Comparing Non-Thermal Plasma and Cold Stratification: Which Pre-Sowing Treatment Benefits Wild Plant Emergence?
Abstract
1. Introduction
2. Results
2.1. Seedling Emergence Analysis
2.2. Assessment of Leaf Length
3. Discussion
4. Materials and Methods
4.1. Seeds
4.2. Experimental Design
4.2.1. NTP Pretreatment
4.2.2. Stratification Pretreatment
4.3. Leaf Length Measurements
4.4. Assessment of Seedling Emergence and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mårtensson, L.-M. Methods of Establishing Species-Rich Meadow Biotopes in Urban Areas. Ecol. Eng. 2017, 103, 134–140. [Google Scholar] [CrossRef]
- Jiang, M.; Hitchmough, J.D. Can Sowing Density Facilitate a Higher Level of Forb Abundance, Biomass, and Richness in Urban, Perennial “Wildflower” Meadows? Urban For. Urban Green. 2022, 74, 127657. [Google Scholar] [CrossRef]
- Isaacs, R.; Tuell, J.; Fiedler, A.; Gardiner, M.; Landis, D. Maximizing Arthropod-Mediated Ecosystem Services in Agricultural Landscapes: The Role of Native Plants. Front. Ecol. Environ. 2009, 7, 196–203. [Google Scholar] [CrossRef]
- Seabrook, L.; Mcalpine, C.A.; Bowen, M.E. Restore, Repair or Reinvent: Options for Sustainable Landscapes in a Changing Climate. Landsc. Urban Plan. 2011, 100, 407–410. [Google Scholar] [CrossRef]
- Klaus, V.H. Urban Grassland Restoration: A Neglected Opportunity for Biodiversity Conservation: Urban Grassland Restoration. Restor. Ecol. 2013, 21, 665–669. [Google Scholar] [CrossRef]
- Garbuzov, M.; Fensome, K.A.; Ratnieks, F.L.W. Public Approval plus More Wildlife: Twin Benefits of Reduced Mowing of Amenity Grass in a Suburban Public Park in Saltdean, UK. Insect. Conserv. Divers. 2015, 8, 107–119. [Google Scholar] [CrossRef]
- Southon, G.E.; Jorgensen, A.; Dunnett, N.; Hoyle, H.; Evans, K.L. Biodiverse Perennial Meadows Have Aesthetic Value and Increase Residents’ Perceptions of Site Quality in Urban Green-Space. Landsc. Urban Plan. 2017, 158, 105–118. [Google Scholar] [CrossRef]
- Kiehl, K.; Kirmer, A.; Donath, T.W.; Rasran, L.; Hölzel, N. Species Introduction in Restoration Projects—Evaluation of Different Techniques for the Establishment of Semi-Natural Grasslands in Central and Northwestern Europe. Basic. Appl. Ecol. 2010, 11, 285–299. [Google Scholar] [CrossRef]
- Hufford, K.M.; Mazer, S.J. Plant Ecotypes: Genetic Differentiation in the Age of Ecological Restoration. Trends Ecol. Evol. 2003, 18, 147–155. [Google Scholar] [CrossRef]
- Giupponi, L.; Leoni, V. Alpine Pioneer Plants in Soil Bioengineering for Slope Stabilization and Restoration: Results of a Preliminary Analysis of Seed Germination and Future Perspectives. Sustainability 2020, 12, 7190. [Google Scholar] [CrossRef]
- Borders, B.D.; Cypher, B.L.; Ritter, N.P.; Kelly, P.A. The Challenge of Locating Seed Sources for Restoration in the San Joaquin Valley, California. Nat. Areas J. 2011, 31, 190–199. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A Classification System for Seed Dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Breaking Seed Dormancy during Dry Storage: A Useful Tool or Major Problem for Successful Restoration via Direct Seeding? Plants 2020, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Just, M.; Wolfgang, A.T.; Lewandrowski, W.; Kingsley, D. Cross Seed dormancy alleviation by warm stratification progressively widens the germination window in Mediterranean climate Rutaceae. Aust. J. Bot. 2023, 71, 55–66. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, Z.; Luo, X.; Yang, L.; Chen, X.; Zhang, Z.; Wang, J.; Hu, X. Cold Stratification Requirements for Seed Dormancy-Break Differ in Soil Moisture Content but Not Duration for Alpine and Desert Species. Plant Soil. 2022, 471, 393–407. [Google Scholar] [CrossRef]
- Bratcher, C.B.; Dole, J.M.; Cole, J.C. Stratification Improves Seed Germination of Five Native Wildflower Species. HortSci 1993, 28, 899–901. [Google Scholar] [CrossRef]
- Holubová, Ľ.; Kyzek, S.; Ďurovcová, I.; Fabová, J.; Horváthová, E.; Ševčovičová, A.; Gálová, E. Non-Thermal Plasma—A New Green Priming Agent for Plants? Int. J. Mol. Sci. 2020, 21, 9466. [Google Scholar] [CrossRef]
- Scholtz, V.; Šerá, B.; Khun, J.; Šerý, M.; Julák, J. Effects of Nonthermal Plasma on Wheat Grains and Products. J. Food Qual. 2019, 2019, 7917825. [Google Scholar] [CrossRef]
- Ekzie, E.F.G.; Chizoba, F.G.; Sun, D.W.; Cheng, J.H. A Review on Recent Advances in Cold Plasma Technology for the Food Industry: Current Applications and Future Trends. Trends Food Sci. Technol. 2017, 69, 46–58. [Google Scholar] [CrossRef]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma Agriculture from Laboratory to Farm: A Review. Processes 2020, 8, 1002. [Google Scholar] [CrossRef]
- Guragain, R.P.; Baniya, H.B.; Pradhan, S.P.; Dhungana, S.; Chhetri, G.K.; Sedhai, B.; Basnet, N.; Panta, G.P.; Joshi, U.M.; Pandey, B.P.; et al. Impact of Non-Thermal Plasma Treatment on the Seed Germination and Seedling Development of Carrot (Daucus carota sativus L.). J. Phys. Commun. 2021, 5, 125011. [Google Scholar] [CrossRef]
- Dufour, T.; Gutierrez, Q.; Bailly, C. Sustainable Improvement of Seeds Vigor Using Dry Atmospheric Plasma Priming: Evidence through Coating Wettability, Water Uptake, and Plasma Reactive Chemistry. J. Appl. Phys. 2021, 129, 084902. [Google Scholar] [CrossRef]
- Šerá, B.; Šerý, M. Non-Thermal Plasma Treatment as a New Biotechnology in Relation to Seeds, Dry Fruits, and Grains. Plasma Sci. Technol. 2018, 20, 044012. [Google Scholar] [CrossRef]
- Pawłat, J.; Starek, A.; Sujak, A.; Terebun, P.; Kwiatkowski, M.; Budzeń, M.; Andrejko, D. Effects of Atmospheric Pressure Plasma Jet Operating with DBD on Lavatera thuringiaca L. Seeds’ Germination. PLoS ONE 2018, 13, e0194349. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Attri, P.; Kamataki, K.; Itagaki, N.; Shiratani, M.; Mildaziene, V. Impact of Radish Sprouts Seeds Coat Color on the Electron Paramagnetic Resonance Signals after Plasma Treatment. Jpn. J. Appl. Phys. 2020, 59, SHHF01. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Y.; Li, J.; Li, L.; He, X.; Shao, H.; Dong, S. Effect of cold plasma treatment on seed germination and growth of wheat. PLoS ONE 2014, 9, e97753. [Google Scholar] [CrossRef]
- Sarapirom, S.; Yu, L.D. Low-Pressure and Atmospheric Plasma Treatments of Sunflower Seeds. Surf. Coat. Technol. 2021, 406, 126638. [Google Scholar] [CrossRef]
- Cui, D.; Yin, Y.; Wang, J.; Wang, Z.; Ding, H.; Ma, R.; Jiao, Z. Research on the Physio-Biochemical Mechanism of Non-Thermal Plasma-Regulated Seed Germination and Early Seedling Development in Arabidopsis. Front. Plant Sci. 2019, 10, 1322. [Google Scholar] [CrossRef]
- Mildažienė, V.; Paužaitė, G.; Naučienė, Z.; Žūkienė, R.; Malakauskienė, A.; Norkevičienė, E.; Šlepetienė, A.; Stukonis, V.; Olšauskaitė, V.; Padarauskas, A.; et al. Effect of Seed Treatment with Cold Plasma and Electromagnetic Field on Red Clover Germination, Growth and Content of Major Isoflavones. J. Phys. D Appl. Phys. 2020, 53, 264001. [Google Scholar] [CrossRef]
- Mildaziene, V.; Ivankov, A.; Sera, B.; Baniulis, D. Biochemical and physiological plant processes affected by seed treatment with non-thermal plasma. Plants 2022, 11, 856. [Google Scholar] [CrossRef]
- Măgureanu, M.; Sîrbu, R.; Dobrin, D.; Gîdea, M. Stimulation of the Germination and Early Growth of Tomato Seeds by Non-Thermal Plasma. Plasma Chem. Plasma Process. 2018, 38, 989–1001. [Google Scholar] [CrossRef]
- Velichko, I.; Gordeev, I.; Shelemin, A.; Nikitin, D.; Brinar, J.; Pleskunov, P.; Choukourov, A.; Pazderů, K.; Pulkrábek, J. Plasma Jet and Dielectric Barrier Discharge Treatment of Wheat Seeds. Plasma Chem. Plasma Process. 2019, 39, 913–928. [Google Scholar] [CrossRef]
- Liu, B.; Honnorat, B.; Yang, H.; Arancibia, J.; Rajjou, L.; Rousseau, A. Non-Thermal DBD Plasma Array on Seed Germination of Different Plant Species. J. Phys. D Appl. Phys. 2019, 52, 025401. [Google Scholar] [CrossRef]
- Ghodsimaab, S.P.; Makarian, H.; Ghasimi Hagh, Z.; Gholipoor, M. Scanning electron microscopy, biochemical and enzymatic studies to evaluate hydro-priming and cold plasma treatment effects on the germination of Salvia leriifolia Benth. seeds. Front. Plant Sci. 2023, 13, 1035296. [Google Scholar] [CrossRef]
- FloraVeg. EU—Database of European Vegetation, Habitats and Flora. Available online: https://floraveg.eu (accessed on 24 March 2023).
- Rašomavičius, V. (Ed.) Lietuvos augalija; Pievos 1; Šviesa: Kaunas, Lithuania; Vilnius, Lithuania, 1998; pp. 163–267. (In Lithuanian) [Google Scholar]
- Rašomavičius, V. (Ed.) Red Data Book of Lithuania; Animals, Plants, Fungi; Lututė: Vilnius, Lithuania, 2021; pp. 35–39. (In Lithuanian) [Google Scholar]
- Hoyle, G.L.; Steadman, K.J.; Good, R.B.; McIntosh, E.J.; Galea, L.M.E.; Nicotra, A.B. Seed Germination Strategies: An Evolutionary Trajectory Independent of Vegetative Functional Traits. Front. Plant Sci. 2015, 6, 731. [Google Scholar] [CrossRef] [PubMed]
- ENSCOBASE: The European Native Seed Conservation Network (ENSCONET) Virtual Seed Bank. Released in October 2009. Last data import: September 2020. Available online: http://enscobase.maich.gr (accessed on 15 April 2023).
- Gomez, G.; Colle, M.L.; Oller, R.; Langohr, K. Tutorial on methods for interval-censored data and their implementation in R. Stat. Model. 2009, 9, 259–297. [Google Scholar] [CrossRef]
- Cochrane, A.; Yates, C.J.; Hoyle, G.L.; Nicotra, A.B. Will Among-Population Variation in Seed Traits Improve the Chance of Species Persistence under Climate Change? Glob. Ecol. Biogeogr. 2015, 24, 12–24. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.; Hilhorst, H.; Nonogaki, H. Seeds. Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 247–258. [Google Scholar]
- Neyts, E.C. Plasma-surface interactions in plasma catalysis. Plasma Chem. Plasma Process. 2016, 36, 185–212. [Google Scholar] [CrossRef]
- Szalai, Z.; Ferschl, B. The Effect of Seed Treatments on the Germination of Different Fabaceae Species of a Natural Meadow-Like Association. Anal. Tech. Szeged. 2016, 10, 58–63. [Google Scholar] [CrossRef][Green Version]
- Šerá, B.; Scholtz, V.; Jirešová, J.; Khun, J.; Julák, J.; Šerý, M. Effects of Non-Thermal Plasma Treatment on Seed Germination and Early Growth of Leguminous Plants—A Review. Plants 2021, 10, 1616. [Google Scholar] [CrossRef]
- Ivankov, A.; Zukiene, R.; Nauciene, Z.; Degutyte-Fomins, L.; Filatova, I.; Lyushkevich, V.; Mildaziene, V. The Effects of Red Clover Seed Treatment with Cold Plasma and Electromagnetic Field on Germination and Seedling Growth Are Dependent on Seed Color. Appl. Sci. 2021, 11, 4676. [Google Scholar] [CrossRef]
- Almarashi, J.Q.M. Second grounded electrode non-equilibrium atmospheric pressure argon plasma jet impact on germination of basil (Ocimum basilicum) seeds. J. Taibah Univ. Sci. 2023, 17, 2194847. [Google Scholar] [CrossRef]
- Ghaemi, M.; Majd, A.; Iranbakhsh, A. Transcriptional responses following seed priming with cold plasma and electromagnetic field in Salvia nemorosa L. J. Theor. Appl. Phys. 2020, 14, 323–328. [Google Scholar] [CrossRef]
- Matilla, A.; Matilla, A.; Gallardo, M.; Puga-Hermida, M.I. Structural, physiological and molecular aspects of heterogeneity in seeds: A review. Seed Sci. Res. 2005, 15, 63–76. [Google Scholar] [CrossRef]
- Meiqiang, Y.; Mingjing, H.; Buzhou, M.; Tengcai, M. Stimulating effects of seed treatment by magnetized plasma on tomato growth and yield. Plasma Sci. Technol. 2005, 7, 3143–3147. [Google Scholar] [CrossRef]
- Mildažienė, V.; Aleknavičiūtė, V.; Žūkienė, R.; Paužaitė, G.; Naučienė, Z.; Filatova, I.; Lyushkevich, V.; Haimi, P.; Tamošiūnė, I.; Baniulis, D. Treatment of Common sunflower (Helianthus annus L.) seeds with radio-frequency electromagnetic field and cold plasma induces changes in seed phytohormone balance, seedling development and leaf protein expression. Sci. Rep. 2019, 9, 6437. [Google Scholar] [CrossRef]
- International Seed Morphology Association. Method for Seed Size Measurement. ISMA Editorial Board for Seed Identification Guide. 2019. Available online: https://www.idseed.org/pages/seed_size_measurement_protocol_new.html (accessed on 27 August 2023).
- Ivankov, A.; Nauciene, Z.; Zukiene, R.; Degutyte-Fomins, L.; Malakauskiene, A.; Kraujalis, P.; Mildaziene, V. Changes in Growth and Production of Non-Psychotropic Cannabinoids Induced by Pre-Sowing Treatment of Hemp Seeds with Cold Plasma, Vacuum and Electromagnetic Field. Appl. Sci. 2020, 10, 8519. [Google Scholar] [CrossRef]
- Onofri, A.; Mesgaran, M.; Ritz, C. A unified framework for the analysis of germination, emergence, and other time-to-event data in weed science. Weed Sci. 2022, 70, 259–271. [Google Scholar] [CrossRef]
- Barreiro-Ures, D.; Francisco-Fernández, M.; Cao, R.; Fraguela, B.B.; Doallo, R.; Gonzá-lez-Andújar, J.L.; Reyes, M. Analysis of interval-grouped data in weed science: The binnednp Rcpp package. Ecol. Evol. 2019, 9, 10903–10915. [Google Scholar] [CrossRef]
- Onofri, A. The ‘Drcte’ Package. Statistical Approaches for Time-to-Event Data in Agriculture. R Package, Version 1.0.6. 2022. Available online: https://www.statforbiology.com (accessed on 15 April 2023).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Gudynienė, V.; Juzėnas, S.; Norkevičienė, E.; Stukonis, V.; Mildažienė, V. Collection of Data on the Impact of NTP on Wild Plant Seeds and Its Comparison with Stratification Effects; Data Set; National Open Access Research Data Archive (MIDAS): Vilnius, Lithuania, 2023. [Google Scholar] [CrossRef]
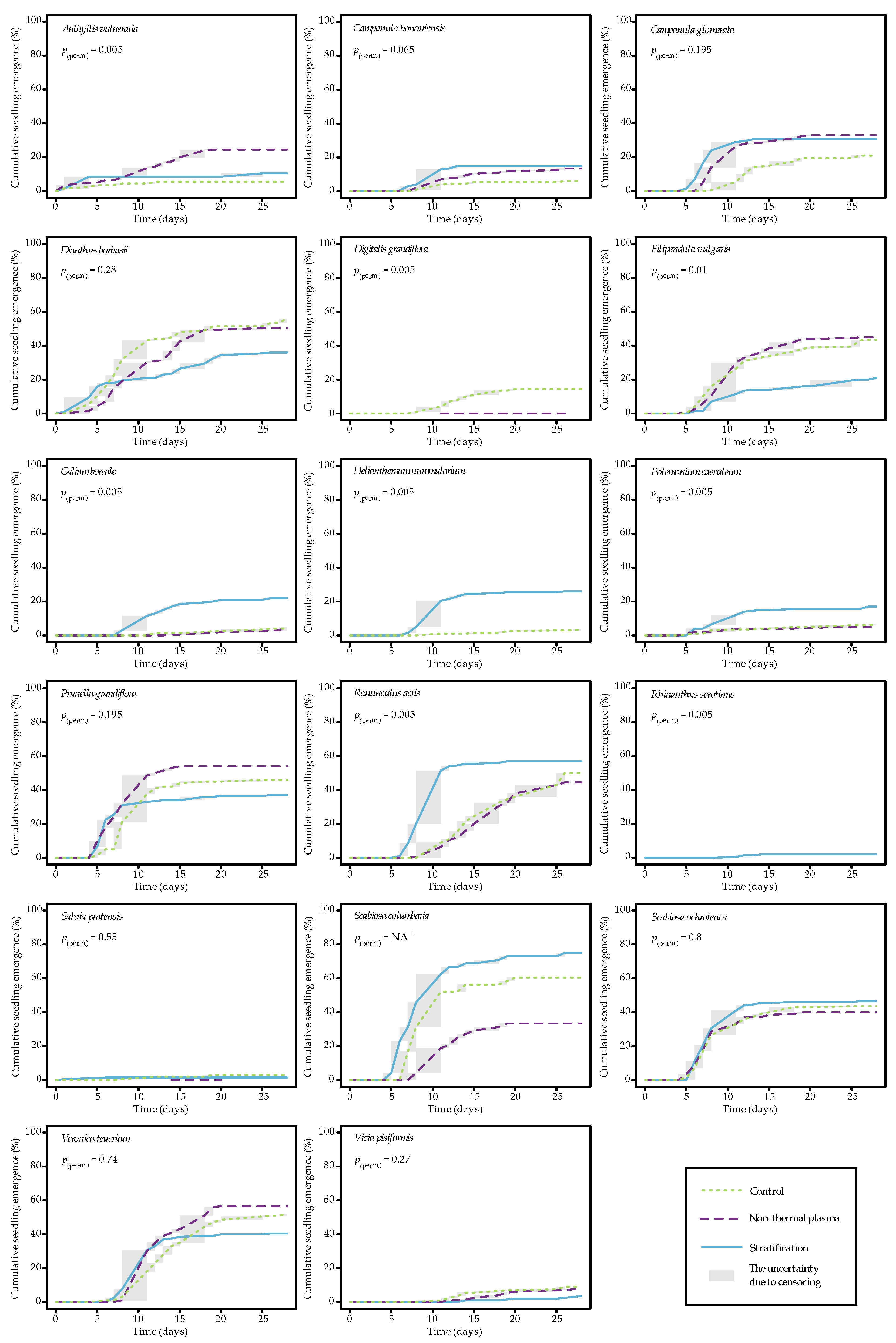
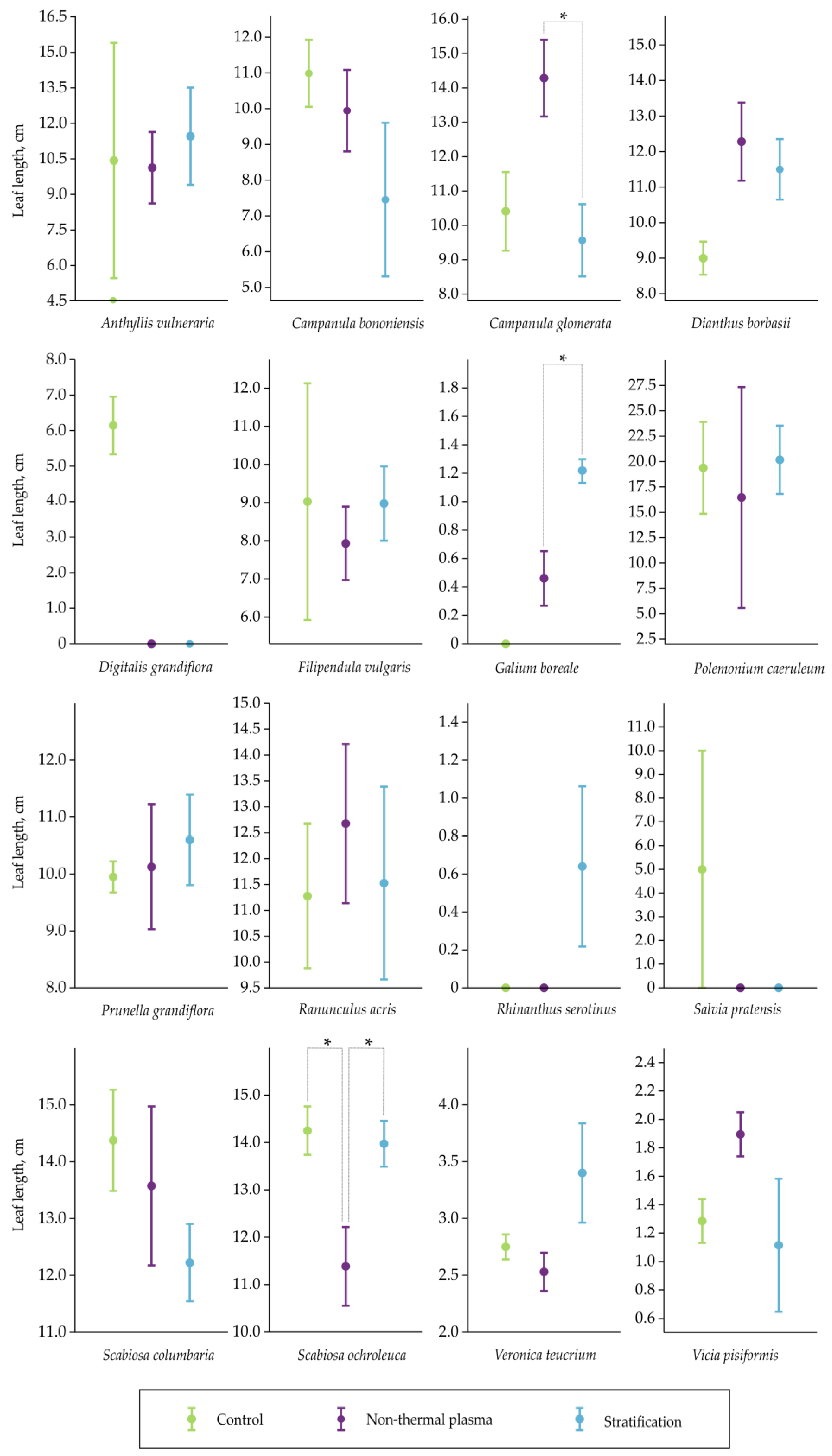
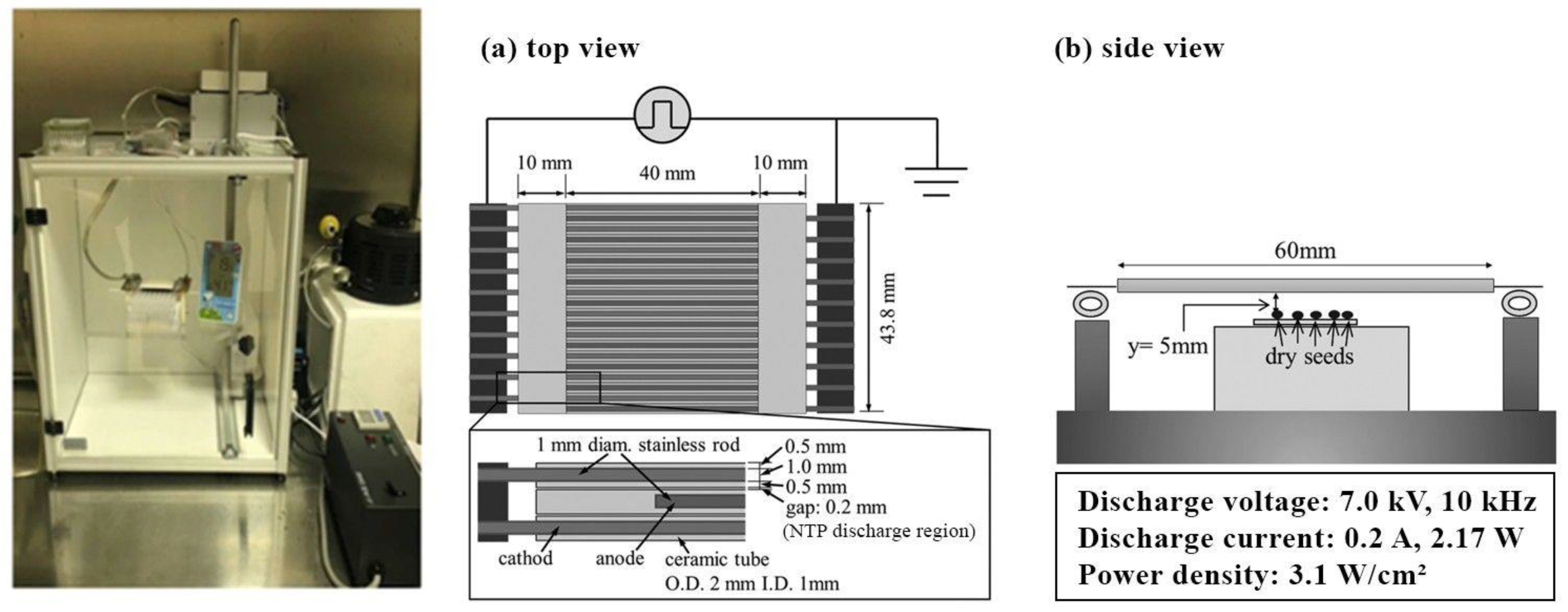
| Plant Species | No Pretreatment | Scarification | Cold Stratification |
|---|---|---|---|
| Anthyllis vulneraria | 100% (MTG 8 d.) | 100% (duration 5 d.) | |
| Campanula bononiensis | 86.7% (duration 63 d.) GA3 | ||
| Campanula glomerata | 8% (duration 27 d.), 95% (MTG 10.4 d.) GA3 | 75% (MTG 26.9 d.) GA3 | |
| Dianthus borbasii (data for D. carthusianorum) | 100% (duration 42 d., MTG 7.4 d.) | 91% (duration unknown) | |
| Digitalis grandiflora | 94% (MTG 9.7 d.) | 99% (duration unknown) GA3 | |
| Filipendula vulgaris | 100% (duration unknown), 95% (MTG 14.7 d.) | 100% (MTG 7 d.) | |
| Galium boreale | 90% (MTG 16.7 d.) GA3 | 65% (MTG 12.9 d.) | |
| Helianthemum nummularium | 100% (duration 6 d.) | ||
| Polemonium caeruleum | 89% (MTG 11.1 d.) | 100% (MTG 7 d.) GA3 | |
| Prunella grandiflora | P. vulgaris 100% (MTG 9.8 d.) | ||
| Ranunculus acris | 98% (MTG 17.6 d.) | ||
| Rhinanthus serotinus (data for R. minor) | 100% (MTG 52.6 d.) | 100% (MTG 1.4 d.) | |
| Salvia pratensis | 100% (MTG 7 d.) | ||
| Scabiosa columbaria | 79% (MTG 17.3 d.) | 79% (MTG 14.5 d.) | |
| Scabiosa ochroleuca (data for S. columbaria) | 79% (MTG 17.3 d.) | 79% (MTG 14.5 d.) | |
| Veronica teucrium (data for V. prostrata) | 90% (duration 62 d.) | ||
| Vicia pisiformis | (data for V. dumetorum) 35% (duration unknown) | (data for V. sylvatica) 100% (duration unknown) |
| Species | Pretreatment | EP14 *, % | EP28 *, % | T10, Days | T30, Days |
|---|---|---|---|---|---|
| Anthyllis vulneraria | C | 5.5 (1.0–9.0) a | 5.5 (1.0–9.0) a | 9.2 (7.3–12.0) a | – |
| N | 17.5 (12.0–23.0) a | 24.5 (17.0–31.0) b | 9.6 (7.0–13.5) a | 17.0 (14.0–19.0) nt | |
| S | 8.5 (3.5–12.0) a | 10.5 (8.5–12.0) a | 12.4 (2.8–25.0) a | – | |
| Campanula bononiensis | C | 4.5 (2.5–5.5) a | 6.0 (4.5–7.0) a | 16.8 (12.9–26.0) a | – |
| N | 9.5 (8.0–11.0) a | 13.5 (9.5–16.0) a | 16.0 (10.7–25.6) a | – | |
| S | 15.0 (8.0–20.0) b | 15.0 (8.0–20.0) a | 10.1 (8.9–12.3) a | 12.5 (12.5–12.5) nt | |
| Campanula glomerata | C | 14.5 (10.0–17.5) a | 21.0 (12.0–28.0) a | 12.4 (10.7–16.2) a | 24.2 (17.5–26.0) a |
| N | 28.5 (19.5–34.5) a | 33.0 (22.0–41.0) a | 7.7 (7.0–9.1) b | 13.5 (9.7–19.3) b | |
| S | 30.5 (20.0–39.0) a | 30.5 (20.0–39.0) a | 6.4 (5.8–7.1) b | 9.6 (7.5–12.9) b | |
| Dianthus borbasii | C | 45.0 (35.0–54.0) a | 56.0 (46.0–64.0) a | 5.0 (3.7–6.5) ab | 8.4 (6.6–11.4) a |
| N | 37.0 (24.0–44.0) a | 50.5 (32.0–36.5) a | 6.4 (5.3–7.6) a | 11.8 (8.9–15.8) ab | |
| S | 23.5 (19.0–27.0) a | 36.0 (34.0–38.0) a | 4.1 (3.0–5.4) b | 17.9 (13.0–25.1) b | |
| Digitalis grandiflora | C | 10.0 (3.5–15.5) a | 14.5 (9.0–20.0) a | 14.8 (11.5–20.0) a | – |
| N | 1.0 (0.0–1.5) b | 12.0 (8.5–15.5) a | 25.7 (25.5–26.0) b | – | |
| S | 0 nt | 0 nt | – | – | |
| Filipendula vulgaris | C | 33.0 (28.5–37.0) a | 43.5 (40.0–47.0) a | 7.1 (6.1–8.0) a | 13.1 (10.4–19.1) a |
| N | 36.0 (30.0–40.0) a | 45.0 (40.0–49.0) a | 7.9 (7.0–8.7) a | 11.6 (10.0–14.7) a | |
| S | 14.0 (10.0–16.0) b | 21.0 (16.5–24.5) b | 10.8 (7.7–19.0) a | 27.1 (25.6–27.7) b | |
| Galium boreale | C | 1.5 (0.5–2.0) a | 4.0 (2.0–5.5) a | 25.8 (25.6–26.0) a | – |
| N | 0.5 (0.0–1.0) a | 5.0 (2.0–7.0) a | 27.8 (27.5–28.0) b | – | |
| S | 17.0 (11.0–22.0) b | 22.0 (14.5–27.0) b | 10.9 (8.7–14.4) c | 20.9 (14.6–26.0) nt | |
| Helianthemum nummularium | C | 1.0 (0–1.5) a | 3.5 (2.0–4.0) a | – | – |
| N | 0.5 (0.0–1.0) a | 0.5 (0.0–1.0) a | – | – | |
| S | 24.5 (16.5–35.5) b | 26.0 (18.0–33.0) b | 9.2 (8.1–10.8) nt | 14.2 (10.3–25.9) nt | |
| Polemonium caeruleum | C | 4.0 (2.0–5.0) a | 6.5 (4.5–7.5) a | 23.6 (16.6–28.0) ab | – |
| N | 4.0 (2.5–5.0) a | 5.0 (2.0–7.0) a | 23.0 (18.5–25.0) a | – | |
| S | 15.0 (9.0–18.0) b | 17.0 (12.0–20.0) b | 10.8 (7.2–26.3) b | – | |
| Prunella grandiflora | C | 42.0 (36.0–48.0) a | 46.0 (40.5–50.5) ab | 7.4 (7.1–7.6) a | 9.7 (8.4–11.1) a |
| N | 53.0 (49.0–57.0) b | 54.0 (48.5–58.5) b | 5.1 (4.7–5.8) b | 7.8 (6.8–9.0) a | |
| S | 34.0 (32.5–35.5) a | 37.0 (36.0–37.5) a | 5.3 (4.8–5.8) b | 9.4 (5.9–19.2) a | |
| Ranunculus acris | C | 21.5 (18.0–25.5) a | 50.0 (48.0–51.0) ab | 11.5 (10.1–13.0) a | 17.3 (14.4–21.3) a |
| N | 16.0 (8.5–22.0) b | 44.5 (39.5–48.0) b | 12.3 (10.2–14.4) a | 18.1 (15.4–20.8) a | |
| S | 55.5 (48.0–64.0) ab | 57.0 (51.0–63.0) a | 7.2 (6.7–7.7) b | 9.0 (8.2–9.8) b | |
| Rhinanthus serotinus | C | 0 nt | 0 nt | – | – |
| N | 0 nt | 0 nt | – | – | |
| S | 2.0 (0.0–3.0) nt | 2.0 (0.0–3.0) nt | – | – | |
| Salvia pratensis | C | 2.0 (0.5–3.0) a | 3.0 (2.0–3.5) a | – | – |
| N | 1.0 (0.0–1.5) a | 1.5 (0.0–2.5) a | – | – | |
| S | 1.5 (0.0–3.0) a | 1.5 (0.0–3.0) a | – | – | |
| Scabiosa columbaria | C | 56.2 (39.6–66.7) a | 60.4 (39.55–75.0) ab | 6.7 (6.4–7.7) a | 8.2 (7.0–10.5) ab |
| N | 27.1 (18.8–31.2) b | 33.3 (27.08–37.5) b | 9.5 (7.9–12.4) a | 14.6 (10.5–18.7) a | |
| S | 68.8 (54.2–79.2) a | 75.0 (62.48–83.4) a | 5.4 (4.9–5.9) b | 6.8 (5.7–8.3) b | |
| Scabiosa ochroleuca | C | 39.0 (26.0–50.0) a | 43.5 (35.5–50.0) a | 6.4 (5.9–6.9) a | 10.3 (7.5–16.6) a |
| N | 37.0 (31.0–42.0) a | 40.0 (35.0–43.0) a | 6.2 (5.4–7.1) a | 9.6 (7.4–14.8) a | |
| S | 45.5 (36.0–53.0) a | 46.5 (36.5–51.5) a | 6.0 (5.6–6.5) a | 8.2 (7.2–10.5) a | |
| Veronica teucrium | C | 33.0 (23.0–43.0) a | 52.0 (41.5–61.0) a | 9.4 (8.7–10.4) a | 13.7 (11.7–16.8) a |
| N | 41.0 (25.0–52.0) a | 56.5 (44.0–64.5) a | 9.0 (8.7–9.4) a | 11.5 (10.1–15.6) a | |
| S | 37.5 (29.5–44.0) a | 40.5 (32.5–44.5) a | 8.3 (7.7–8.9) a | 11.5 (10.1–14.7) a | |
| Vicia pisiformis | C | 5.5 (2.5–8.0) a | 9.0 (6.0–12.0) a | 22.9 (13.9–26.0) a | – |
| N | 1.5 (0.0–2.5) a | 7.5 (4.0–11.0) a | 23.4 (17.2–27.0) a | – | |
| S | 1.0 (0.0–1.5) a | 3.5 (0.5–6.0) a | – | – |
| Plant Species | Location Geographical Coordinates ** | Seed Collection Dates |
|---|---|---|
| Anthyllis vulneraria | Sibirka, Trakai distr. 54.667517, 24.899277 | 11 August 2020 |
| Campanula bononiensis * EN | Dotnuva, Kėdainiai distr. 55.396349, 23.865125 | 21 August 2020 |
| Campanula glomerata | Krekenava, Panevėžys distr. 55.540838, 24.111416 | 21 August 2020 |
| Dianthus borbasii * EN | Dotnuva, Kėdainiai distr. | 21 August 08 2020 |
| Digitalis grandiflora | Sibirka, Trakai distr. 54.667517, 24.899277 | 11 August 08 2020 |
| Filipendula vulgaris | Bradeliškės, Vilnius distr. 54.82638, 24.949936 | 1 October 2020 |
| Galium boreale | Bradeliškės, Vilnius distr. 54.82638, 24.949936 | 1 October 2020 |
| Helianthemum nummularium | Bradeliškės, Vilnius distr. 54.82638, 24.949936 | 4 October 2020 |
| Polemonium caeruleum * VU | Bradeliškės, Vilnius distr. 54.82638, 24.949936 | 1 October 2020 |
| Prunella grandiflora * EN | Dotnuva, Kėdainiai distr. 55.396349, 23.865125 | 21 August 2020 |
| Ranunculus acris | Liubavas, Vilnius distr. 54.85014, 25.32080 | 10 October 2020 |
| Rhinanthus serotinus | Rusnė, Šilutė distr. 55.3252, 21.455061 | 30 September 2020 |
| Salvia pratensis * VU | Jurbarkas distr. 55.08005, 23.40588 | 3 November 2020 |
| Scabiosa columbaria | Vilnius distr. 54.91008, 25.32236 | 23 August 08 2020 |
| Scabiosa ochroleuca | Sibirka, Trakai distr. 54.667517, 24.899277 | 2 October 2020 |
| Veronica teucrium | Krekenava, Panevėžys distr. 55.540838, 24.111416 | 21 August 2020 |
| Vicia pisiformis * NT | Dotnuva, Kėdainiai distr. 55.396349, 23.865125 | 15 July 2020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudyniene, V.; Juzenas, S.; Stukonis, V.; Mildaziene, V.; Ivankov, A.; Norkeviciene, E. Comparing Non-Thermal Plasma and Cold Stratification: Which Pre-Sowing Treatment Benefits Wild Plant Emergence? Plants 2023, 12, 3220. https://doi.org/10.3390/plants12183220
Gudyniene V, Juzenas S, Stukonis V, Mildaziene V, Ivankov A, Norkeviciene E. Comparing Non-Thermal Plasma and Cold Stratification: Which Pre-Sowing Treatment Benefits Wild Plant Emergence? Plants. 2023; 12(18):3220. https://doi.org/10.3390/plants12183220
Chicago/Turabian StyleGudyniene, Vilma, Sigitas Juzenas, Vaclovas Stukonis, Vida Mildaziene, Anatolii Ivankov, and Egle Norkeviciene. 2023. "Comparing Non-Thermal Plasma and Cold Stratification: Which Pre-Sowing Treatment Benefits Wild Plant Emergence?" Plants 12, no. 18: 3220. https://doi.org/10.3390/plants12183220
APA StyleGudyniene, V., Juzenas, S., Stukonis, V., Mildaziene, V., Ivankov, A., & Norkeviciene, E. (2023). Comparing Non-Thermal Plasma and Cold Stratification: Which Pre-Sowing Treatment Benefits Wild Plant Emergence? Plants, 12(18), 3220. https://doi.org/10.3390/plants12183220






