naturalized flora of continental Portugal. A reassessment. Bot. Complut. 2006, 30, 117. [Google Scholar]
- McWhorter, C.G. Introduction and Spread of Johnsongrass in the United States. Weed Sci. 1971, 19, 496–500. [Google Scholar] [CrossRef]
- Holm, L.G.; Pancho, J.V.; Herberger, J.P.; Plucknett, D.L. A Geographic Atlas of World Weeds. Krieger Publishing Co.: Malabar, FL, USA, 1991; p. 391. [Google Scholar]
- Jacques-félix, H. The Gramineae of Tropical Africa. I. Generalities, Classification and Description of Genera; Institut de Recherches Agronomiques Tropicales: Paris, France, 1962. [Google Scholar]
- Parsons, W.T.; Cuthbertson, E.G. Noxious Weeds of Australia; Inkarta Press: Melbourne, Australia, 1992. [Google Scholar]
- Puchalka, R.; Dyderski, M.K.; Vitkova, M.; Sadlo, J.; Klisz, M.; Netsvetov, M.; Prokopuk, Y.; Matisons, R.; Mionskowski, M.; Wojda, T.; et al. Black locust (Robinia pseudoacacia L.) range contraction and expansion in Europe under changing climate. Glob. Chang. Biol. 2021, 27, 1587–1600. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.V.; Stokes, K.E.; van Klinken, R.D. Predicting the potential distribution of a riparian invasive plant: The effects of changing climate, flood regimes and land-use patterns. Glob. Chang. Biol. 2012, 18, 1738–1753. [Google Scholar] [CrossRef]
- Gottfried, M.; Pauli, H.; Futschik, A.; Akhalkatsi, M.; Barančok, P.; Benito Alonso, J.L.; Coldea, G.; Dick, J.; Erschbamer, B.; Fernández Calzado, M.a.R.; et al. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Chang. 2012, 2, 111–115. [Google Scholar] [CrossRef]
- Bonnamour, A.; Gippet, J.M.W.; Bertelsmeier, C. Insect and plant invasions follow two waves of globalisation. Ecol. Lett. 2021, 24, 2418–2426. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Nishi, T.; Asai, M.; Muranaka, T.; Konuma, A.; Tominaga, T.; Shimono, Y. The role of weed seed contamination in grain commodities as propagule pressure. Biol. Invasions 2022, 24, 1707–1723. [Google Scholar] [CrossRef]
- Godber, O.F.; Wall, R. Livestock and food security: Vulnerability to population growth and climate change. Glob. Chang. Biol. 2014, 20, 3092–3102. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Chen, Y.; Wang, T.; Jiang, X.; Hu, X.; Feng, J. Double-edged effects of climate change on plant invasions: Ecological niche modeling global distributions of two invasive alien plants. Sci. Total Environ. 2020, 740, 139933. [Google Scholar] [CrossRef]
- Sirami, C.; Caplat, P.; Popy, S.; Clamens, A.; Arlettaz, R.; Jiguet, F.; Brotons, L.; Martin, J.-L. Impacts of global change on species distributions: Obstacles and solutions to integrate climate and land use. Glob. Ecol. Biogeogr. 2017, 26, 385–394. [Google Scholar] [CrossRef]
- Liu, Y.; Oduor, A.M.O.; Zhang, Z.; Manea, A.; Tooth, I.M.; Leishman, M.R.; Xu, X.; van Kleunen, M. Do invasive alien plants benefit more from global environmental change than native plants? Glob. Chang. Biol. 2017, 23, 3363–3370. [Google Scholar] [CrossRef]
- Lenoir, J.; Gégout, J.C.; Marquet, P.A.; de Ruffray, P.; Brisse, H. A Significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Molina-Montenegro, M.; Naya, D. Latitudinal patterns in phenotypic plasticity and fitness-related traits: Assessing the climatic variability hypothesis (CVH) with an invasive plant species. PLoS ONE 2012, 7, e47620. [Google Scholar] [CrossRef] [PubMed]
- Bongaarts, J. IPBES, 2019. Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Popul. Dev. Rev. 2019, 45, 680–681. [Google Scholar] [CrossRef]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Finch, D.M.; Butler, J.L.; Runyon, J.B.; Fettig, C.J.; Kilkenny, F.F.; Jose, S.; Frankel, S.J.; Cushman, S.A.; Cobb, R.C.; Dukes, J.S.; et al. Effects of Climate Change on Invasive Species. In Invasive Species in Forests and Rangelands of the United States: A Comprehensive Science Synthesis for the United States Forest Sector; Poland, T.M., Patel-Weynand, T., Finch, D.M., Miniat, C.F., Hayes, D.C., Lopez, V.M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 57–83. [Google Scholar]
- Ravi, S.; Law, D.J.; Caplan, J.S.; Barron-Gafford, G.A.; Dontsova, K.M.; Espeleta, J.F.; Villegas, J.C.; Okin, G.S.; Breshears, D.D.; Huxman, T.E. Biological invasions and climate change amplify each other’s effects on dryland degradation. Glob. Chang. Biol. 2022, 28, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Okin, G.S.; Ravi, S.; D’Odorico, P. Potential of grass invasions in desert shrublands to create novel ecosystem states under variable climate. Ecohydrology 2016, 9, 1496–1506. [Google Scholar] [CrossRef]
- Barney, J.N.; DiTomaso, J.M. Global climate niche estimates for bioenergy crops and invasive species of agronomic origin: Potential problems and opportunities. PLoS ONE 2011, 6, e17222. [Google Scholar] [CrossRef]
- Grassini, P.; Eskridge, K.M.; Cassman, K.G. Distinguishing between yield advances and yield plateaus in historical crop production trends. Nat. Commun. 2013, 4, 2918. [Google Scholar] [CrossRef]
- Bren d’Amour, C.; Reitsma, F.; Baiocchi, G.; Barthel, S.; Güneralp, B.; Erb, K.-H.; Haberl, H.; Creutzig, F.; Seto, K.C. Future urban land expansion and implications for global croplands. Proc. Natl. Acad. Sci. USA 2017, 114, 8939–8944. [Google Scholar] [CrossRef]
- Thebo, A.; Drechsel, P.; Lambin, E. Global assessment of urban and peri-urban agriculture: Irrigated and rainfed croplands. Environ. Res. Lett. 2014, 9, 114002. [Google Scholar] [CrossRef]
- Johnson, W.G.; Li, J.; Wait, J.D. Johnsongrass control, total nonstructural carbohydrates in rhizomes, and regrowth after application of herbicides used in herbicide-resistant corn (Zea mays). Weed Technol. 2003, 17, 36–41. [Google Scholar] [CrossRef]
- Defelice, M.S.; Witt, W.W.; Martin, J.R. Johnsongrass (Sorghum halepense) control and soil moisture relationships in no-tillage, doublecropped soybeans (Glycine max). Weed Sci. 1987, 35, 108–114. [Google Scholar] [CrossRef]
- Brown, S.M.; Chandler, J.M.; Morrison, J.E. Glyphosate for johnsongrass (Sorghum halepense) control in no-till Sorghum (Sorghum bicolor). Weed Sci. 1988, 36, 510–513. [Google Scholar] [CrossRef]
- Tóth, V.; Lehoczky, É. The analysis of effect of different herbicides on Johnson-grass in maize. Commun. Agric. Appl. Biol. Sci. 2007, 72, 279–282. [Google Scholar] [PubMed]
- Warren, D.L.; Matzke, N.J.; Cardillo, M.; Baumgartner, J.B.; Beaumont, L.J.; Turelli, M.; Glor, R.E.; Huron, N.A.; Simões, M.; Iglesias, T.L.; et al. ENMTools 1.0: An R package for comparative ecological biogeography. Ecography 2021, 44, 504–511. [Google Scholar] [CrossRef]
- Chen, G.; Li, X.; Liu, X. Global land projection based on plant functional types with a 1-km resolution under socio-climatic scenarios. Sci. Data 2022, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Stehfest, E.; Vuuren, D.; Kram, T.; Bouwman, A.; Alkemade, R.; Bakkenes, M.; Biemans, H.; Bouwman, A.; Elzen, M.; Janse, J.; et al. Integrated Assessment of Global Environmental Change with IMAGE 3.0. Model Description and Policy Applications; Netherlands Environmental Assessment Agency (PBL): Zuid-Holland, The Netherlands, 2014. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Schwalm, C.R.; Glendon, S.; Duffy, P.B. RCP8.5 tracks cumulative CO2 emissions. Proc. Natl. Acad. Sci. USA 2020, 117, 19656–19657. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Marotzke, J.; Bala, G.; Cao, L.; Corti, S.; Dunne, J.P.; Engelbrecht, F.; Fischer, E.; Fyfe, J.C.; Jones, C.; et al. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F. Ensemble Platform for Species Distribution Modeling; R Package, 2023.
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
| Variable | Description | Unit | Importance |
|---|---|---|---|
| bio2 | Mean diurnal range (mean of monthly max temp-min temp) | °C | 0.078 |
| bio5 | Max temperature of warmest month | °C | 0.067 |
| bio6 | Min temperature of coldest month | °C | 0.091 |
| bio12 | Annual precipitation | mm | 0.085 |
| bio15 | Precipitation seasonality (coefficient of variation×1) | - | 0.048 |
| bio17 | Precipitation of driest quarter | mm | 0.108 |
| bio19 | Precipitation of coldest quarter | mm | 0.317 |
| LUC | Land-use change | 0.019 |
| Land Use (×104 km) | Cropland | Grassland | Urban | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Suitable Area | Total Area | Suitable Area | Total Area | Suitable Area | Total Area | ||||
| Near-current | 921.99 | 2177.42 | 42.34% | 280.41 | 1675.80 | 16.73% | 50.42 | 61.89 | 81.47% |
| 2030s, SSP1-2.6 | 1026.27 | 2150.03 | 47.73% | 297.00 | 1555.53 | 19.09% | 75.24 | 89.92 | 83.67% |
| 2030s, SSP2-4.5 | 1073.00 | 2279.08 | 47.08% | 317.87 | 1638.99 | 19.39% | 72.92 | 87.09 | 83.73% |
| 2030s, SSP5-8.5 | 1079.24 | 2340.77 | 46.11% | 315.38 | 1613.94 | 19.54% | 78.81 | 93.78 | 84.04% |
| 2050s, SSP1-2.6 | 1060.59 | 2178.33 | 48.69% | 281.28 | 1448.65 | 19.42% | 85.00 | 101.57 | 83.69% |
| 2050s, SSP2-4.5 | 1091.27 | 2345.28 | 46.53% | 315.51 | 1577.10 | 20.01% | 82.24 | 99.20 | 82.90% |
| 2050s, SSP5-8.5 | 1113.91 | 2377.42 | 46.85% | 334.58 | 1609.21 | 20.79% | 95.63 | 112.74 | 84.82% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Zhao, H.; Xian, X.; Qi, Y.; Li, Q.; Guo, J.; Chen, L.; Liu, W. Reconstructed Global Invasion and Spatio-Temporal Distribution Pattern Dynamics of Sorghum halepense under Climate and Land-Use Change. Plants 2023, 12, 3128. https://doi.org/10.3390/plants12173128
Yang M, Zhao H, Xian X, Qi Y, Li Q, Guo J, Chen L, Liu W. Reconstructed Global Invasion and Spatio-Temporal Distribution Pattern Dynamics of Sorghum halepense under Climate and Land-Use Change. Plants. 2023; 12(17):3128. https://doi.org/10.3390/plants12173128
Chicago/Turabian StyleYang, Ming, Haoxiang Zhao, Xiaoqing Xian, Yuhan Qi, Qiao Li, Jianying Guo, Li Chen, and Wanxue Liu. 2023. "Reconstructed Global Invasion and Spatio-Temporal Distribution Pattern Dynamics of Sorghum halepense under Climate and Land-Use Change" Plants 12, no. 17: 3128. https://doi.org/10.3390/plants12173128
APA StyleYang, M., Zhao, H., Xian, X., Qi, Y., Li, Q., Guo, J., Chen, L., & Liu, W. (2023). Reconstructed Global Invasion and Spatio-Temporal Distribution Pattern Dynamics of Sorghum halepense under Climate and Land-Use Change. Plants, 12(17), 3128. https://doi.org/10.3390/plants12173128








