Are Basic Substances a Key to Sustainable Pest and Disease Management in Agriculture? An Open Field Perspective
Abstract
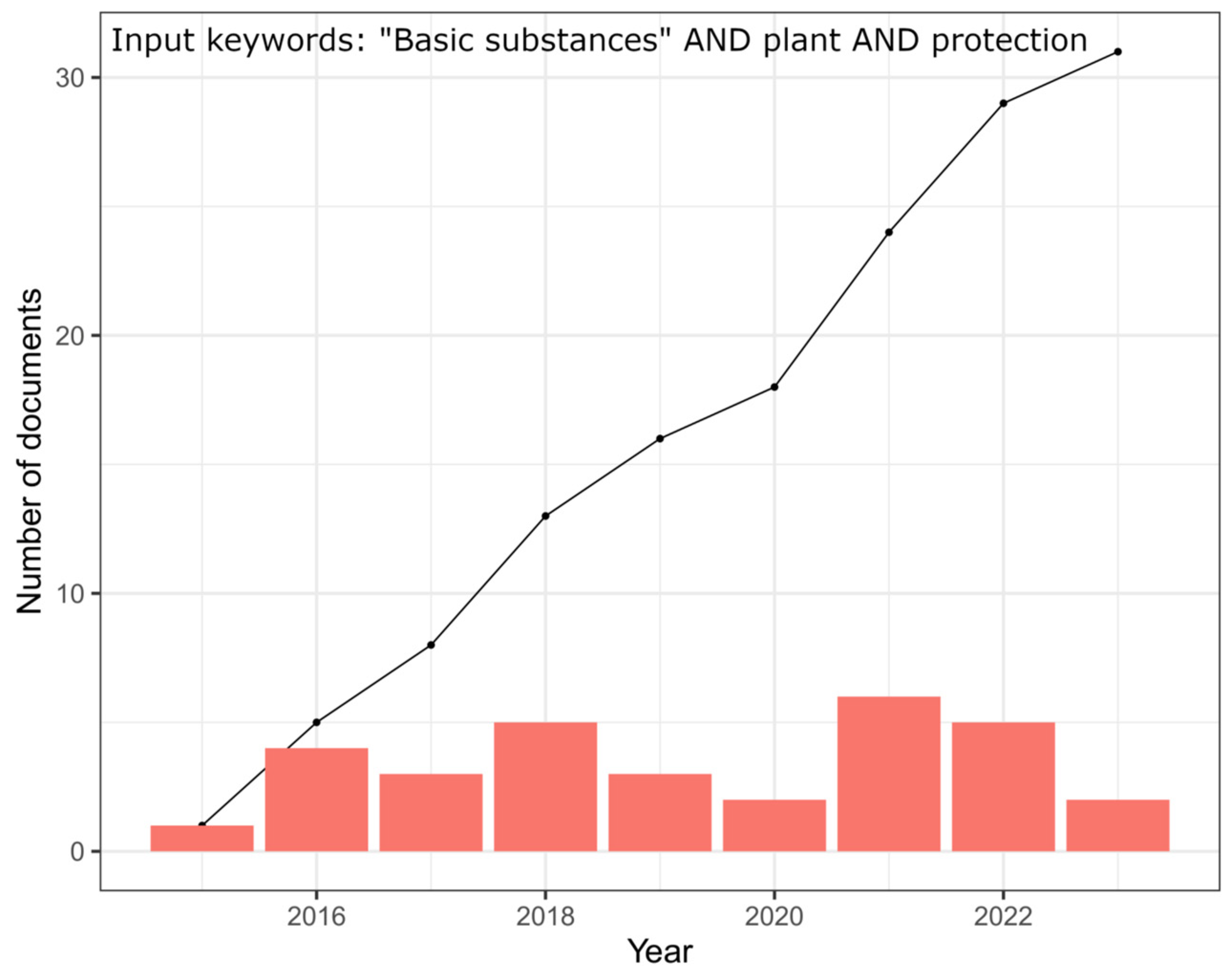
1. Introduction
2. Activity of Approved Basic Substances against Fungal Diseases
2.1. Grapevine
2.1.1. Grapevine Downy Mildew
2.1.2. Grey Mold on Table Grape
2.2. Potato Leaf Diseases
2.3. Pre-Harvest Treatment Affecting the Post-Harvest Diseases of Fruits
| Crop (Species) | Disease (Pathogen) | Basic Substance | Reference |
|---|---|---|---|
| Grapevine (Vitis vinifera) | Downy mildew (Plasmopara viticola) | Chitosan | [42,43] |
| Botrytis bunch rot (Botrytis cinerea) | Chitosan | [66] | |
| Potato (Solanum tuberosum) | Early blight (Alternaria alternata) | Nettle slurry (Urtica dioica) and broad-leaf hopbush (Dodonaea viscosa) methanolic extracts | [86] |
| Early blight (Alternaria solani) | Water solutions of Allium cepa | [87] | |
| Late blight (Phytophthora infestans) | Chitosan | [78,79,80,82] | |
| Strawberry (Fragaria x ananassa and Fragaria chiloensis) | Grey mold (Botrytis cinerea) | Chitosan | [90,95,96,97] |
| Sweet cherry (Prunus avium) | Storage decay | Chitosan | [98] |
| Botrytis rot (B. cinerea) | Sodium bicarbonate salts | [108] | |
| Date palm fruit (Phoenix dactylifera) | Storage decay | Chitosan | [99] |
| Kiwifruit (Actinidia deliciosa) | Soft rot (Botryosphaeria dothidea and Phomopsis sp.) | Chitosan | [100] |
| Apricot (Prunus armeniaca) | Decay (A. alternata) | Chitosan | [101,102] |
| Peach (Prunus persica) | Decay (A. alternata) | Chitosan | [103,104] |
| Jujube (Zizyphus jujuba) | Storage decay | Chitosan | [105] |
| Tomato (Solanum lycopersicum) | Storage decay | Chitosan | [106] |
| Pear (Pyrus communis) | Storage decay | Onion (Allium cepa) extract | [109] |
3. Activity of Approved Basic Substances against Insects
3.1. Nettle
3.2. Sucrose and Fructose
3.3. Talc
3.4. Diammonium Phosphate
3.5. Onion Oil
3.6. Chitosan
4. Basic Substances as Partners in Disease Management
5. Potential Basic Substances: Approval Procedure and Issues
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucas, J.A. Foresight Project on Global Food and Farming Futures: Advances in Plant Disease and Pest Management. J. Agric. Sci. 2011, 149, 91–114. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Lykogianni, M.; Bempelou, E.; Karamaouna, F.; Aliferis, K.A. Do Pesticides Promote or Hinder Sustainability in Agriculture? The Challenge of Sustainable Use of Pesticides in Modern Agriculture. Sci. Total Environ. 2021, 795, 148625. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, F.; Jeuffroy, M.H.; Jouan, J.; Le Cadre, E.; Litrico, I.; Malausa, T.; Reboud, X.; Huyghe, C. Pesticide-Free Agriculture as a New Paradigm for Research. Agron. Sustain. Dev. 2022, 42, 8. [Google Scholar] [CrossRef]
- Pierlot, F.; Marks-Perreau, J.; Soulé, E.; Keichinger, O.; Bedos, C.; Prevost, L.; Van Dijk, P.; Bockstaller, C. An Indicator to Assess Risks on Water and Air of Pesticide Spraying in Crop Fields. Sci. Total Environ. 2023, 870, 161000. [Google Scholar] [CrossRef]
- van den Berg, F.; Jacobs, C.M.J.; Butler Ellis, M.C.; Spanoghe, P.; Doan Ngoc, K.; Fragkoulis, G. Modelling Exposure of Workers, Residents and Bystanders to Vapour of Plant Protection Products after Application to Crops. Sci. Total Environ. 2016, 573, 1010–1020. [Google Scholar] [CrossRef]
- Tudi, M.; Li, H.; Li, H.; Wang, L.; Lyu, J.; Yang, L.; Tong, S.; Yu, Q.J.; Ruan, H.D.; Atabila, A.; et al. Exposure Routes and Health Risks Associated with Pesticide Application. Toxics 2022, 10, 335. [Google Scholar] [CrossRef]
- Kenko, D.B.N.; Ngameni, N.T.; Awo, M.E.; Njikam, N.A.; Dzemo, W.D. Does Pesticide Use in Agriculture Present a Risk to the Terrestrial Biota? Sci. Total Environ. 2023, 861, 160715. [Google Scholar] [CrossRef]
- European Parliament Regulation (EC). No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. Eur. Union 2009, L309, 1–50. [Google Scholar]
- Hillocks, R.J. Farming with Fewer Pesticides: EU Pesticide Review and Resulting Challenges for UK Agriculture. Crop. Prot. 2012, 31, 85–93. [Google Scholar] [CrossRef]
- Wilson, C.; Tisdell, C. Why Farmers Continue to Use Pesticides despite Environmental, Health and Sustainability Costs. Ecol. Econ. 2001, 39, 449–462. [Google Scholar] [CrossRef]
- Gehen, S.; Corvaro, M.; Jones, J.; Ma, M.; Yang, Q. Challenges and Opportunities in the Global Regulation of Crop Protection Products. Org. Process. Res. Dev. 2019, 23, 2225–2233. [Google Scholar] [CrossRef]
- McDougall, P. The Cost of New Agrochemical Product Discovery, Development and Registration in 1995, 2000, 2005–2008 and 2010–2014. Available online: https://croplife.org/wp-content/uploads/2016/04/Cost-of-CP-report-FINAL.pdf (accessed on 26 July 2023).
- Hawkins, N.J.; Bass, C.; Dixon, A.; Neve, P. The Evolutionary Origins of Pesticide Resistance. Biol. Rev. 2019, 94, 135–155. [Google Scholar] [CrossRef]
- Marchand, P.A. Basic Substances: An Opportunity for Approval of Low-Concern Substances under EU Pesticide Regulation. Pest Manag. Sci. 2015, 71, 1197–1200. [Google Scholar] [CrossRef]
- Romanazzi, G.; Orçonneau, Y.; Moumni, M.; Davillerd, Y.; Marchand, P.A. Basic Substances, a Sustainable Tool to Complement and Eventually Replace Synthetic Pesticides in the Management of Pre and Postharvest Diseases: Reviewed Instructions for Users. Molecules 2022, 27, 3484. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Directive (EU) 2019/782 of 15 May 2019 Amending Directive 2009/128/EC of the European Parliament and of the Council as Regards the Establishment of Harmonized Risk Indicators. Off. J. Eur. Union 2019, L127, 4–10. [Google Scholar]
- Sun, C.; Zhu, C.; Tang, Y.; Ren, D.; Cai, Y.; Zhou, G.; Wang, Y.; Xu, L.; Zhu, P. Inhibition of Botrytis cinerea and Control of Gray Mold on Table Grapes by Calcium Propionate. Food Qual. Saf. 2021, 5, 1–12. [Google Scholar] [CrossRef]
- FAO Transforming Food and Agriculture to Achieve the Sustainable Development Goals (SDGs). Available online: https://www.fao.org/sustainability/en/ (accessed on 26 July 2023).
- Trebbi, G.; Negri, L.; Bosi, S.; Dinelli, G.; Cozzo, R.; Marotti, I. Evaluation of Equisetum Arvense (Horsetail Macerate) as a Copper Substitute for Pathogen Management in Field-Grown Organic Tomato and Durum Wheat Cultivations. Agriculture 2021, 11, 5. [Google Scholar] [CrossRef]
- Trouvelot, S.; Héloir, M.C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in Plant Immunity and Plant Protection: Roles and Potential Application as Foliar Sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Sivakumar, D. Chitosan, a Biopolymer with Triple Action on Postharvest Decay of Fruit and Vegetables: Eliciting, Antimicrobial and Film-Forming Properties. Front. Microbiol. 2018, 9, 2745. [Google Scholar] [CrossRef] [PubMed]
- Llamazares De Miguel, D.; Mena-Petite, A.; Díez-Navajas, A.M. Toxicity and Preventive Activity of Chitosan, Equisetum Arvense, Lecithin and Salix Cortex against Plasmopara Viticola, the Causal Agent of Downy Mildew in Grapevine. Agronomy 2022, 12, 3139. [Google Scholar] [CrossRef]
- Langa-Lomba, N.; Buzón-Durán, L.; Martín-Ramos, P.; Casanova-Gascón, J.; Martín-Gil, J.; Sánchez-Hernández, E.; González-García, V. Assessment of Conjugate Complexes of Chitosan and Urtica dioica or Equisetum Arvense Extracts for the Control of Grapevine Trunk Pathogens. Agronomy 2021, 11, 976. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Recent Advances of Chitosan Applications in Plants. Polymers 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, J.; Tyburski, J.; Matysiak, K.; Jakubowska, M.; Łukaszyk, J.; Krzymińska, J. Cinnamon as a Useful Preventive Substance for the Care of Human and Plant Health. Molecules 2021, 26, 5299. [Google Scholar] [CrossRef]
- Matyjaszczyk, E. Plant Protection Means Used in Organic Farming throughout the European Union. Pest Manag. Sci. 2018, 74, 505–510. [Google Scholar] [CrossRef]
- Eurostat. Key Figures on the European Food Chain—2021 Edition; Publications Office of the European Union: Bietlot, Belgium, 2021; ISBN 9789276415152. [Google Scholar]
- Eurostat. Agriculture, Forestry and Fishery Statistics—2020 Edition; Publications Office of the European Union: Bietlot, Belgium, 2020; ISBN 978-92-76-21521-9. [Google Scholar]
- Bois, B.; Zito, S.; Calonnec, A.; Ollat, N. Climate vs Grapevine Pests and Diseases Worldwide: The First Results of a Global Survey. J. Int. Sci. Vigne Vin 2017, 51, 133–139. [Google Scholar] [CrossRef]
- Gessler, C.; Pertot, I.; Perazzolli, M. Plasmopara viticola: A Review of Knowledge on Downy Mildew of Grapevine and Effective Disease Management. Phytopathol. Mediterr. 2011, 50, 3–44. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) 2018/1981 of 13 December 2018 Renewing the Approval of the Active Substances Copper Compounds, as Candidates for Substitution, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Co. Off. J. Eur. Union 2018, L317, 16–20. [Google Scholar]
- Maia, A.J.; Leite, C.D.; Botelho, R.V.; Faria, C.M.D.R.; Machado, D. Quitosana Como Opção de Controle Do Míldio Para Viticultura Sustentável. Semin. Cienc. Agrar. 2012, 33, 2519–2530. [Google Scholar] [CrossRef]
- Puopolo, G.; Giovannini, O.; Pertot, I. Lysobacter capsici AZ78 Can Be Combined with Copper to Effectively Control Plasmopara Viticola on Grapevine. Microbiol. Res. 2014, 169, 633–642. [Google Scholar] [CrossRef]
- Rosa, S.; Pesaresi, P.; Mizzotti, C.; Bulone, V.; Mezzetti, B.; Baraldi, E.; Masiero, S. Game-Changing Alternatives to Conventional Fungicides: Small RNAs and Short Peptides. Trends Biotechnol. 2022, 40, 320–337. [Google Scholar] [CrossRef] [PubMed]
- Lachhab, N.; Sanzani, S.M.; Adrian, M.; Chiltz, A.; Balacey, S.; Boselli, M.; Ippolito, A.; Poinssot, B. Soybean and Casein Hydrolysates Induce Grapevine Immune Responses and Resistance against Plasmopara viticola. Front. Plant. Sci. 2014, 5, 716. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bézier, A.; Lambert, B.; Joubert, J.M.; Pugin, A. Laminarin Elicits Defense Responses in Grapevine and Induces Protection Against Botrytis cinerea and Plasmopara viticola. Mol. Plant-Microbe Interact. 2003, 16, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Gabaston, J.; Cantos-Villar, E.; Biais, B.; Waffo-Teguo, P.; Renouf, E.; Corio-Costet, M.F.; Richard, T.; Mérillon, J.M. Stilbenes from Vitis vinifera L. Waste: A Sustainable Tool for Controlling Plasmopara viticola. J. Agric. Food Chem. 2017, 65, 2711–2718. [Google Scholar] [CrossRef] [PubMed]
- Dagostin, S.; Formolo, T.; Giovannini, O.; Pertot, I.; Schmitt, A. Salvia Officinalis Extract Can Protect Grapevine Against Plasmopara viticola. Plant Dis. 2010, 94, 575–580. [Google Scholar] [CrossRef]
- La Torre, A.; Mandalà, C.; Pezza, L.; Caradonia, F.; Battaglia, V. Evaluation of Essential Plant Oils for the Control of Plasmopara viticola. J. Essent. Oil Res. 2014, 26, 282–291. [Google Scholar] [CrossRef]
- Rienth, M.; Crovadore, J.; Ghaffari, S.; Lefort, F. Oregano Essential Oil Vapour Prevents Plasmopara viticola Infection in Grapevine (Vitis Vinifera) and Primes Plant Immunity Mechanisms. PLoS ONE 2019, 14, e0222854. [Google Scholar] [CrossRef]
- Romanazzi, G.; Mancini, V.; Feliziani, E.; Servili, A.; Endeshaw, S.; Neri, D. Impact of Alternative Fungicides on Grape Downy Mildew Control and Vine Growth and Development. Plant Dis. 2016, 100, 739–748. [Google Scholar] [CrossRef]
- Romanazzi, G.; Mancini, V.; Foglia, R.; Marcolini, D.; Kavari, M.; Piancatelli, S. Use of Chitosan and Other Natural Compounds Alone or in Different Strategies with Copper Hydroxide for Control of Grapevine Downy Mildew. Plant Dis. 2021, 105, 3261–3268. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper Environmental Toxicology, Recent Advances, and Future Outlook: A Review. Environ. Sci. Pollut. Res. 2019, 26, 18003–18016. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Mancini, V.; Carrasco-Quiroz, M.; Servili, A.; Gutiérrez-Gamboa, G.; Foglia, R.; Pérez-Álvarez, E.P.; Romanazzi, G. Chitosan and Laminarin as Alternatives to Copper for Plasmopara viticola Control: Effect on Grape Amino Acid. J. Agric. Food Chem. 2017, 65, 7379–7386. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Mundy, D.C.; Elmer, P.; Wood, P.; Agnew, R. A Review of Cultural Practices for Botrytis Bunch Rot Management in New Zealand Vineyards. Plants 2022, 11, 3004. [Google Scholar] [CrossRef]
- Plesken, C.; Pattar, P.; Reiss, B.; Noor, Z.N.; Zhang, L.; Klug, K.; Huettel, B.; Hahn, M. Genetic Diversity of Botrytis cinerea Revealed by Multilocus Sequencing, and Identification of B. Cinerea Populations Showing Genetic Isolation and Distinct Host Adaptation. Front. Plant Sci. 2021, 12, 663027. [Google Scholar] [CrossRef]
- Fedele, G.; Brischetto, C.; Rossi, V. Biocontrol of Botrytis cinerea on Grape Berries as Influenced by Temperature and Humidity. Front. Plant Sci. 2020, 11, 1232. [Google Scholar] [CrossRef]
- Bigot, G.; Mosetti, D.; Cargnus, E.; Freccero, A.; Moosavi, F.K.; Lujan, C.; Stecchina, M.; Tacoli, F.; Zandigiacomo, P.; Sivilotti, P.; et al. Influence of Vineyard Inter-Row Management and Clone on “Sauvignon Blanc” Performance in Friuli Venezia Giulia (North-Eastern Italy). Vitis-J. Grapevine Res. 2022, 61, 53–62. [Google Scholar] [CrossRef]
- Habib, W.; Khalil, J.; Mincuzzi, A.; Saab, C.; Gerges, E.; Tsouvalakis, H.; Ippolito, A.; Sanzani, S. Fungal Pathogens Associated with Harvested Table Grapes in Lebanon, and Characterization of the Mycotoxigenic Genera. Phytopathol. Mediterr. 2021, 60, 427–439. [Google Scholar] [CrossRef]
- Hahn, M. The Rising Threat of Fungicide Resistance in Plant Pathogenic Fungi: Botrytis as a Case Study. J. Chem. Biol. 2014, 7, 133–141. [Google Scholar] [CrossRef]
- Fan, F.; Zhu, Y.; Wu, M.; Yin, W.-X.; Li, G.-Q.; Hanh, M.; Hamada, M.S.; Luo, C.-X. Mitochondrial Inner Membrane ABC Transporter Bcmdl1 Is Involved in Conidial Germination, Virulence, and Resistance to Anilinopyrimidine Fungicides in Botrytis cinerea. Microbiol. Spectr. 2023, 11, e0010823. [Google Scholar] [CrossRef]
- Kretschmer, M.; Leroch, M.; Mosbach, A.; Walker, A.-S.; Fillinger, S. Fungicide-Driven Evolution and Molecular Basis of Multidrug Resistance in Field Populations of the Grey Mould Fungus Botrytis cinerea. PLoS Pathog. 2009, 5, 1000696. [Google Scholar] [CrossRef]
- Saito, S.; Michailides, T.J.; Xiao, C.L. Fungicide-Resistant Phenotypes in Botrytis cinerea Populations and Their Impact on Control of Gray Mold on Stored Table Grapes in California. Eur. J. Plant Pathol. 2019, 154, 203–213. [Google Scholar] [CrossRef]
- Testempasis, S.; Puckett, R.D.; Michailides, T.J.; Karaoglanidis, G.S. Genetic Structure and Fungicide Resistance Profile of Botrytis spp. Populations Causing Postharvest Gray Mold of Pomegranate Fruit in Greece and California. Postharvest Biol. Technol. 2020, 170, 111319. [Google Scholar] [CrossRef]
- Mincuzzi, A.; Sanzani, S.M.; Palou, L.; Ragni, M.; Ippolito, A. Postharvest Rot of Pomegranate Fruit in Southern Italy: Characterization of the Main Pathogens. J. Fungi 2022, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yang, H. Effect of Preharvest Chitosan-g-Salicylic Acid Treatment on Postharvest Table Grape Quality, Shelf Life, and Resistance to Botrytis cinerea-Induced Spoilage. Sci. Hortic. 2017, 224, 367–373. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Tzortzakis, N.; Petropoulos, S.A. Sustainable Agriculture Systems in Vegetable Production Using Chitin and Chitosan as Plant Biostimulants. Biomolecules 2021, 11, 819. [Google Scholar] [CrossRef]
- EFSA National Summary Reports on Pesticide Residue Analysis Performed in 2018. EFSA Support. Publ. 2020, 17, 1814E. [CrossRef]
- Zhang, Z.; Zhao, P.; Zhang, P.; Su, L.; Jia, H.; Wei, X.; Fang, J.; Jia, H. Integrative Transcriptomics and Metabolomics Data Exploring the Effect of Chitosan on Postharvest Grape Resistance to Botrytis cinerea. Postharvest Biol. Technol. 2020, 167, 111248. [Google Scholar] [CrossRef]
- Youssef, K.; de Oliveira, A.G.; Tischer, C.A.; Hussain, I.; Roberto, S.R. Synergistic Effect of a Novel Chitosan/Silica Nanocomposites-Based Formulation against Gray Mold of Table Grapes and Its Possible Mode of Action. Int. J. Biol. Macromol. 2019, 141, 247–258. [Google Scholar] [CrossRef]
- Youssef, K.; Roberto, S.R. Chitosan/Silica Nanocomposite-Based Formulation Alleviated Gray Mold through Stimulation of the Antioxidant System in Table Grapes. Int. J. Biol. Macromol. 2021, 168, 242–250. [Google Scholar] [CrossRef]
- De Simone, N.; Pace, B.; Grieco, F.; Chimienti, M.; Tyibilika, V.; Santoro, V.; Capozzi, V.; Colelli, G.; Spano, G.; Russo, P.; et al. Botrytis cinerea and Table Grapes: A Review of the Main Physical, Chemical, and Bio-Based Control Treatments in Post-Harvest. Foods 2020, 9, 1138. [Google Scholar] [CrossRef]
- Reglinski, T.; Elmer, P.A.G.; Taylor, J.T.; Wood, P.N.; Hoyte, S.M. Inhibition of Botrytis cinerea Growth and Suppression of Botrytis Bunch Rot in Grapes Using Chitosan. Plant Pathol. 2010, 59, 882–890. [Google Scholar] [CrossRef]
- Rajestary, R.; Xylia, P.; Chrysargyris, A.; Romanazzi, G.; Tzortzakis, N. Preharvest Application of Commercial Products Based on Chitosan, Phosphoric Acid Plus Micronutrients, and Orange Essential Oil on Postharvest Quality and Gray Mold Infections of Strawberry. Int. J. Mol. Sci. 2022, 23, 15472. [Google Scholar] [CrossRef] [PubMed]
- Palou, L.; Usall, J.; Munoz, A.; Smilanick, J.; Vinas, I. Hot Water, Sodium Carbonate, and Sodium Bicarbonate for the Control of Postharvest Green and Blue Molds of clementine Mandarins. Postharvest Biol. Technol. 2002, 24, 93–96. [Google Scholar] [CrossRef]
- Nigro, F.; Schena, L.; Ligorio, A.; Pentimone, I.; Ippolito, A.; Salerno, M.G. Control of Table Grape Storage Rots by Pre-Harvest Applications of Salts. Postharvest Biol. Technol. 2006, 42, 142–149. [Google Scholar] [CrossRef]
- Ding, S.; Meinholz, K.; Cleveland, K.; Jordan, S.A.; Gevens, A.J. Diversity and Virulence of Alternaria spp. Causing Potato Early Blight and Brown Spot in Wisconsin. Phytopathology 2019, 109, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Kumar Yadav, V.; Kumar, V.; Mani, A. Evaluation of Fungicides, Biocontrol Agents and Plant Extracts against Early Blight of Potato Caused by Alternaria solani. Int. J. Chem. Stud. 2018, 6, 1227–1230. [Google Scholar] [CrossRef]
- He, D.C.; He, M.H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration. Pathogens 2021, 10, 1311. [Google Scholar] [CrossRef]
- Palmieri, D.; Ianiri, G.; Del Grosso, C.; Barone, G.; De Curtis, F.; Castoria, R.; Lima, G. Advances and Perspectives in the Use of Biocontrol Agents against Fungal Plant Diseases. Horticulturae 2022, 8, 577. [Google Scholar] [CrossRef]
- Kowalska, J.; Tyburski, J.; Matysiak, K.; Tylkowski, B.; Malusá, E. Field Exploitation of Multiple Functions of Beneficial Microorganisms for Plant Nutrition and Protection: Real Possibility or Just a Hope? Front. Microbiol. 2020, 11, 1904. [Google Scholar] [CrossRef]
- Dong, S.M.; Zhou, S.Q. Potato Late Blight Caused by Phytophthora infestans: From Molecular Interactions to Integrated Management Strategies. J. Integr. Agric 2022, 21, 3456–3466. [Google Scholar] [CrossRef]
- Huang, X.; You, Z.; Luo, Y.; Yang, C.; Ren, J.; Liu, Y.; Wei, G.; Dong, P.; Ren, M. Antifungal Activity of Chitosan against Phytophthora infestans, the Pathogen of Potato Late Blight. Int. J. Biol. Macromol. 2021, 166, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Amborabé, B.E.; Bonmort, J.; Fleurat-Lessard, P.; Roblin, G. Early Events Induced by Chitosan on Plant Cells. J. Exp. Bot. 2008, 59, 2317–2324. [Google Scholar] [CrossRef] [PubMed]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in Plant Protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as Antimicrobial Agent: Applications and Mode of Action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Acar, O.; Aki, C.; Erdugan, H. Fungal and Bacterial Diseases Control with Elexa Plant Booster. Fresenius Environ. Bull. 2008, 17, 797–802. [Google Scholar] [CrossRef]
- Nechwatal, J.; Zellner, M. Potential Suitability of Various Leaf Treatment Products as Copper Substitutes for the Control of Late Blight (Phytophthora infestans) in Organic Potato Farming. Potato Res. 2015, 58, 261–276. [Google Scholar] [CrossRef]
- Hadwiger, L.A.; McBride, P.O. Low-Level Copper Plus Chitosan Applications Provide Protection Against Late Blight of Potato. Plant Health Prog. 2006, 6, 7. [Google Scholar] [CrossRef]
- Žabka, M.; Pavela, R. The Dominance of Chitosan Hydrochloride over Modern Natural Agents or Basic Substances in Efficacy against Phytophthora infestans, and Its Safety for the Non-Target Model Species Eisenia fetida. Horticulturae 2021, 7, 366. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, K.; Cheng, W.; Li, J.; Liang, X.; Shen, J.; Dou, D.; Yin, M.; Yan, S. Field Application of Star Polymer-Delivered Chitosan to Amplify Plant Defense against Potato Late Blight. Chem. Eng. J. 2021, 417, 129327. [Google Scholar] [CrossRef]
- Wasternack, C.; Feussner, I. The Oxylipin Pathways: Biochemistry and Function. Annu. Rev. Plant Biol. 2018, 69, 363–386. [Google Scholar] [CrossRef]
- Garmendia, A.; Raigoń, M.D.; Marques, O.; Ferriol, M.; Royo, J.; Merle, H. Effects of Nettle Slurry (Urtica dioica L.) Used as Foliar Fertilizer on Potato (Solanum tuberosum L.) Yield and Plant Growth. PeerJ 2018, 6, 4729. [Google Scholar] [CrossRef]
- Behiry, S.I.; Philip, B.; Salem, M.Z.M.; Amer, M.A.; El-Samra, I.A.; Abdelkhalek, A.; Heflish, A. Urtica dioica and Dodonaea Viscosa Leaf Extracts as Eco-Friendly Bioagents against Alternaria Alternata Isolate TAA-05 from Tomato Plant. Sci. Rep. 2022, 12, 16468. [Google Scholar] [CrossRef] [PubMed]
- Catuna (Petrar), T.; Odagiu, A.; Balint, C.; Darjan, S.; Bordea, D.; Mihaiescu, R. Testing the Anti-Alternariosis Effect of Aqueous Extract of Allium cepa L. in Potato. ProEnvironment 2021, 14, 87–90. [Google Scholar]
- Wianowska, D.; Olszowy-Tomczyk, M.; Garbaczewska, S. A Central Composite Design in Increasing the Quercetin Content in the Aqueous Onion Waste Isolates with Antifungal and Antioxidant Properties. Eur. Food Res. Technol. 2022, 248, 497–505. [Google Scholar] [CrossRef]
- Narayanasamy, P. Ecology of Postharvest Microbial Pathogens. In Postharvest Pathogens and Disease Management; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 79–116. [Google Scholar]
- Aharoni, Y.; Barkai-Golan, R. Pre-Harvest Fungicide Sprays and Polyvinyl Wraps to Control Botrytis Rot and Prolong the Post-Harvest Storage Life of Strawberries. J. Hortic. Sci. 1987, 62, 177–181. [Google Scholar] [CrossRef]
- Romanazzi, G.; Gabler, F.M.; Smilanick, J.L. Preharvest Chitosan and Postharvest UV Irradiation Treatments Suppress Gray Mold of Table Grapes. Plant Dis. 2006, 90, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, B.; Liu, J.; Tian, S. Physiological Responses and Quality Attributes of Table Grape Fruit to Chitosan Preharvest Spray and Postharvest Coating during Storage. Food Chem. 2008, 106, 501–508. [Google Scholar] [CrossRef]
- Ehtesham Nia, A.; Taghipour, S.; Siahmansour, S. Pre-Harvest Application of Chitosan and Postharvest Aloe Vera Gel Coating Enhances Quality of Table Grape (Vitis vinifera L. Cv. ‘Yaghouti’) during Postharvest Period. Food Chem. 2021, 347, 129012. [Google Scholar] [CrossRef]
- Bhaskara Reddy, M.V.; Belkacemi, K.; Corcuff, R.; Castaigne, F.; Arul, J. Effect of Pre-Harvest Chitosan Sprays on Post-Harvest Infection by Botrytis cinerea Quality of Strawberry Fruit. Postharvest Biol. Technol. 2000, 20, 39–51. [Google Scholar] [CrossRef]
- Mazaro, S.M.; Deschamps, C.; May de Mio, L.L.; Biasi, L.A.; de Gouvea, A.; Sautter, C.K. Comportamento Pós-Colheita de Frutos de Morangueiro Após a Aplicação Pré-Colheita de Quitosana e Acibenzolar-S-Metil. Rev. Bras. Frutic. 2008, 30, 185–190. [Google Scholar] [CrossRef]
- He, Y.; Bose, S.K.; Wang, W.; Jia, X.; Lu, H.; Yin, H. Pre-Harvest Treatment of Chitosan Oligosaccharides Improved Strawberry Fruit Quality. Int. J. Mol. Sci. 2018, 19, 2194. [Google Scholar] [CrossRef] [PubMed]
- Mazur, S.; Waksmundzka, A. Effect of Some Compounds on the Decay of Strawberry Fruits Caused by Botrytis cinerea Pers. Meded. Rijksuniv. Gent Fak. Landbouwkd. Toegep. Biol. Wet. 2001, 66, 227–231. [Google Scholar] [PubMed]
- Romanazzi, G.; Nigro, F.; Ippolito, A. Short Hypobaric Treatments Potentiate the Effect of Chitosan in Reducing Storage Decay of Sweet Cherries. Postharvest Biol. Technol. 2003, 29, 73–80. [Google Scholar] [CrossRef]
- Ahmed, Z.F.R.; Kaur, N.; Maqsood, S.; Schmeda-Hirschmann, G. Preharvest Applications of Chitosan, Salicylic Acid, and Calcium Chloride Have a Synergistic Effect on Quality and Storability of Date Palm Fruit (Phoenix dactylifera L.). HortScience 2022, 57, 422–430. [Google Scholar] [CrossRef]
- Zhang, C.; Long, Y.H.; Wang, Q.P.; Li, J.H.; An, H.M.; Wu, X.M.; Li, M. The Effect of Preharvest 28.6% Chitosan Composite Film Sprays for Controlling the Soft Rot on Kiwifruit and Its Defence Responses. Hortic. Sci. 2019, 46, 180–194. [Google Scholar] [CrossRef]
- Cui, K.; Shu, C.; Zhao, H.; Fan, X.; Cao, J.; Jiang, W. Preharvest Chitosan Oligochitosan and Salicylic Acid Treatments Enhance Phenol Metabolism and Maintain the Postharvest Quality of Apricots (Prunus armeniaca L.). Sci. Hortic. 2020, 267, 109334. [Google Scholar] [CrossRef]
- Elmenofy, H.M.; Okba, S.K.; Salama, A.M.; Alam-Eldein, S.M. Yield, Fruit Quality, and Storability of ‘Canino’ Apricot in Response to Aminoethoxyvinylglycine, Salicylic Acid, and Chitosan. Plants 2021, 10, 1838. [Google Scholar] [CrossRef]
- El-Badawy, H.E.M. Effect of Chitosan and Calcium Chloride Spraying on Fruits Quality of Florida Prince Peach under Cold Storage. Res. J. Agric. Biol. Sci. 2012, 8, 272–281. [Google Scholar]
- Gayed, A.A.N.A.; Shaarawi, S.A.M.A.; Elkhishen, M.A.; Elsherbini, N.R.M. Pre-Harvest Application of Calcium Chloride and Chitosan on Fruit Quality and Storability of ‘Early Swelling’ Peach during Cold Storage. Ciênc. Agrotecnol. 2017, 41, 220–231. [Google Scholar] [CrossRef]
- Yan, J.; Cao, J.; Jiang, W.; Zhao, Y. Effects of Preharvest Oligochitosan Sprays on Postharvest Fungal Diseases, Storage Quality, and Defense Responses in Jujube (Zizyphus Jujuba Mill. Cv. Dongzao) Fruit. Sci. Hortic. 2012, 142, 196–204. [Google Scholar] [CrossRef]
- Migliori, C.A.; Salvati, L.; Di Cesare, L.F.; Lo Scalzo, R.; Parisi, M. Effects of Preharvest Applications of Natural Antimicrobial Products on Tomato Fruit Decay and Quality during Long-Term Storage. Sci. Hortic. 2017, 222, 193–202. [Google Scholar] [CrossRef]
- Tezotto-Uliana, J.V.; Fargoni, G.P.; Geerdink, G.M.; Kluge, R.A. Chitosan Applications Pre- or Postharvest Prolong Raspberry Shelf-Life Quality. Postharvest Biol. Technol. 2014, 91, 72–77. [Google Scholar] [CrossRef]
- Ippolito, A.; Schena, L.; Pentimone, I.; Nigro, F. Control of Postharvest Rots of Sweet Cherries by Pre- and Postharvest Applications of Aureobasidium pullulans in Combination with Calcium Chloride or Sodium Bicarbonate. Postharvest Biol. Technol. 2005, 36, 245–252. [Google Scholar] [CrossRef]
- Mahmoud, G.; Ahmed, S.; Abbas, M.; Soliman, A.S. Effect of Garlic and Onion Extracts as a Preharvest Applications on the Post-Harvest Quality and Oxidative Enzyme Activity of Pearfruit during Cold Storage. J. Biol. Chem. Environ. Sci. 2018, 13, 329–356. [Google Scholar]
- Ahn, S.E.; Lee, A.Y.; Wang, M.H.; Hwang, Y.S. Increase of Strawberry Fruit Shelf-Life through Preharvest Spray of Calcium-Chitosan and Post-Harvest Treatment with High Pressure CO2. Hortic. Sci. Technol. 2014, 32, 636–644. [Google Scholar] [CrossRef]
- Saavedra, G.M.; Figueroa, N.E.; Poblete, L.A.; Cherian, S.; Figueroa, C.R. Effects of Preharvest Applications of Methyl Jasmonate and Chitosan on Postharvest Decay, Quality and Chemical Attributes of Fragaria Chiloensis Fruit. Food Chem. 2016, 190, 448–453. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority) Outcome of the Consultation with Member States and EFSA on the Basic Substance Application for Calcium Hydroxide and the Conclusions Drawn by EFSA on the Specific Points Raised; EN-488; 2013; 41p. Available online: www.efsa.europa.eu/publications (accessed on 4 August 2023).
- Gaspari, M.; Lykouressis, D.; Perdikis, D.; Polissiou, M. Nettle Extract Effects on the Aphid Myzus Persicae and Its Natural Enemy, the Predator Macrolophus Pygmaeus (Hem., Miridae). J. Appl. Entomol. 2007, 131, 652–657. [Google Scholar] [CrossRef]
- Thacker, J. An Introduction to Arthropod Pest Control; Cambridge University Press: Cambridge, UK, 2002; ISBN 9780521567879. [Google Scholar]
- González-Macedo, M.; Cabirol, N.; Rojas-Oropeza, M. Assessment of the Ancestral Use of Garlic (Allium Sativum) and Nettle (Urtica dioica) as Botanical Insecticides in the Protection of Mesquite (Prosopis laevigata) Seeds against Bruchins. J. Plant Prot. Res. 2021, 61, 170–175. [Google Scholar] [CrossRef]
- Bozsik, A. Studies on Aphicidal Efficiency of Different Stinging Nettle Extracts. Anz. Schädl. Pflanzenschutz Umweltschutz 1996, 69, 21–22. [Google Scholar] [CrossRef]
- Bozsik, A. Effect of Fermented Nettle Extract on Callaphis Juglandis. Godolloi Agrartud. Egyet. 1992, 28, 71–73. [Google Scholar]
- Dąbrowski, Z.T.; Seredyńska, U. Characterisation of the Two-Spotted Spider Mite (Tetranychus Urticae KOCH, Acari: Tetranychidae) Response to Aqueous Extracts from Selected Plant Species. J. Plant Prot. Res. 2007, 47, 113–124. [Google Scholar]
- Kapsoot, E.; Mwangi, M.; Kamau, A. Repellence and Toxicity Effect of Crude Plant Extracts on the Two-Spotted Spider Mite Tetranychus Urticae on Roses. Acta Hortic. 2015, 1077, 155–164. [Google Scholar] [CrossRef]
- Pavela, R. History, Presence and Perspective of Using Plant Extracts as Commercial Botanical Insecticides and Farm Products for Protection against Insects—A Review. Plant Prot. Sci. 2016, 52, 229–241. [Google Scholar] [CrossRef]
- Jairoce, C.F.; Teixeira, C.M.; Nunes, C.F.P.; Nunes, A.M.; Pereira, C.M.P.; Garcia, F.R.M. Insecticide Activity of Clove Essential Oil on Bean Weevil and Maize Weevil. Rev. Bras. Eng. Agric. Ambient. 2016, 20, 72–77. [Google Scholar] [CrossRef]
- Isman, M.B. Commercial Development of Plant Essential Oils and Their Constituents as Active Ingredients in Bioinsecticides. Phytochem. Rev. 2020, 19, 235–241. [Google Scholar] [CrossRef]
- Karkanis, A.C.; Athanassiou, C.G. Natural Insecticides from Native Plants of the Mediterranean Basin and Their Activity for the Control of Major Insect Pests in Vegetable Crops: Shifting from the Past to the Future. J. Pest Sci. 2021, 94, 187–202. [Google Scholar] [CrossRef]
- Mkenda, P.; Mwanauta, R.; Stevenson, P.C.; Ndakidemi, P.; Mtei, K.; Belmain, S.R. Extracts from Field Margin Weeds Provide Economically Viable and Environmentally Benign Pest Control Compared to Synthetic Pesticides. PLoS ONE 2015, 10, e0143530. [Google Scholar] [CrossRef]
- Bolouri Moghaddam, M.; Van den Ende, W. Sugars and Plant Innate Immunity. J. Exp. Bot. 2012, 63, 3989–3998. [Google Scholar] [CrossRef]
- Tarkowski, Ł.P.; Van de Poel, B.; Höfte, M.; Van den Ende, W. Sweet Immunity: Inulin Boosts Resistance of Lettuce (Lactuca sativa) against Grey Mold (Botrytis cinerea) in an Ethylene-Dependent Manner. Int. J. Mol. Sci. 2019, 20, 1052. [Google Scholar] [CrossRef]
- Derridj, S.; Cabanat, I.; Cochet, E.; Couzi, P.; Lombarkia, N.; Wu, B. Incidence Des Métabolites Présents à La Surface Des Organes Du Pommier Sur Le Comportement de Cydia Pomonella (Lepidoptera, Tortricidae). In Proceedings of the ANPP—5ème Conférence Internationale sur les Ravageurs en Agriculture, Montpellier, France, 7–9 December 1999; pp. 279–286. [Google Scholar]
- Lombarkia, N.; Derridj, S. Incidence of Apple Fruit and Leaf Surface Metabolites on Cydia pomonella Oviposition. Entomol. Exp. Appl. 2002, 104, 79–87. [Google Scholar] [CrossRef]
- Derridj, S.; Moulin, F.; Ferré, E.; Galy, H.; Bergougnoux, A.; Arnaud, I.; Auger, J. Sucrose as an Apple Tree Resistance Inducer against Cydia Pomonella L. In Proceedings of the 7th International Conference on Integrated Fruit Production, Avignon, France, 27–30 October 2008; Cross, J., Brown, M., Fitzgerald, J., Fountain, M., Yohalem, D., Eds.; IOBC Working Groups “Integrated Fruit Protection in Fruit Crops″: Avignon, France, 2008; p. 66. [Google Scholar]
- Lombarkia, N.; Derridj, S. Resistance of Apple Trees to Cydia Pomonella Egg-Laying Due to Leaf Surface Metabolites. Entomol Exp. Appl. 2008, 128, 57–65. [Google Scholar] [CrossRef]
- Arnault, I.; Lombarkia, N.; Joy-Ondet, S.; Romet, L.; Brahim, I.; Meradi, R.; Nasri, A.; Auger, J.; Derridj, S. Foliar Application of Microdoses of Sucrose to Reduce Codling Moth Cydia pomonella L. (Lepidoptera: Tortricidae) Damage to Apple Trees. Pest Manag. Sci. 2016, 72, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Tiffrent, A.E.K.; Lombarkia, N. Assessement of Control Strategy by Spraying Low Doses of Sugars on Apple Orchard against Cydia pomonella (Linnaeus, 1758.). Acta Agric. Slov. 2021, 117, 1–6. [Google Scholar] [CrossRef]
- Tiffrent, A.-K.; Lombarkia, N. Effect of Foliar Application of Glucose and Fructose to Reduce Codling Moth (Cydia pomonella [L., 1758]) Damages on Apple Orchard. Acta Agric. Slov. 2022, 118, 1–6. [Google Scholar] [CrossRef]
- Tiffrent, A.; Lombarkia, N. Effect of the Exogenous Foliar Sprays of Micro-Doses of Fructose and Glucose, on Egg-Laying of Cydia pomonella L. and its Oviposition Site Selection in Apple Orchard. J. Bioresour. Manag. 2022, 9, 85–91. [Google Scholar]
- Derridj, S.; Fiala, V. Soluble Sugars of Maize Leaves (Zea mays L.) and Oviposition of the European Corn Borer (Ostrinia Nubilalis Hbn.). C. R. Seances L’acad. D’agric. Fr. 1983, 69, 465–472. [Google Scholar]
- Fiala, V.; Derridj, S.; Jolivet, E. Influence de La Teneur En Glucides Solubles Des Feuilles de Zea mays L. Sur Le Choix Du Site de Ponte de La Pyrale, Ostrinia Nubilalis Hbn. (Lepid. Pyralidae). Agronomie 1985, 5, 927–932. [Google Scholar] [CrossRef]
- Derridj, S.; Gregoire, V.; Boutin, J.P.; Fiala, V. Plant Growth Stages in the Interspecific Oviposition Preference of the European Corn Borer and Relations with Chemicals Present on the Leaf Surfaces. Entomol. Exp. Appl. 1989, 53, 267–276. [Google Scholar] [CrossRef]
- Derridj, S.; Fiala, V.; Barry, P.; Robert, P.; Roessingh, P.; Städler, E. Role of Nutrients Found in the Phylloplane, in the Insect Host-Plant Selection for Oviposition. In Proceedings of the 8th International Symposium on Insect-Plant Relationships; Springer: Dordrecht, The Netherlands, 1992; Volume 49, pp. 139–140. [Google Scholar] [CrossRef]
- Suverkropp, B.P.; Dutton, A.; Bigler, F.; Van Lenteren, J.C. Oviposition Behaviour and Egg Distribution of the European Corn Borer, Ostrinia Nubilalis, on Maize, and Its Effect on Host Finding by Trichogramma Egg Parasitoids. Bull. Insectol. 2008, 61, 303–312. [Google Scholar]
- Arnault, I.; Zimmermann, M.; Furet, A.; Chovelon, M.; Thibord, J.; Derridj, S. Fructose and Sucrose as Priming Molecules against Pathogens and Pests? In Proceedings of the Ecological Perspectives of Induced Resistance in Plants and Multitrophic Interactions in Soil, Riva del Garda (TN), Italy, 18–20 October 2017; Perazzolli, M., Puopolo, G., Pertot, I., Pieterse, C., Mauch-Mani, B., Schmitt, A., Flors, V., Eds.; IOBC-WPRS Bulletin: Riva del Garda, Italy, 2018; Volume 135, pp. 110–112. [Google Scholar]
- Derridj, S.; Lombarkia, N.; Garrec, J.P.; Galy, H.; Ferré, E. Sugars on Leaf Surfaces Used as Signals by the Insect and the Plant: Implications in Orchard Protection against Cydia pomonella L. (Lepidoptera, Tortricidae). In Moths: Types, Ecological Significance and Control; Nova Science Publishers Inc.: Hauppage, NY, USA, 2012; pp. 1–38. ISBN 9781614706267. [Google Scholar]
- Mijailovic, N.; Nesler, A.; Perazzolli, M.; Aït Barka, E.; Aziz, A. Rare Sugars: Recent Advances and Their Potential Role in Sustainable Crop Protection. Molecules 2021, 26, 1720. [Google Scholar] [CrossRef]
- Station d’experimentation La Pugere. The TALC Efficiency Evaluation in a Preventive Control Strategy of the Pear Psylla Year; Station d’experimentation La Pugere: Mallemort, France, 2011; pp. 1–68. [Google Scholar]
- Marchand, P. Basic Substances under EC 1107/2009 Phytochemical Regulation: Experience with Non-Biocide and Food Products as Biorationals. J. Plant Prot. Res. 2016, 56, 312–318. [Google Scholar] [CrossRef]
- Warlop, F. Évaluation de l’efficacité de Produits Naturels Vis-à-Vis de La Mouche de L’olivier. Available online: http://www.grab.fr/wp-content/uploads/2014/07/CR_mouche_olive_20131.pdf (accessed on 26 July 2023).
- De Smedt, C.; Someus, E.; Spanoghe, P. Potential and Actual Uses of Zeolites in Crop Protection. Pest Manag. Sci. 2015, 71, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, N. A Review: Insecticidal Potential of Zeolite (Clinoptilolite), Toxicity Ratings and General Properties of Turkish Zeolites. In Proceedings of the 11th International Working Conference on Stored Product Protection, Chiang Mai, Thailand, 24–28 November 2014; pp. 755–767. [Google Scholar]
- Barrer, R.M. Zeolites and Clay Minerals as Sorbents and Molecular Sieves; Academic Press: London, UK, 1978; ISBN 0120793504. [Google Scholar]
- Christidis, G.E.; Moraetis, D.; Keheyan, E.; Akhalbedashvili, L.; Kekelidze, N.; Gevorkyan, R.; Yeritsyan, H.; Sargsyan, H. Chemical and Thermal Modification of Natural HEU-Type Zeolitic Materials from Armenia, Georgia and Greece. Appl. Clay Sci. 2003, 24, 79–91. [Google Scholar] [CrossRef]
- Joint FAO/WHO Food Standards Programme. Codex Alimentarius Commission; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Haryadi, Y.; Syarief, R.; Hubeis, M.; Herawati, I. Effect of Zeolite on the Development of Sitophilus Zeamais Motsch. In Proceedings of the 6th International Working Conference on Stored-Product Protection, Canberra, Australia, 17–23 April 1994; Highley, E., Wright, E.J., Banks, H.J., Champ, B.R., Eds.; CAB International: Wallingford, UK, 2010; pp. 633–634. [Google Scholar]
- Kljajic, P. Protection of Stored Plant Products from Harmful Organisms; Institut za Pesticide i Zaštitu Životne Sredine: Beograd, Serbia, 2008; ISBN 9788686869029. [Google Scholar]
- Kljajić, P.; Andrić, G.; Adamović, M.; Bodroža-Solarov, M.; Marković, M.; Perić, I. Laboratory Assessment of Insecticidal Effectiveness of Natural Zeolite and Diatomaceous Earth Formulations against Three Stored-Product Beetle Pests. J. Stored Prod. Res. 2010, 46, 1–6. [Google Scholar] [CrossRef]
- Kljajić, P.; Andrić, G.; Adamović, M.; Golić, M.P. Laboratory Evaluation of Insecticidal Effectiveness of a Natural Zeolite Formulation against Sitophilus oryzae (L.), Rhyzopertha dominica (F.) and Tribolium castaneum (Herbst) in Treated Wheat. In Proceedings of the 10th International Working Conference on Stored Product Protection, 27 June–2 July 2010, Estoril, Portugal; Julius-Kuhn-Archiv: Quedlinburg, Germany; pp. 863–868.
- Kljajić, P.J.; Andrić, G.G.; Adamović, M.; Golić, M.P. Possibilities of Application of Natural Zeolites in Stored Wheat Grain Protection against Pest Insects. J. Process. Energy Agric. 2011, 15, 12–15. [Google Scholar]
- Andrić, G.G.; Marković, M.M.; Adamović, M.; Daković, A.; Golić, M.P.; Kljajić, P.J. Insecticidal Potential of Natural Zeolite and Diatomaceous Earth Formulations against Rice Weevil (Coleoptera: Curculionidae) and Red Flour Beetle (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2012, 105, 670–678. [Google Scholar] [CrossRef]
- Daniel, C.; Dierauer, H.; Clerc, M. The Potential of Silicate Rock Dust to Control Pollen Beetles (Meligethes spp.). IOBC wprs Bull. 2013, 96, 47–55. [Google Scholar]
- Rumbos, C.I.; Sakka, M.; Berillis, P.; Athanassiou, C.G. Insecticidal Potential of Zeolite Formulations against Three Stored-Grain Insects, Particle Size Effect, Adherence to Kernels and Influence on Test Weight of Grains. J. Stored Prod. Res. 2016, 68, 93–101. [Google Scholar] [CrossRef]
- Lü, J.; Sehgal, B.; Subramanyam, B. Insecticidal Potential of a Synthetic Zeolite against the Cowpea Weevil, Callosobruchus Maculatus (Fabricius) (Coleoptera: Bruchidae). J. Stored Prod. Res. 2017, 72, 28–34. [Google Scholar] [CrossRef]
- Eroglu, N.; Sakka, M.K.; Emekci, M.; Athanassiou, C.G. Effects of Zeolite Formulations on the Mortality and Progeny Production of Sitophilus Oryzae and Oryzaephilus Surinamensis at Different Temperature and Relative Humidity Levels. J. Stored Prod. Res. 2019, 81, 40–45. [Google Scholar] [CrossRef]
- Checchia, I.; Perin, C.; Mori, N.; Mazzon, L. Oviposition Deterrent Activity of Fungicides and Low-Risk Substances for the Integrated Management of the Olive Fruit Fly Bactrocera Oleae (Diptera, Tephritidae). Insects 2022, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Dickens, J.C. Olfaction in the Boll Weevil, Anthonomus Grandis Boh. (Coleoptera: Curculionidae): Electroantennogram Studies. J. Chem. Ecol. 1984, 10, 1759–1785. [Google Scholar] [CrossRef] [PubMed]
- Visser, J. Host Odor Perception in Phytophagous Insects. Annu. Rev. Entomol. 1986, 31, 121–144. [Google Scholar] [CrossRef]
- Katsoyannos, B.I.; Papadopoulos, N.T.; Stavridis, D. Evaluation of Trap Types and Food Attractants for Rhagoletis Cerasi (Diptera: Tephritidae). J. Econ. Entomol. 2000, 93, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Mazor, M.; Gothfil, S.; Galun, R. The Role of Ammonia in the Attraction of Females of the Mediterranean Fruit Fly to Protein Hydrolysate Baits. Entomol. Exp. Appl. 1987, 43, 25–29. [Google Scholar] [CrossRef]
- Epsky, N.; Heath, R. Exploiting the Interactions of Chemical and Visual Cues in Behavioral Control Measures for Pest Tephritid Fruit Flies. Fla. Entomol. 2010, 81, 273–282. [Google Scholar] [CrossRef]
- Hull, C.D.; Cribb, B.W. Olfaction in the Queensland Fruit Fly, Bactrocera Tryoni. I: Identification of Olfactory Receptor Neuron Types Responding to Environmental Odors. J. Chem. Ecol. 2001, 27, 871–887. [Google Scholar] [CrossRef]
- Sarles, L.; Verhaeghe, A.; Francis, F.; Verheggen, F.J. Semiochemicals of Rhagoletis Fruit Flies: Potential for Integrated Pest Management. Crop Prot. 2015, 78, 114–118. [Google Scholar] [CrossRef]
- Shelly, T.; Nishimoto, J.; Kurashima, R. Trap Capture of Three Economically Important Fruit Fly Species (Diptera: Tephritidae): Evaluation of a Solid Formulation Containing Multiple Male Lures in a Hawaiian Coffee Field. J. Econ. Entomol. 2012, 105, 1186–1193. [Google Scholar] [CrossRef]
- Montiel Bueno, A.; Jones, O. Alternative Methods for Controlling the Olive Fly, Bactrocera Oleae, Involving Semiochemicals. Bull. OILB/SROP 2002, 25, 147–155. [Google Scholar]
- EFSA. Outcome of the Consultation with Member States and EFSA on the Basic Substance Application for Onion Oil for Use in Plant Protection as Repellent. EFSA Support. Publ. 2017, 14, 1315E. [Google Scholar] [CrossRef][Green Version]
- Sharif, R.; Mujtaba, M.; Rahman, M.U.; Shalmani, A.; Ahmad, H.; Anwar, T.; Tianchan, D.; Wang, X. The Multifunctional Role of Chitosan in Horticultural Crops; a Review. Molecules 2018, 23, 872. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.I.; Rogge, T.M.; Stevens, C.V.; Höfte, M.; Steurbaut, W.; Smagghe, G. Insecticidal and Fungicidal Activity of New Synthesized Chitosan Derivatives. Pest Manag. Sci. 2005, 61, 951–960. [Google Scholar] [CrossRef]
- Casals, P.; Cardenas, G.; Galvez, G.; Villar, A.; Cabrera, G. Agricultural Applications of Chitosan and Derivatives. In Proceedings of the Proceedings 10th IUPAC International Congress on the Chemistry of Crop Protection, Basel, Switzerland, 4–9 August 2002; p. 228. [Google Scholar]
- Zhang, M.; Tan, T.; Yuan, H.; Rui, C. Insecticidal and Fungicidal Activities of Chitosan and Oligo-Chitosan. J. Bioact. Compat. Polym. 2003, 18, 391–400. [Google Scholar] [CrossRef]
- Li, Y.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Li, K.; Li, P. Preparation, Characterization, and Insecticidal Activity of Avermectin-Grafted-Carboxymethyl Chitosan. Biomed. Res. Int. 2016, 2016, 9805675. [Google Scholar] [CrossRef] [PubMed]
- Abbey, J.; Percival, D.; Abbey, L.; Asiedu, S.; Prithiviraj, B.; Schilder, A. Biofungicides as Alternative to Synthetic Fungicide Control of Grey Mould (Botrytis cinerea)–Prospects and Challenges. Biocontrol Sci. Technol. 2019, 29, 241–262. [Google Scholar] [CrossRef]
- Fravel, D.R.; Deahl, K.L.; Stommel, J.R. Compatibility of the Biocontrol Fungus Fusarium oxysporum Strain CS-20 with Selected Fungicides. Biol. Control 2005, 34, 165–169. [Google Scholar] [CrossRef]
- Lima, G.; De Curtis, F.; De Cicco, V. Interaction of Microbial Biocontrol Agents and Fungicides in the Control of Postharvest Diseases. Stewart Postharvest Rev. 2008, 1, 4. [Google Scholar] [CrossRef]
- Wedajo, B. Compatibility Studies of Fungicides with Combination of Trichoderma Species under In Vitro Conditions. Virol. Mycol. 2015, 4, 2. [Google Scholar] [CrossRef]
- Xu, X.M.; Jeffries, P.; Pautasso, M.; Jeger, M.J. Combined Use of Biocontrol Agents to Manage Plant Diseases in Theory and Practice. Phytopathology 2011, 101, 1024–1031. [Google Scholar] [CrossRef]
- Guetsky, R.; Shtienberg, D.; Elad, Y.; Fischer, E.; Dinoor, A. Improving Biological Control by Combining Biocontrol Agents Each with Several Mechanisms of Disease Suppression. Phytopathology 2002, 92, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Abo-Elyousr, K.A.M.; Hashem, M.; Ali, E.H. Integrated Control of Cotton Root Rot Disease by Mixing Fungal Biocontrol Agents and Resistance Inducers. Crop Prot. 2009, 28, 295–301. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.M.; El-Hendawy, H.H. Integration of Pseudomonas Fluorescens and Acibenzolar-S-Methyl to Control Bacterial Spot Disease of Tomato. Crop Prot. 2008, 27, 1118–1124. [Google Scholar] [CrossRef]
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Babaki, S.A.; Barka, E.A. Chitosan as a Potential Natural Compound to Manage Plant Diseases. Int. J. Biol. Macromol. 2022, 220, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Stasinska-Jakubas, M.; Hawrylak-Novak, B. Protective, Biostimulating, and Eliciting Effects of Chitosan and Its Derivatives on Crop Plants. Molecules 2022, 27, 2801. [Google Scholar] [CrossRef] [PubMed]
- Chittenden, C.; Singh, T. In Vitro Evaluation of Combination of Trichoderma harzianum and Chitosan for the Control of Sapstain Fungi. Biol. Control 2009, 50, 262–266. [Google Scholar] [CrossRef]
- Rkhaila, A.; Chtouki, T.; Erguig, H.; El Haloui, N.; Ounine, K. Chemical Proprieties of Biopolymers (Chitin/Chitosan) and Their Synergic Effects with Endophytic Bacillus Species: Unlimited Applications in Agriculture. Molecules 2021, 26, 1117. [Google Scholar] [CrossRef]
- Kishore, G.K.; Pande, S. Chitin-Supplemented Foliar Application of Chitinolytic Bacillus Cereus Reduces Severity of Botrytis Gray Mold Disease in Chickpea under Controlled Conditions. Lett. Appl. Microbiol. 2007, 44, 98–105. [Google Scholar] [CrossRef]
- Sid Ahmed, A.; Ezziyyani, M.; Pérez Sánchez, C.; Candela, M.E. Effect of Chitin on Biological Control Activity of Bacillus spp. and Trichoderma harzianum against Root Rot Disease in Pepper (Capsicum annuum) Plants. Eur. J. Plant Pathol. 2003, 109, 633–637. [Google Scholar] [CrossRef]
- Prasad, R.D.; Chandrika, K.S.V.P.; Godbole, V. A Novel Chitosan Biopolymer Based Trichoderma Delivery System: Storage Stability, Persistence and Bio Efficacy against Seed and Soil Borne Diseases of Oilseed Crops. Microbiol. Res. 2020, 237, 126487. [Google Scholar] [CrossRef]
- Ruiz-de-la-Cruz, G.; Leobardo Aguirre-Mancilla, C.; Aracely Godínez-Garrido, N.; Monserrat Osornio-Flores Jorge Ariel Torres-Castillo, N. Chitosan Mixed with Beneficial Fungal Conidia or Fungicide for Bean (Phaseolus vulgaris L.) Seed Coating. Interciencia 2017, 42, 307–312. [Google Scholar]
- Orconneau, Y.; Taylor, A.; Marchand, P.A. Basic Substances in Organic Agriculture: Current Status. Chron. Bioresour. Manag. 2022, 6, 76–83. [Google Scholar]
- European Commission Working Document on the Procedure for Application of Basic Substances to Be Approved in Compliance with Article 23 of Regulation (EC) No 1107/2009 2021. Available online: www.efsa.europa.eu/sites/default/files/2022-10/basic-substances-applications-procedure-an-overview.pdf (accessed on 31 August 2023).
- European Commission Working Document Regulation (EC) No 1107/2009—Scope and Bordeline Issues 2022. Available online: https://food.ec.europa.eu/system/files/2022-09/pesticides_ppp_app-proc_guide_scope_reg-1107-2019.pdf (accessed on 31 August 2023).
- European Commission Commission Implementing Regulation (EU) 2023/121 of 17 January 2023. Off. J. Eur. Union 2023, 16, 24–31.
- Dodini, M.; Fantini, M. The EU Neighbourhood Policy: Implications for Economic Growth and Stability. J. Common Mark. Stud. 2006, 44, 507–532. [Google Scholar] [CrossRef]
- Costantini, E.; La Torre, A. Regulatory Framework in the European Union Governing the Use of Basic Substances in Conventional and Organic Production. J. Plant Dis. Prot. 2022, 129, 715–743. [Google Scholar] [CrossRef]
- Deguine, J.P.; Aubertot, J.N.; Flor, R.J.; Lescourret, F.; Wyckhuys, K.A.G.; Ratnadass, A. Integrated Pest Management: Good Intentions, Hard Realities. A Review. Agron. Sustain. Dev. 2021, 41, 38. [Google Scholar] [CrossRef]

| Basic Substance | BSA or Extension | Regulatory Stage | Issue |
|---|---|---|---|
| NaCl | Ext. | Vote | Positive |
| Willow bark and stem extract | BSA | Vote | Negative |
| H2O2 silver stabilized | BSA | Vote | Negative |
| Yucca schidigera extract | BSA | Vote | Negative |
| CaOH2 | Ext. | Vote | Stop clock |
| Sodium hypochlorite | BSA | Vote | Uncertain |
| Caffeine | BSA | Vote | Stop clock |
| Ozone | BSA | Vote | Stop clock |
| Chitosan HCl | Ext. | Vote | Uncertain |
| Sainfoin (Onobrychis viciifolia var. Perly) dried pellet | BSA | EFSA outcome | To be determined |
| Quassia amara | BSA | EFSA outcome | To be determined |
| Magnesium hydroxide | BSA | EFSA outcome | To be determined |
| Moringa oleifera | BSA | Submitted | Questions for admissibility |
| Psidium guajava L. leaf extract | BSA | Submitted | Questions for admissibility |
| Organic polyphenolic botanical compost | BSA | Submitted | Questions for admissibility |
| Grape seed extract | BSA | Evaluation | To be determined |
| Allium fistulosum extract | BSA | Evaluation | To be determined |
| Eggshell | BSA | Evaluation | To be determined |
| Water | BSA | Submitted | - |
| Pepper dust | BSA | Submitted | Questions for admissibility |
| Ocimum gratissimum extract | BSA | Submitted | Questions for admissibility |
| Chitosan | Ext. | Vote | Uncertain |
| Equisetum arvense | Ext. | Vote | Negative |
| NaCl | Ext. | Vote | Uncertain |
| Urtica sp. | Ext. | Submitted | Uncertain |
| Urtica sp. | Ext. | Submitted | Uncertain |
| Sunflower oil | Ext. | Submitted | Abandoned |
| Equisetum arvense | Ext. | Submitted | Abandoned |
| Lecithin | Ext. | Submitted | Abandoned |
| Salix cortex | Ext. | Submitted | Uncertain |
| Basic Substance | BSA or Extension | Regulatory Stage | Issue |
|---|---|---|---|
| Ginger extract | BSA | Submitted | Questions for admissibility |
| Capsicum oleoresin | BSA | Submitted | Questions for admissibility |
| Vinegar | Ext. | Submitted | |
| Plectranthus amboinicus | BSA | Submitted | |
| Plantago major | BSA | Submitted | |
| NaCl | Ext. | Ongoing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toffolatti, S.L.; Davillerd, Y.; D’Isita, I.; Facchinelli, C.; Germinara, G.S.; Ippolito, A.; Khamis, Y.; Kowalska, J.; Maddalena, G.; Marchand, P.; et al. Are Basic Substances a Key to Sustainable Pest and Disease Management in Agriculture? An Open Field Perspective. Plants 2023, 12, 3152. https://doi.org/10.3390/plants12173152
Toffolatti SL, Davillerd Y, D’Isita I, Facchinelli C, Germinara GS, Ippolito A, Khamis Y, Kowalska J, Maddalena G, Marchand P, et al. Are Basic Substances a Key to Sustainable Pest and Disease Management in Agriculture? An Open Field Perspective. Plants. 2023; 12(17):3152. https://doi.org/10.3390/plants12173152
Chicago/Turabian StyleToffolatti, Silvia Laura, Yann Davillerd, Ilaria D’Isita, Chiara Facchinelli, Giacinto Salvatore Germinara, Antonio Ippolito, Youssef Khamis, Jolanta Kowalska, Giuliana Maddalena, Patrice Marchand, and et al. 2023. "Are Basic Substances a Key to Sustainable Pest and Disease Management in Agriculture? An Open Field Perspective" Plants 12, no. 17: 3152. https://doi.org/10.3390/plants12173152
APA StyleToffolatti, S. L., Davillerd, Y., D’Isita, I., Facchinelli, C., Germinara, G. S., Ippolito, A., Khamis, Y., Kowalska, J., Maddalena, G., Marchand, P., Marcianò, D., Mihály, K., Mincuzzi, A., Mori, N., Piancatelli, S., Sándor, E., & Romanazzi, G. (2023). Are Basic Substances a Key to Sustainable Pest and Disease Management in Agriculture? An Open Field Perspective. Plants, 12(17), 3152. https://doi.org/10.3390/plants12173152














