Using Plant Functional Traits to Define the Biomass Energy Potential of Invasive Alien Plant Species
Abstract
:1. Introduction
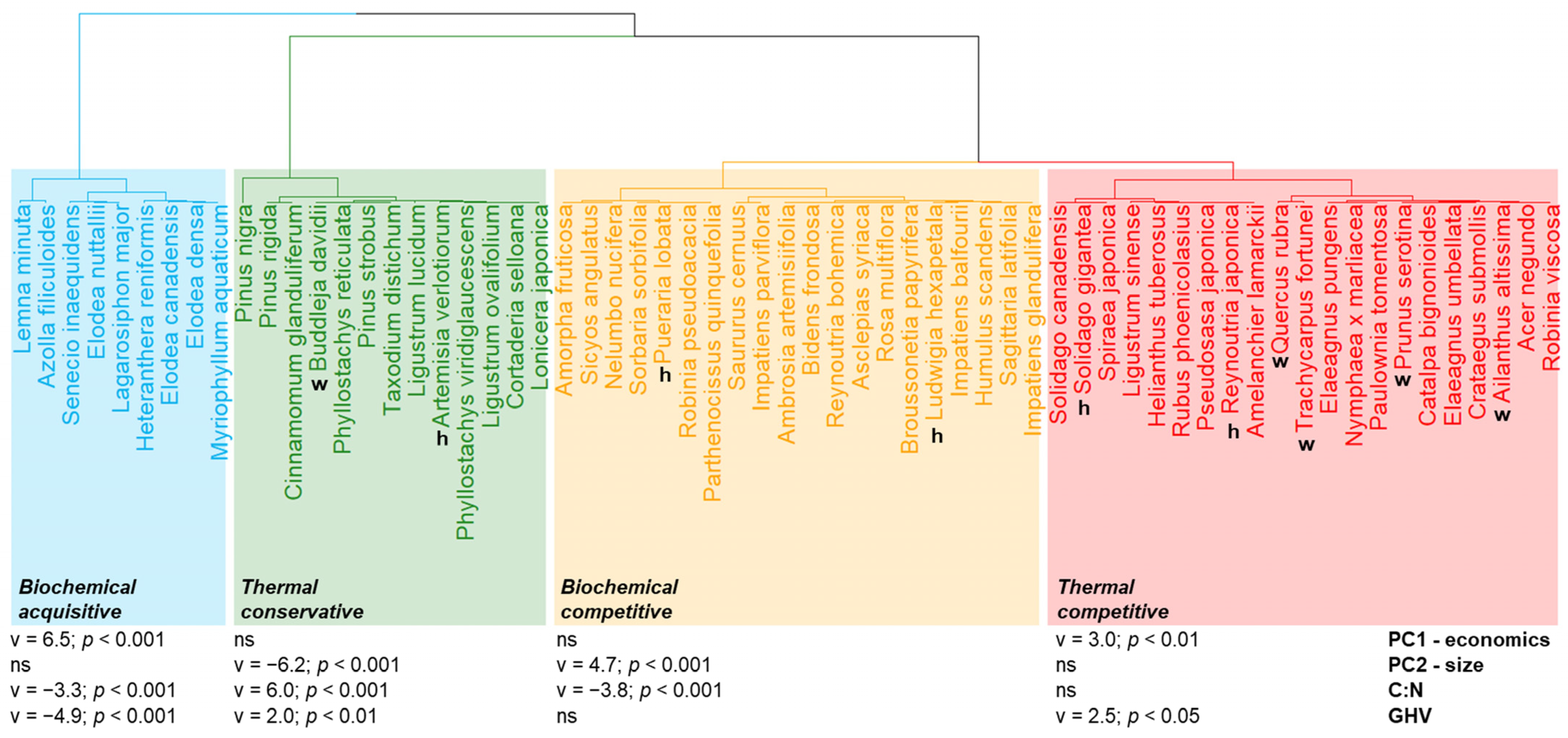
2. Results
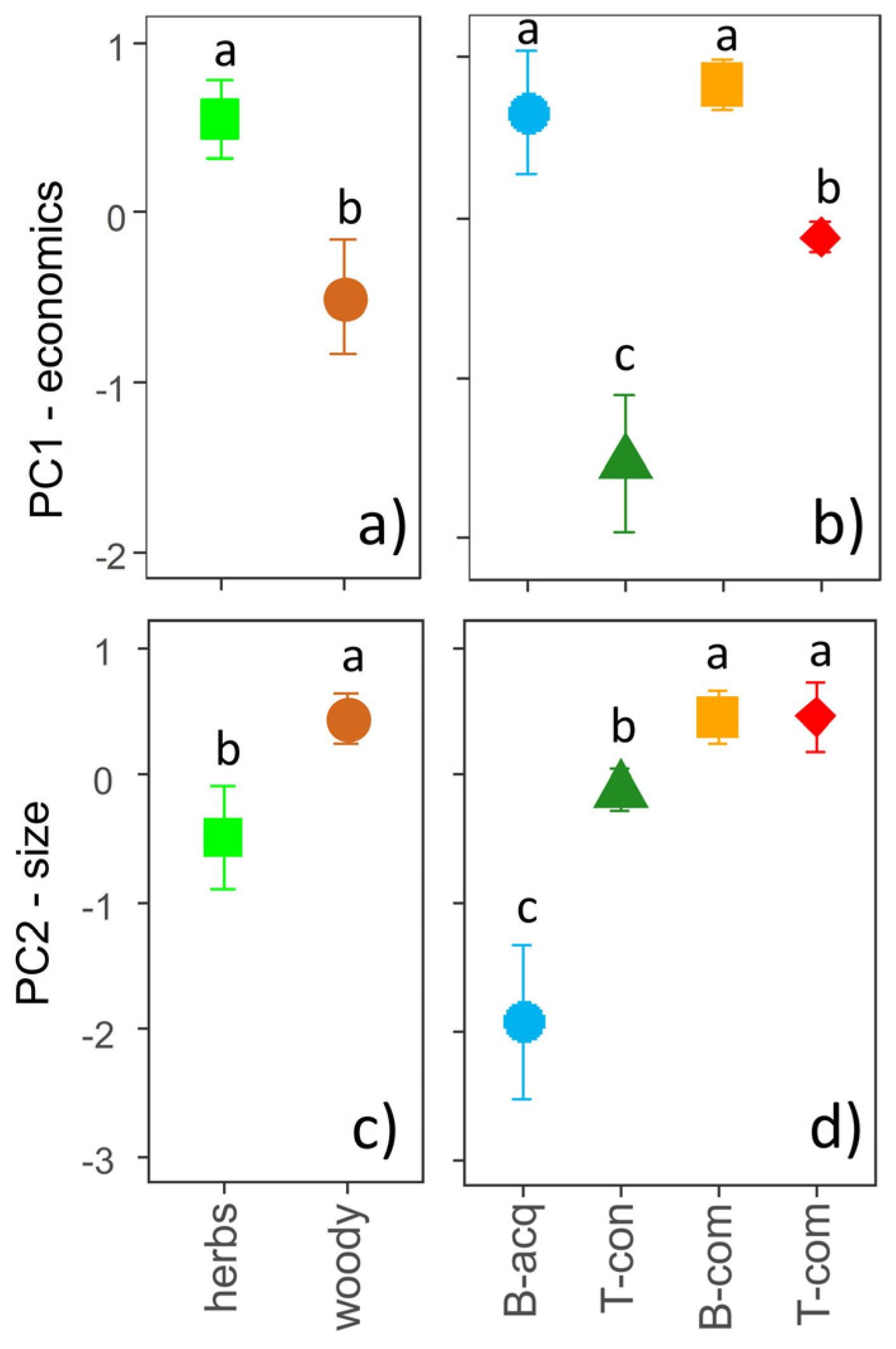
2.1. Principal Component Analysis
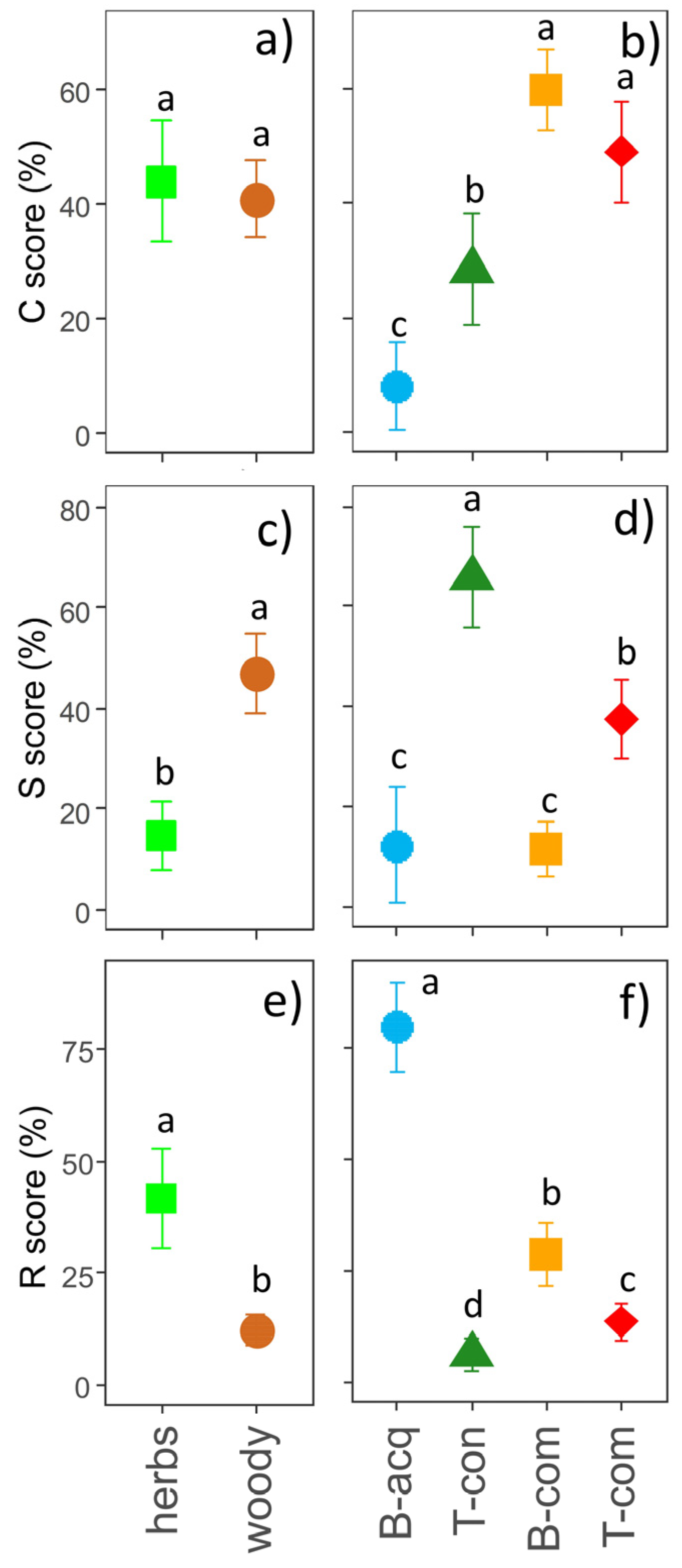
2.2. CSR Plant Strategies
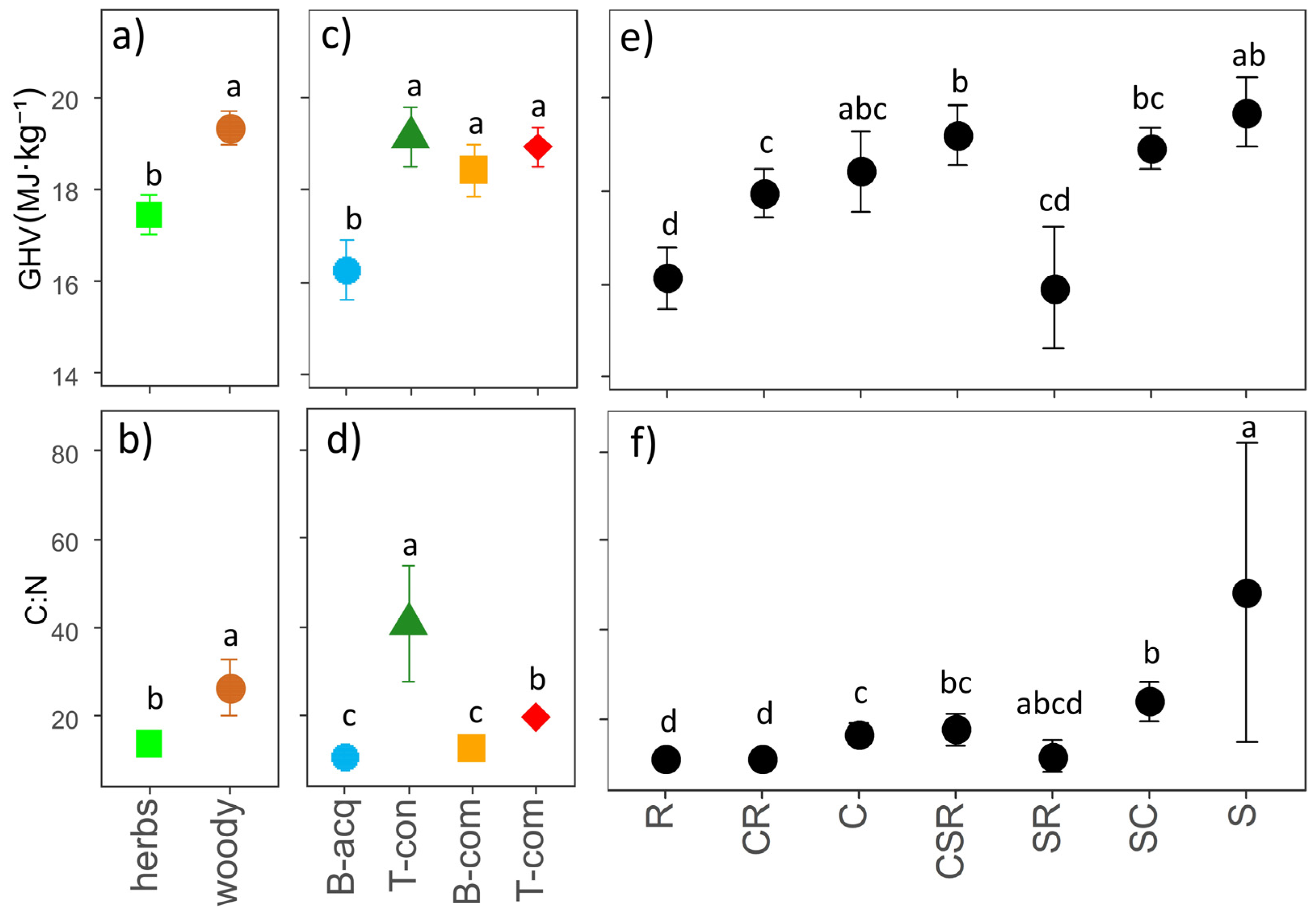
2.3. Carbon-to-Nitrogen Ratio and Gross Heating Value
2.4. Aboveground Biomass C:N and GHV Relationships with Leaf Traits and Plant Strategies
3. Discussion
4. Materials and Methods
4.1. Dataset
4.2. Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meyerson, L.A.; Mooney, H.A. Invasive alien species in an era of globalization. Front. Ecol. Evol. 2007, 5, 199–208. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pysek, P.; Rejmánek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and invasion of alien plants: Concepts and definitions. Divers. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M.; Rejmánek, M.; Webster, G.L.; Williamson, M.; Kirschner, J. Alien plants in checklists and floras: Towards better communication between taxonomists and ecologists. Taxon 2004, 53, 131–143. [Google Scholar] [CrossRef]
- Dalle Fratte, M.; Bolpagni, R.; Brusa, G.; Caccianiga, M.; Pierce, S.; Zanzottera, M.; Cerabolini, B.E.L. Alien plant species invade by occupying similar functional spaces to native species. Flora 2019, 257, 151419. [Google Scholar] [CrossRef]
- Liao, H.; Pal, R.W.; Niinemets, Ü.; Bahn, M.; Cerabolini, B.E.L.; Peng, S. Different functional characteristics can explain different dimensions of plant invasion success. J. Ecol. 2021, 109, 1524–1536. [Google Scholar] [CrossRef]
- Dimitrova, A.; Csilléry, K.; Klisz, M.; Lévesque, M.; Heinrichs, S.; Cailleret, M.; Andivia, E.; Madsen, P.; Bōhenius, H.; Cvjetkovic, B.; et al. Risks, benefits, and knowledge gaps of non-native tree species in Europe. Front. Ecol. Evol. 2022, 10, 908464. [Google Scholar] [CrossRef]
- Diagne, C.; Leroy, B.; Vaissière, A.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–585. [Google Scholar] [CrossRef]
- Nackley, L.L.; Lieu, V.H.; Garcia, B.B.; Richardson, J.J.; Isaac, E.; Spies, K.; Rigdon, S.; Schwartz, D.T. Bioenergy that supports ecological restoration. Front. Ecol. Evol. 2013, 11, 535–540. [Google Scholar] [CrossRef]
- Guo, W.Y.; van Kleunen, M.; Winter, M.; Weigelt, P.; Stein, A.; Pierce, S.; Pergl, J.; Moser, D.; Maurel, N.; Lenzner, B.; et al. The role of adaptive strategies in plant naturalization. Ecol. Lett. 2018, 21, 1380–1389. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2006; pp. 1–464. [Google Scholar]
- Pierce, S.; Negreiros, D.; Cerabolini, B.E.L.; Kattge, J.; Sandra, D.; Kleyer, M.; Shipley, B.; Wright, S.J.; Soudzilovskaia, N.A.; Onipchenko, G.; et al. A global method for calculating plant CSR ecological strategies applied across biomes world-wide. Funct. Ecol. 2017, 31, 444–457. [Google Scholar] [CrossRef]
- Wiatrowska, B.M.; Wawro, A.; Gieparda, W.; Waliszewska, B. Bioethanol Production Potential and Other Biomass Energy Properties of Invasive Reynoutria, Solidago, and Spiraea Plants. Forest 2022, 13, 1582. [Google Scholar] [CrossRef]
- van Meerbeek, K.; Appels, L.; Dewil, R.; Calmeyn, A.; Lemmens, P.; Muys, B.; Hermy, M. Biomass of invasive plant species as a potential feedstock for bioenergy production. Biofuel Bioprod. Biorefin. 2015, 9, 273–282. [Google Scholar] [CrossRef]
- Kothari, R.; Pandey, A.K.; Kumar, S.; Tyagi, V.V.; Tyagi, S.K. Different aspects of dry anaerobic digestion for bio-energy: An overview. Renew. Sustain. Energy Rev. 2014, 39, 174–195. [Google Scholar] [CrossRef]
- Fernández, M.J.; Mediavilla, I.; Barro, R.; Borjabad, E.; Ramos, R.; Carrasco, J.E. Sintering reduction of herbaceous biomass when blended with woody biomass: Predictive and combustion tests. Fuel 2019, 239, 1115–1124. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Jewiarz, M.; Dziedzic, K. Assessment of energy parameters of biomass and biochars, leachability of heavy metals and phytotoxicity of their ashes. J. Mater. Cycles Waste Manag. 2019, 21, 786–800. [Google Scholar] [CrossRef]
- Le Bauer, D.; Kooper, R.; Mulrooney, P.; Rohde, S.; Wang, D.; Long, S.P.; Dietze, M.C. BETYdb: A yield, trait, and ecosystem service database applied to second-generation bioenergy feedstock production. Glob. Chang. Biol. Bioenergy 2018, 10, 61–71. [Google Scholar] [CrossRef]
- Rojas-Sandoval, J.; Ackerman, J.D.; Marcano-Vega, H.; Willig, M.R. Alien species affect the abundance and richness of native species in tropical forests: The role of adaptive strategies. Ecosphere 2022, 13, 1–18. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Weber, E.; Fischer, M. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 2010, 13, 235–245. [Google Scholar] [CrossRef]
- Guo, K.; Pyšek, P.; Chytrý, M.; Divíšek, J.; Lososová, Z.; van Kleunen, M.; Pierce, S.; Guo, W.Y. Ruderals naturalize, competitors invade: Varying roles of plant adaptive strategies along the invasion continuum. Funct. Ecol. 2022, 36, 2469–2479. [Google Scholar] [CrossRef]
- Ordonez, A.; Wright, I.J.; Olff, H. Functional differences between native and alien species: A global-scale comparison. Funct. Ecol. 2010, 24, 1353–1361. [Google Scholar] [CrossRef]
- Raghu, S.; Anderson, R.C.; Daehler, C.C.; Davis, A.S.; Wiedenmann, R.N.; Simberloff, D.; Mack, R.N. Adding Biofuels to the Invasive Species Fire? Science 2006, 313, 1742. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B. The world-wide ‘fast–slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Dalle Fratte, M.; Pierce, S.; Zanzottera, M.; Cerabolini, B.E.L. The association of leaf sulfur content with the leaf economics spectrum and plant adaptive strategies. Funct. Plant Biol. 2021, 48, 924–935. [Google Scholar] [CrossRef]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- Friedl, A.; Padouvas, E.; Rotter, H.; Varmuza, K. Prediction of heating values of biomass fuel from elemental composition. Anal. Chim. Acta 2005, 544, 191–198. [Google Scholar] [CrossRef]
- Vernin, G.A. GC/MS Analysis of Artemisia verlotiorum Lamotte Oil. J. Essent. Oil Res. 2011, 12, 143–146. [Google Scholar] [CrossRef]
- Zanzottera, M.; Dalle Fratte, M.; Caccianiga, M.; Pierce, S.; Cerabolini, B.E.L. Towards a functional phytosociology: The functional ecology of woody diagnostic species and their vegetation classes in Northern Italy. I Forest 2021, 14, 522–530. [Google Scholar] [CrossRef]
- Manikandan, K.; Viruthagiri, T. Optimization of C/N ratio of the medium and Fermentation conditions of Ethanol Production from Tapioca Starch using Co—Culture of Aspergillus niger and Sachormyces cerevisiae. Int. J. Chemtech Res. 2010, 2, 947–955. [Google Scholar]
- Pierce, S.; Brusa, G.; Vagge, I.; Cerabolini, B.E.L. Allocating CSR plant functional types: The use of leaf economics and size traits to classify woody and herbaceous vascular plants. Funct. Ecol. 2013, 27, 1002–1010. [Google Scholar] [CrossRef]
- Abbasi, S.A.; Nipaney, P.C.; Schaumberg, G.D. Bioenergy Potential of Eight Common Aquatic Weeds. Biol. Wastes 1990, 34, 359–366. [Google Scholar] [CrossRef]
- Minarchenko, V.; Karpiuk, U.; Kovalska, N.; Tymchenko, I.; Dvirna, T.; Cholak, I. Diagnostic micromorphological features of leaf surface of selected species of the genus Artemisia L. (Asteraceae). Hacquetia 2023, 22, 131–141. [Google Scholar] [CrossRef]
- Suárez, L.; Dìaz, T.E.; Benavente-ferraces, I.; Plaza, C.; Almeida, M.; Centeno, T.A. Hydrothermal treatment as a complementary tool to control the invasive Pampas grass (Cortaderia selloana). Sci. Total. Environ. 2022, 807, 150796. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.; Zhang, Z.; Tahir, N.; Zhang, Q. Biohydrogen production from Humulus scandens by dark fermentation: Potential evaluation and process optimization. Int. J. Hydrogen Energy 2019, 45, 3760–3768. [Google Scholar] [CrossRef]
- Terzopoulou, P.; Kamperidou, V. Chemical characterization of wood and bark biomass of the invasive species of tree-of-heaven (Ailanthus altissima (Mill.) Swingle), focusing on its chemical composition horizontal variability assessment. Wood Mater. Sci. Eng. 2021, 17, 469–477. [Google Scholar] [CrossRef]
- Lambers, H.; Poorter, H. Inherent variation in growth rate between higher plants: A search for ecological causes and consequences. Adv. Ecol. Res. 1992, 23, 187–261. [Google Scholar] [CrossRef]
- Kettenring, K.M.; Adams, C.R. Lessons learned from invasive plant control experiments: A systematic review and meta-analysis. J. Appl. Ecol. 2011, 48, 970–979. [Google Scholar] [CrossRef]
- Van Meerbeek, K.; Appels, L.; Dewil, R.; Van Beek, J.; Bellings, L.; Liebert, K.; Muys, B.; Hermy, M. Energy potential for combustion and anaerobic digestion of biomass from low-input high-diversity systems in conservation areas. Glob. Chang. Biol. Bioenergy 2015, 7, 888–898. [Google Scholar] [CrossRef]
- Tilman, D.; Socolow, R.; Foley, J.A.; Hill, J.; Larson, E.; Lynd, L.; Pacala, S.; Reilly, J.; Searchinger, T.; Somerville, C.; et al. Beneficial Biofuels—The Food, Energy, and Environment Trilemma. Science 2009, 325, 270–271. [Google Scholar] [CrossRef] [PubMed]
- Cerabolini, B.E.L.; Brusa, G.; Ceriani, R.M.; De Andreis, R.; Luzzaro, A.; Pierce, S. Can CSR classification be generally applied outside Britain? Plant Ecol. 2010, 210, 253–261. [Google Scholar] [CrossRef]
- Schrodt, F.; Kattge, J.; Shan, H.; Fazayeli, F.; Karpatne, A.; Banerjee, A.; Reichstein, M.; Boenisch, M.; Diaz, S.; Dickie, J.; et al. BHPMF—A hierarchical Bayesian approach to gap-filling and trait prediction for macroecology and functional biogeography. Glob. Ecol. Biogeogr. 2015, 24, 1510–1521. [Google Scholar] [CrossRef]
- Bisi, F.; Montagnani, C.; Cardarelli, E.; Manenti, R.; Trasforini, S.; Gentili, R.; Ardenghi, N.M.G.; Citterio, S.; Bogliani, G.; Ficetola, F.; et al. Strategia di Azione e Degli Interventi per il Controllo e Gestione Delle Specie Alloctone. Documento Aggiornato da Regione Lombardia (Versione Novembre 2022); Milan, Italy, 2018. [Google Scholar]
- Galasso, G.; Conti, F.; Peruzzi, L.; Ardenghi, N.M.G.; Banfi, E.; Albano, A.; Alessandrini, A.; Bacchetta, G.; Ballelli, S.; Mazzanti, M.B.; et al. An updated checklist of the vascular flora alien to Italy. Plant Biosyst. 2018, 152, 556–592. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research; R Package Version 2.3.3; Northwestern University: Evanston, IL, USA, 2023; Available online: https://CRAN.Rproject.org/package=psych (accessed on 20 June 2023).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: A Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Hamilton, N.E.; Ferry, M. ggtern: Ternary Diagrams Using ggplot2. J. Stat. Softw. 2018, 87, 1–17. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, G.F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 20 June 2023).
- Ricotta, C.; Dalle Fratte, M.; Pierce, S.; Carboni, M.; Cerabolini, B.E.L. Quantifying the extent of plant functional specialization using Grime’s CSR strategies. Ecol. Indic. 2023, 148, 110066. [Google Scholar] [CrossRef]
- Wheeler, B.; Torchiano, M. lmPerm Permutation Tests for Linear Models Package Version 2.1.0. 2016. Available online: https://github.com/mtorchiano/lmPerm (accessed on 20 June 2023).



| LNC | SLA | H | LA | GHV | SM | C | S | R | PC1-economics | PC2-size | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C:N | −0.99 *** | −0.70 *** | −0.66 *** | 0.0 ns | 0.48 *** | 0.42 *** | −0.12 ns | 0.75 *** | −0.63 *** | −0.97 *** | 0.18 ns |
| LNC | −0.48 *** | 0.07 ns | −0.32 ** | −0.35 ** | 0.18 ns | −0.73 *** | 0.56 *** | 0.97 *** | −0.06 ns | ||
| SLA | −0.66 *** | −0.30 * | −0.49 *** | −0.47 *** | −0.22 ns | −0.67 *** | 0.88 *** | 0.71 *** | −0.46 *** | ||
| H | 0.46 *** | 0.62 *** | 0.73 *** | 0.23 ns | 0.53 *** | −0.74 *** | −0.48 *** | 0.76 *** | |||
| LA | 0.41 *** | 0.44 *** | 0.91 *** | −0.29 * | −0.56 *** | 0.20 ns | 0.84 *** | ||||
| GHV | 0.51 *** | 0.18 ns | 0.43 *** | −0.60 *** | −0.34 ** | 0.70 *** | |||||
| SM | 0.30 * | 0.32 ** | −0.60 *** | −0.31 * | 0.75 *** | ||||||
| C | −0.47 *** | −0.47 *** | 0.31 * | 0.66 *** | |||||||
| S | −0.56 *** | −0.83 *** | 0.06 ns | ||||||||
| R | 0.54 *** | −0.69 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceriani, A.; Dalle Fratte, M.; Agosto, G.; Montagnoli, A.; Cerabolini, B.E.L. Using Plant Functional Traits to Define the Biomass Energy Potential of Invasive Alien Plant Species. Plants 2023, 12, 3198. https://doi.org/10.3390/plants12183198
Ceriani A, Dalle Fratte M, Agosto G, Montagnoli A, Cerabolini BEL. Using Plant Functional Traits to Define the Biomass Energy Potential of Invasive Alien Plant Species. Plants. 2023; 12(18):3198. https://doi.org/10.3390/plants12183198
Chicago/Turabian StyleCeriani, Alex, Michele Dalle Fratte, Gustavo Agosto, Antonio Montagnoli, and Bruno Enrico Leone Cerabolini. 2023. "Using Plant Functional Traits to Define the Biomass Energy Potential of Invasive Alien Plant Species" Plants 12, no. 18: 3198. https://doi.org/10.3390/plants12183198
APA StyleCeriani, A., Dalle Fratte, M., Agosto, G., Montagnoli, A., & Cerabolini, B. E. L. (2023). Using Plant Functional Traits to Define the Biomass Energy Potential of Invasive Alien Plant Species. Plants, 12(18), 3198. https://doi.org/10.3390/plants12183198







