Potato Non-Specific Lipid Transfer Protein StnsLTPI.33 Is Associated with the Production of Reactive Oxygen Species, Plant Growth, and Susceptibility to Alternaria solani
Abstract
:1. Introduction
2. Results
2.1. The Gene PGSC0003DMG400031236 Encodes a Putative nsLTP
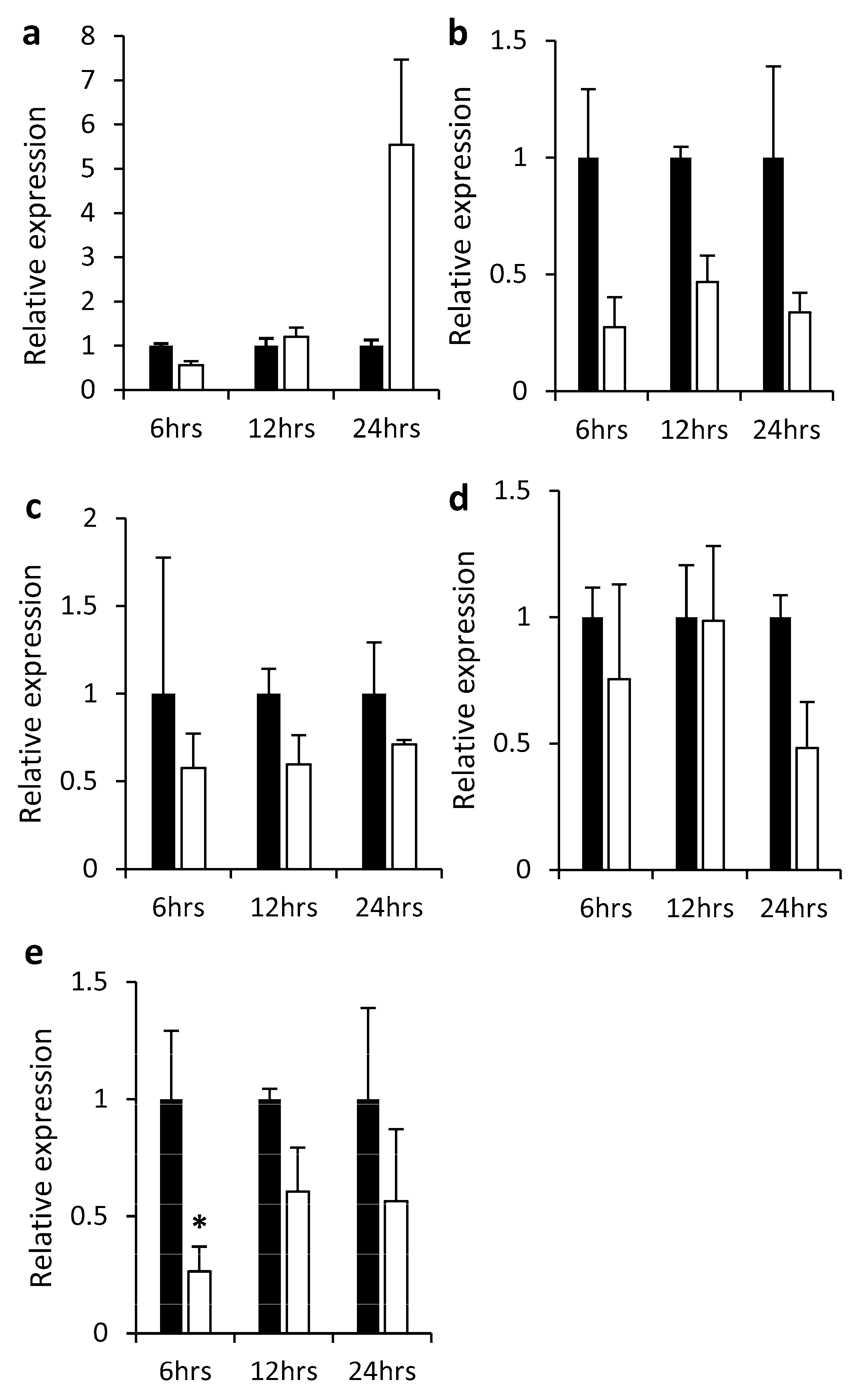
2.2. Gene Expression in Different Plant Tissues and in Response to Hormones
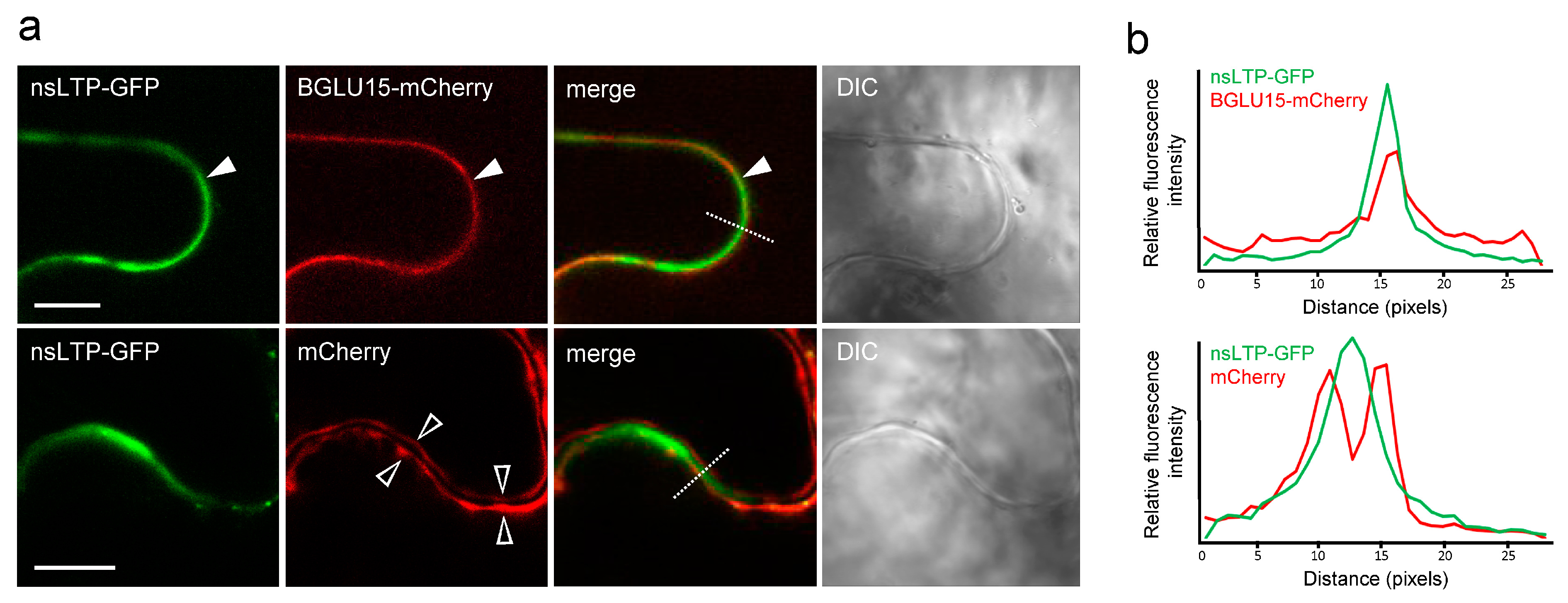
2.3. Subcellular Localization of StnsLTPI.33
2.4. Phenotypic Characterization of Transgenic Lines Overexpressing StnsLTPI.33
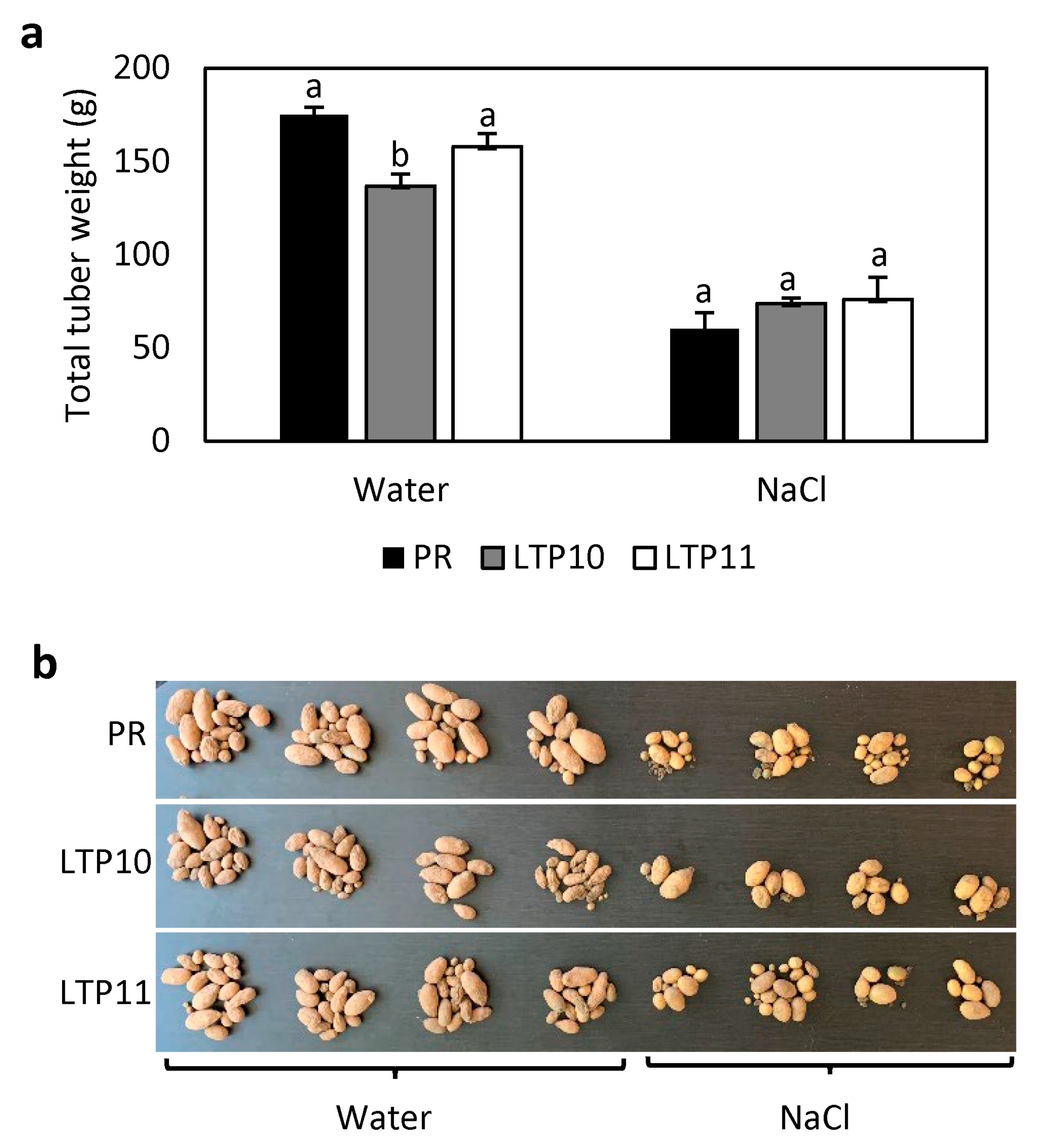
2.5. Response of StnsLTPI.33-Overexpression Lines to Salt Stress
2.6. Response of StnsLTPI.33-Overexpression Lines to PVY
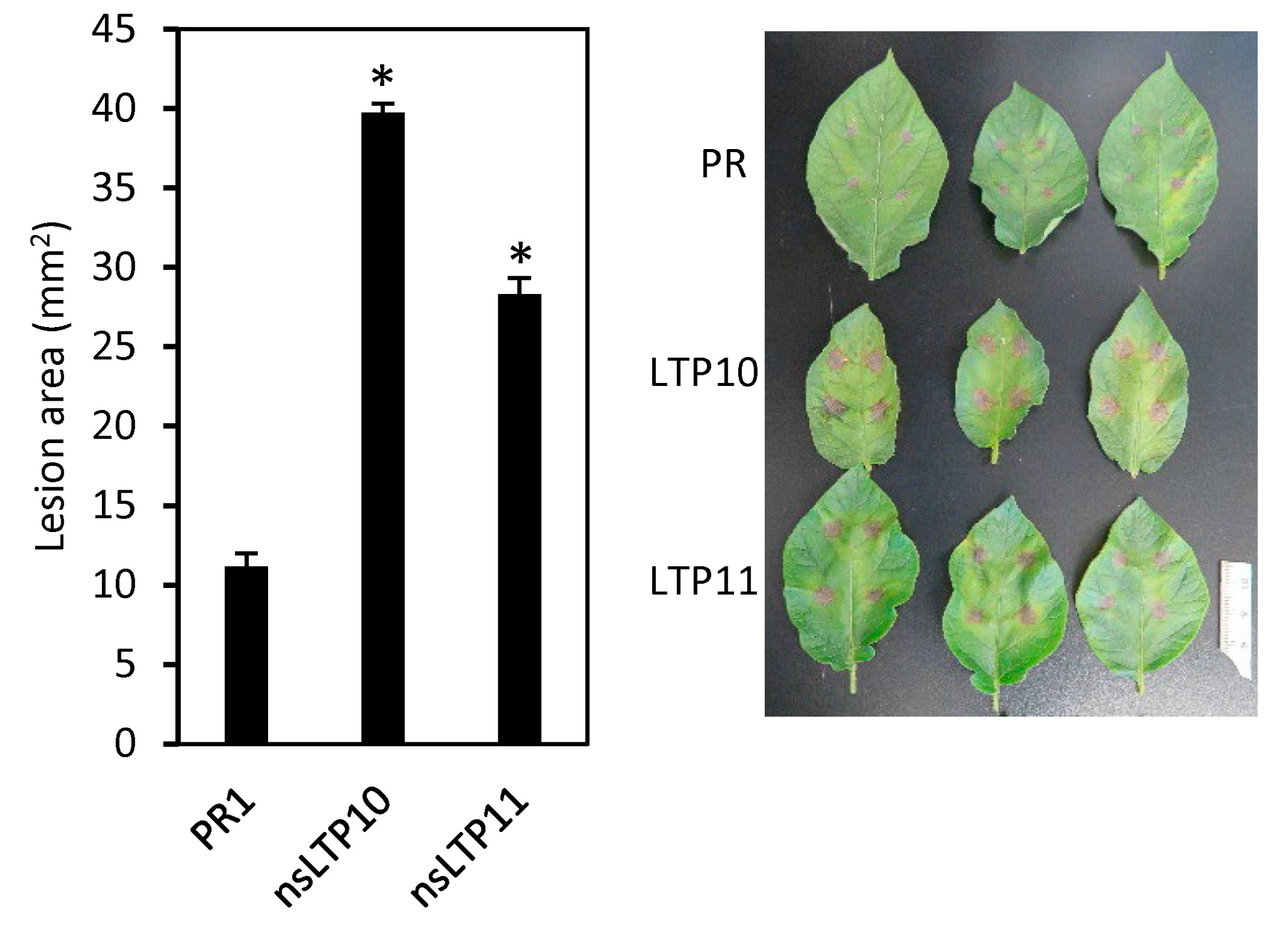
2.7. Response of StnsLTPI.33-Overexpression Lines to Alternaria Solani
2.8. StnsLTPI.33-Overexpression Lines Accumulate Superoxide Anions
3. Discussion
4. Materials and Methods
4.1. Phylogenetic Analysis
4.2. Plant Growth Conditions
4.3. Generation of StnsLTPI.33-Overexpressing Potato Plants
4.4. Quantitative Reverse Transcription PCR
4.5. Hormone Treatments
4.6. Subcellular Localization
4.7. Detection of Hydrogen Peroxide and Superoxide Anion Radicals
4.8. Metabolite Analysis by GC-MS
4.9. PVY Strains, Inoculation and Detection
4.10. Alternaria Solani Disease Assays
4.11. Statistical Analysis of the Results
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kader, J.C. Lipid-transfer proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 627–654. [Google Scholar] [CrossRef] [PubMed]
- Kader, J.C. Lipid-transfer proteins: A puzzling family of plant proteins. Trends Plant Sci. 1997, 2, 66–70. [Google Scholar] [CrossRef]
- Charvolin, D.; Douliez, J.P.; Marion, D.; Cohen-Addad, C.; Pebay-Peyroula, E. The crystal structure of a wheat nonspecific lipid transfer protein (ns-LTP1) complexed with two molecules of phospholipid at 2.1 angstrom resolution. Eur. J. Biochem. 1999, 264, 562–568. [Google Scholar] [CrossRef]
- Han, G.W.; Lee, J.Y.; Song, H.K.; Chang, C.S.; Min, K.; Moon, J.; Shin, D.H.; Kopka, M.L.; Sawaya, M.R.; Yuan, H.S.; et al. Structural basis of non-specific lipid binding in maize lipid-transfer protein complexes revealed by high-resolution X-ray crystallography. J. Mol. Biol. 2001, 308, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Salminen, T.A.; Blomqvist, K.; Edqvist, J. Lipid transfer proteins: Classification, nomenclature, structure, and function. Planta 2016, 244, 971–997. [Google Scholar] [CrossRef]
- Gincel, E.; Simorre, J.P.; Caille, A.; Marion, D.; Ptak, M.; Vovelle, F. Three-dimensional structure in solution of a wheat lipid-transfer protein from multidimensional 1H-NMR data. Eur. J. Biochem. 1994, 226, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Lee, J.Y.; Hwang, K.Y.; Kim, K.K.; Suh, S.W. High-resolution crystal structure of the non-specific lipid transfer protein from maize seedlings. Structure 1995, 3, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Boutrot, F.; Chantret, N.; Gautier, M.F. Genome-wide analysis of the rice and arabidopsis non-specific lipid transfer protein (nsLtp) gene families and identification of wheat nsLtp genes by EST data mining. BMC Genom. 2008, 9, 19. [Google Scholar] [CrossRef]
- Wang, N.J.; Lee, C.C.; Cheng, C.S.; Lo, W.C.; Yang, Y.F.; Chen, M.N.; Lyu, P.C. Construction and analysis of a plant non-specific lipid transfer protein database (nsLTPDB). BMC Genom. 2012, 13, 9. [Google Scholar] [CrossRef]
- Edqvist, J.; Blomqvist, K.; Nieuwland, J.; Salminen, T.A. Plant lipid transfer proteins: Are we finally closing in on the roles of these enigmatic proteins? J. Lipid Res. 2018, 59, 1374–1382. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.B.; Lu, C.M.; Zeng, X.H.; Li, Y.J.; Fu, D.H.; Wu, G. Non-specific lipid transfer proteins in plants: Presenting new advances and an integrated functional analysis. J. Exp. Bot. 2015, 66, 5663–5681. [Google Scholar] [CrossRef] [PubMed]
- Sossountzov, L.; Ruizavila, L.; Vignols, F.; Jolliot, A.; Arondel, V.; Tchang, F.; Grosbois, M.; Guerbette, F.; Miginiac, E.; Delseny, M.; et al. Spatial and temporal expression of a maize lipid transfer protein gene. Plant Cell 1991, 3, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Sels, J.; Mathys, J.; De Coninck, B.M.A.; Cammue, B.P.A.; De Bolle, M.F.C. Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol. Biochem. 2008, 46, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Wu, J.H.; Ng, T.B.; Ye, X.Y.; Rao, P.F. A non-specific lipid transfer protein with antifungal and antibacterial activities from the mung bean. Peptides 2004, 25, 1235–1242. [Google Scholar] [CrossRef]
- Molina, A.; Segura, A.; Garciaolmedo, F. Lipid transfer proteins (nsLTPs) from barley and maize leaves are potent inhibitors of bacterial and fungal plant pathogens. FEBS Lett. 1993, 316, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Cammue Bruno, P.A.; Thevissen, K.; Hendriks, M.; Eggermont, K.; Goderis, I.J.; Proost, P.; Vandamme, J.; Osborn, R.W.; Guerbette, F.; Kader, J.C.; et al. A potent antimicrobial protein from onion seeds showing sequence homology to plant lipid transfer proteints. Plant Physiol. 1995, 109, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, N.M.; Steffes, K.; Schlappi, M.R.; Gifford, A.N.; Greenberg, J.T. Arabidopsis AZI1 family proteins mediate signal mobilization for systemic defence priming. Nat. Commun. 2015, 6, 7658. [Google Scholar] [CrossRef]
- Champigny, M.J.; Shearer, H.; Mohammad, A.; Haines, K.; Neumann, M.; Thilmony, R.; He, S.Y.; Fobert, P.; Dengler, N.; Cameron, R.K. Localization of DIR1 at the tissue, cellular and subcellular levels during Systemic Acquired Resistance in Arabidopsis using DIR1:GUS and DIR1:EGFP reporters. BMC Plant Biol. 2011, 11, 125. [Google Scholar] [CrossRef]
- Sarowar, S.; Kim, Y.J.; Kim, K.D.; Hwang, B.K.; Ok, S.H.; Shin, J.S. Overexpression of lipid transfer protein (LTP) genes enhances resistance to plant pathogens and LTP functions in long-distance systemic signaling in tobacco. Plant Cell Rep. 2009, 28, 419–427. [Google Scholar] [CrossRef]
- Wang, C.C.; Gao, H.J.; Chu, Z.H.; Ji, C.Q.; Xu, Y.; Cao, W.L.; Zhou, S.M.; Song, Y.Z.; Liu, H.M.; Zhu, C.X. A nonspecific lipid transfer protein, StLTP10, mediates resistance to Phytophthora infestans in potato. Mol. Plant Pathol. 2021, 22, 48–63. [Google Scholar] [CrossRef]
- Dhar, N.; Caruana, J.; Erdem, I.; Raina, R. An Arabidopsis DISEASE RELATED NONSPECIFIC LIPID TRANSFER PROTEIN 1 is required for resistance against various phytopathogens and tolerance to salt stress. Gene 2020, 753, 144802. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yang, H.B.; Zhang, X.Y.; Yang, S.H. Lipid transfer protein 3 as a target of MYB96 mediates freezing and drought stress in Arabidopsis. J. Exp. Bot. 2013, 64, 1755–1767. [Google Scholar] [CrossRef]
- Pitzschke, A.; Datta, S.; Persak, H. Salt stress in Arabidopsis: Lipid Transfer Protein AZI1 and its control by Mitogen-Activated Protein Kinase MPK3. Mol. Plant 2014, 7, 722–738. [Google Scholar] [CrossRef]
- Guo, C.K.; Ge, X.C.; Ma, H. The rice OsDIL gene plays a role in drought tolerance at vegetative and reproductive stages. Plant Mol. Biol. 2013, 82, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.W.; Kim, K.D.; Hwang, B.K. Identification of pathogen-responsive regions in the promoter of a pepper lipid transfer protein gene (CALTPI) and the enhanced resistance of the CALTPI transgenic Arabidopsis against pathogen and environmental stresses. Planta 2005, 221, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.N.; Xing, Y.; Wang, Q.B.; Wang, C.C.; Zhang, X.Y.; Chen, D.Y.; Song, Y.Z.; Zhu, C.X. Expression of tobacco Lipid Transfer Protein NtLTP4 enhances tolerance to abiotic and biotic stresses in transgenic potato lines. Potato Res. 2022, 65, 631–647. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, X.X.; Song, Y.Z.; Zhu, L.F.; Yu, Z.P.; Gan, L.M.; Zhou, S.M.; Liu, H.M.; Wen, F.J.; Zhu, C.X. NtLTP4, a lipid transfer protein that enhances salt and drought stresses tolerance in Nicotiana tabacum. Sci. Rep. 2018, 8, 8873. [Google Scholar] [CrossRef] [PubMed]
- Goyer, A.; Hamlin, L.; Crosslin, J.M.; Buchanan, A.; Chang, J.H. RNA-Seq analysis of resistant and susceptible potato varieties during the early stages of Potato Virus Y infection. BMC Genom. 2015, 16, 472. [Google Scholar] [CrossRef] [PubMed]
- Karasev, A.V.; Gray, S.M. Continuous and emerging challenges of Potato virus Y in potato. Annu. Rev. Phytopathol. 2013, 51, 571–586. [Google Scholar] [CrossRef]
- Li, G.J.; Hou, M.L.; Liu, Y.X.; Pei, Y.; Ye, M.H.; Zhou, Y.; Huang, C.X.; Zhao, Y.Q.; Ma, H.L. Genome-wide identification, characterization and expression analysis of the non-specific lipid transfer proteins in potato. BMC Genom. 2019, 20, 14. [Google Scholar] [CrossRef]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef]
- Roepke, J.; Gordon, H.O.W.; Neil, K.J.A.; Gidda, S.; Mullen, R.T.; Coutin, J.A.F.; Bray-Stone, D.; Bozzo, G.G. An apoplastic beta-glucosidase is essential for the degradation of flavonol 3-O-beta-glucoside-7-O-alpha-rhamnosides in Arabidopsis. Plant Cell Physiol. 2017, 58, 1030–1047. [Google Scholar] [CrossRef] [PubMed]
- Yubero-Serrano, E.M.; Moyano, E.; Medina-Escobar, N.; Munoz-Blanco, J.; Caballero, J.L. Identification of a strawberry gene encoding a non-specific lipid transfer protein that responds to ABA, wounding and cold stress. J. Exp. Bot. 2003, 54, 1865–1877. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.H.; Robertson, A.J.; Liu, X.J.; Zheng, P.; Wilen, R.W.; Nesbitt, N.T.; Gusta, L.V. A lipid transfer protein gene BG-14 is differentially regulated by abiotic stress, ABA, anisomycin, and sphingosine in bromegrass (Bromus inermis). J. Plant Physiol. 2004, 161, 449–458. [Google Scholar] [CrossRef]
- Goyer, A.; Bvindi, C. Overexpression of VQ motif-containing gene does not affect infection rates of potato with Potato Virus Y. Am. J. Potato Res. 2023, 100, 233–239. [Google Scholar] [CrossRef]
- Gao, G.; Jin, L.P.; Xie, K.Y.; Qu, D.Y. The potato StLTPa7 gene displays a complex Ca-associated pattern of expression during the early stage of potato-Ralstonia solanacearum interaction. Mol. Plant Pathol. 2009, 10, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Park, C.J.; Shin, R.; Park, J.M.; Lee, G.J.; Youl, J.S.; Paek, K.H. Induction of pepper cDNA encoding a lipid transfer protein during the resistance response to tobacco mosaic virus. Plant Mol. Biol. 2002, 48, 243–254. [Google Scholar] [CrossRef]
- Shang, K.J.; Xu, Y.; Cao, W.L.; Xie, X.Y.; Zhang, Y.R.; Zhang, J.F.; Liu, H.M.; Zhou, S.M.; Zhu, X.P.; Zhu, C.X. Potato (Solanum tuberosum L.) non-specific lipid transfer protein StLTP6 promotes viral infection by inhibiting virus-induced RNA silencing. Planta 2022, 256, 54. [Google Scholar] [CrossRef]
- Molina, A.; GarciaOlmedo, F. Enhanced tolerance to bacterial pathogens caused by the transgenic expression of barley lipid transfer protein LTP2. Plant J. 1997, 12, 669–675. [Google Scholar] [CrossRef]
- Subhankar, R.B.; Sautter, C.; Chattoo, B.B.; Sautter, C. Expression of the lipid transfer protein Ace-AMP1 in transgenic wheat enhances antifungal activity and defense responses. Transgenic Res. 2006, 15, 435–446. [Google Scholar] [CrossRef]
- Patkar, R.N.; Chattoo, B.B. Transgenic indica rice expressing ns-LTP-Like protein shows enhanced resistance to both fungal and bacterial pathogens. Mol. Breed. 2006, 17, 159–171. [Google Scholar] [CrossRef]
- Yang, X.Y.; Xiao, Y.H.; Wang, X.W.; Pei, Y. Expression of a novel small antimicrobial protein from the seeds of motherwort (Leonurus japonicus) confers disease resistance in tobacco. Appl. Environ. Microbiol. 2007, 73, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.C.; Gou, J.Q.; Sun, Y.M.; Yuan, L.; Tang, Q.A.; Yang, X.Y.; Pei, Y.; Luo, K.M. Enhanced resistance to fungal pathogens in transgenic Populus tomentosa Carr. by overexpression of an nsLTP-like antimicrobial protein gene from motherwort (Leonurus japonicus). Tree Physiol. 2010, 30, 1599–1605. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yoshioka, M.; Asai, S.; Nomura, H.; Kuchimura, K.; Mori, H.; Doke, N.; Yoshioka, H. StCDPK5 confers resistance to late blight pathogen but increases susceptibility to early blight pathogen in potato via reactive oxygen species burst. New Phytol. 2012, 196, 223–237. [Google Scholar] [CrossRef]
- van Baarlen, P.; Woltering, E.J.; Staats, M.; van Kan, J.A.L. Histochemical and genetic analysis of host and non-host interactions of Arabidopsis with three Botrytis species: An important role for cell death control. Mol. Plant Pathol. 2007, 8, 41–54. [Google Scholar] [CrossRef]
- Achard, P.; Renou, J.P.; Berthome, R.; Harberd, N.P.; Genschik, P. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr. Biol. 2008, 18, 656–660. [Google Scholar] [CrossRef]
- Bernsdorff, F.; Doring, A.C.; Gruner, K.; Schuck, S.; Brautigam, A.; Zeier, J. Pipecolic acid orchestrates plant systemic acquired resistance and defense priming via salicylic acid-dependent and -independent pathways. Plant Cell 2016, 28, 102–129. [Google Scholar] [CrossRef]
- Zeier, J. New insights into the regulation of plant immunity by amino acid metabolic pathways. Plant Cell Environ. 2013, 36, 2085–2103. [Google Scholar] [CrossRef]
- Sharma, S.; Choudhary, B.; Yadav, S.; Mishra, A.; Mishra, V.K.; Chand, R.; Chen, C.; Pandey, S.P. Metabolite profiling identified pipecolic acid as an important component of peanut seed resistance against Aspergillus flavus infection. J. Hazard. Mater. 2021, 404, 124155. [Google Scholar] [CrossRef] [PubMed]
- Navarova, H.; Bernsdorff, F.; Doring, A.C.; Zeier, J. Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 2012, 24, 5123–5141. [Google Scholar] [CrossRef] [PubMed]
- Nieuwland, J.; Feron, R.; Huisman, B.A.H.; Fasolino, A.; Hilbers, C.W.; Derksen, J.; Mariani, C. Lipid transfer proteins enhance cell wall extension in tobacco. Plant Cell 2005, 17, 2009–2019. [Google Scholar] [CrossRef]
- Huang, H.L.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Shapiguzov, A.; Vainonen, J.P.; Wrzaczek, M.; Kangasjarvi, J. ROS-talk—How the apoplast, the chloroplast, and the nucleus get the message through. Front. Plant Sci. 2012, 3, 292. [Google Scholar] [CrossRef]
- Mehdy, M.C. Active oxygen species in plant defense against pathogens. Plant Physiol. 1994, 105, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.D.; Shao, Q.S.; Yin, L.H.; Younis, A.; Zheng, B.S. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pan, S.K.; Cheng, S.F.; Zhang, B.; Mu, D.S.; Ni, P.X.; Zhang, G.Y.; Yang, S.; Li, R.Q.; Wang, J.; et al. Genome sequence and analysis of the tuber crop potato. Nature 2011, 475, 189–195. [Google Scholar] [CrossRef]
- Curtis, M.D.; Grossniklaus, U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 2003, 133, 462–469. [Google Scholar] [CrossRef]
- Combest, M.M.; Moroz, N.; Tanaka, K.; Rogan, C.J.; Anderson, J.C.; Thura, L.; Rakotondrafara, A.M.; Goyer, A. StPIP1, a PAMP-induced peptide in potato, elicits plant defenses and is associated with disease symptom severity in a compatible interaction with Potato virus Y. J. Exp. Bot. 2021, 72, 4472–4488. [Google Scholar] [CrossRef]
- Taylor, S.C.; Nadeau, K.; Abbasi, M.; Lachance, C.; Nguyen, M.; Fenrich, J. The ultimate qPCR experiment: Producing publication quality, reproducible data the first time. Trends Biotechnol. 2019, 37, 761–774. [Google Scholar] [CrossRef]
- Cai, Y.Q.; Goodman, J.M.; Pyc, M.; Mullen, R.T.; Dyer, J.M.; Chapman, K.D. Arabidopsis SEIPIN proteins modulate triacylglycerol accumulation and influence lipid droplet proliferation. Plant Cell 2015, 27, 2616–2636. [Google Scholar] [CrossRef]
- McCartney, A.W.; Greenwood, J.S.; Fabian, M.R.; White, K.A.; Mullen, R.T. Localization of the tomato bushy stunt virus replication protein p33 reveals a peroxisome-to-endoplasmic reticulum sorting pathway. Plant Cell 2005, 17, 3513–3531. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, F.K.; Doner, N.M.; Krawczyk, H.E.; Scholz, P.; Schmitt, K.; Valerius, O.; Braus, G.H.; Mullen, R.T.; Ischebeck, T. Identification of low-abundance lipid droplet proteins in seeds and Seedlings. Plant Physiol. 2020, 182, 1326–1345. [Google Scholar] [CrossRef]
- Pyc, M.; Cai, Y.Q.; Gidda, S.K.; Yurchenko, O.; Park, S.; Kretzschmar, F.K.; Ischebeck, T.; Valerius, O.; Braus, G.H.; Chapman, K.D.; et al. Arabidopsis lipid droplet-associated protein (LDAP) interacting protein (LDIP) influences lipid droplet size and neutral lipid homeostasis in both leaves and seeds. Plant J. 2017, 92, 1182–1201. [Google Scholar] [CrossRef]
- Gidda, S.K.; Park, S.; Pyc, M.; Yurchenko, O.; Cai, Y.Q.; Wu, P.; Andrews, D.W.; Chapman, K.D.; Dyer, J.M.; Mullen, R.T. Lipid Droplet-Associated Proteins (LDAPs) are required for the dynamic regulation of neutral lipid compartmentation in plant cells. Plant Physiol. 2016, 170, 2052–2071. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Li, J.B.; Luan, Y.S.; Liu, Z. SpWRKY1 mediates resistance to Phytophthora infestans and tolerance to salt and drought stress by modulating reactive oxygen species homeostasis and expression of defense-related genes in tomato. Plant Cell Tissue Organ. Cult. 2015, 123, 67–81. [Google Scholar] [CrossRef]
- Davies, A.N. The new Automated Mass Spectrometry Deconvolution and Identification System (AMDIS). Spectrosc. Eur. 1998, 10, 24–27. [Google Scholar]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib: Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef]
- Vinchesi, A.C.; Rondon, S.I.; Goyer, A. Priming potato with thiamin to control potato virus Y. Am. J. Potato Res. 2017, 94, 120–128. [Google Scholar] [CrossRef]
- Pang, Z.Q.; Chong, J.; Zhou, G.Y.; Morais, D.A.D.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.E.; Li, S.Z.; Xia, J.G. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]






| Experiment 1 | Experiment 2 | Experiment 3 | |||||
|---|---|---|---|---|---|---|---|
| Treatment | Potato Line | In-Season a | Seedborne b | In-Season a | Seedborne b | In-Season a | Seedborne b |
| Mock | PR | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/5 |
| LTP10 | 0/6 | 0/5 | 1/6 | 0/5 | 0/3 | 0/2 | |
| LTP11 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/4 | |
| PVYO | PR | 0/6 | 0/6 | 0/6 | 0/6 | 1/6 | 0/6 |
| LTP10 | 0/6 | 0/6 | 0/6 | 0/4 | 1/4 | 0/2 | |
| LTP11 | 0/5 | 0/5 | 0/5 | 0/5 | 2/5 | 0/5 | |
| PVYN-Wilga | PR | 0/6 | 0/6 | 4/6 | 3/6 | 2/6 | 0/6 |
| LTP10 | 0/4 | 0/4 | 2/5 | 4/5 | 0/4 | 0/4 | |
| LTP11 | 0/6 | 0/5 | 0/6 | 4/6 | 1/6 | 0/5 | |
| PVYNTN | PR | 4/6 | 4/6 | 5/6 | 5/6 | 5/6 | 5/6 |
| LTP10 | 5/6 | 5/6 | 5/6 | 5/5 | 4/5 | 2/3 | |
| LTP11 | 4/6 | 4/6 | 4/4 | 4/4 | 6/6 | 5/6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bvindi, C.; Howe, K.; Wang, Y.; Mullen, R.T.; Rogan, C.J.; Anderson, J.C.; Goyer, A. Potato Non-Specific Lipid Transfer Protein StnsLTPI.33 Is Associated with the Production of Reactive Oxygen Species, Plant Growth, and Susceptibility to Alternaria solani. Plants 2023, 12, 3129. https://doi.org/10.3390/plants12173129
Bvindi C, Howe K, Wang Y, Mullen RT, Rogan CJ, Anderson JC, Goyer A. Potato Non-Specific Lipid Transfer Protein StnsLTPI.33 Is Associated with the Production of Reactive Oxygen Species, Plant Growth, and Susceptibility to Alternaria solani. Plants. 2023; 12(17):3129. https://doi.org/10.3390/plants12173129
Chicago/Turabian StyleBvindi, Carol, Kate Howe, You Wang, Robert T. Mullen, Conner J. Rogan, Jeffrey C. Anderson, and Aymeric Goyer. 2023. "Potato Non-Specific Lipid Transfer Protein StnsLTPI.33 Is Associated with the Production of Reactive Oxygen Species, Plant Growth, and Susceptibility to Alternaria solani" Plants 12, no. 17: 3129. https://doi.org/10.3390/plants12173129






