Herbivory by Striped Stem Borer Triggers Polyamine Accumulation in Host Rice Plants to Promote Its Larval Growth
Abstract
:1. Introduction
2. Results
2.1. PA Levels in Response to SSB Larval Infestation
2.2. Expression Levels of PA Biosynthesis Genes in Response to SSB Larval Infestation
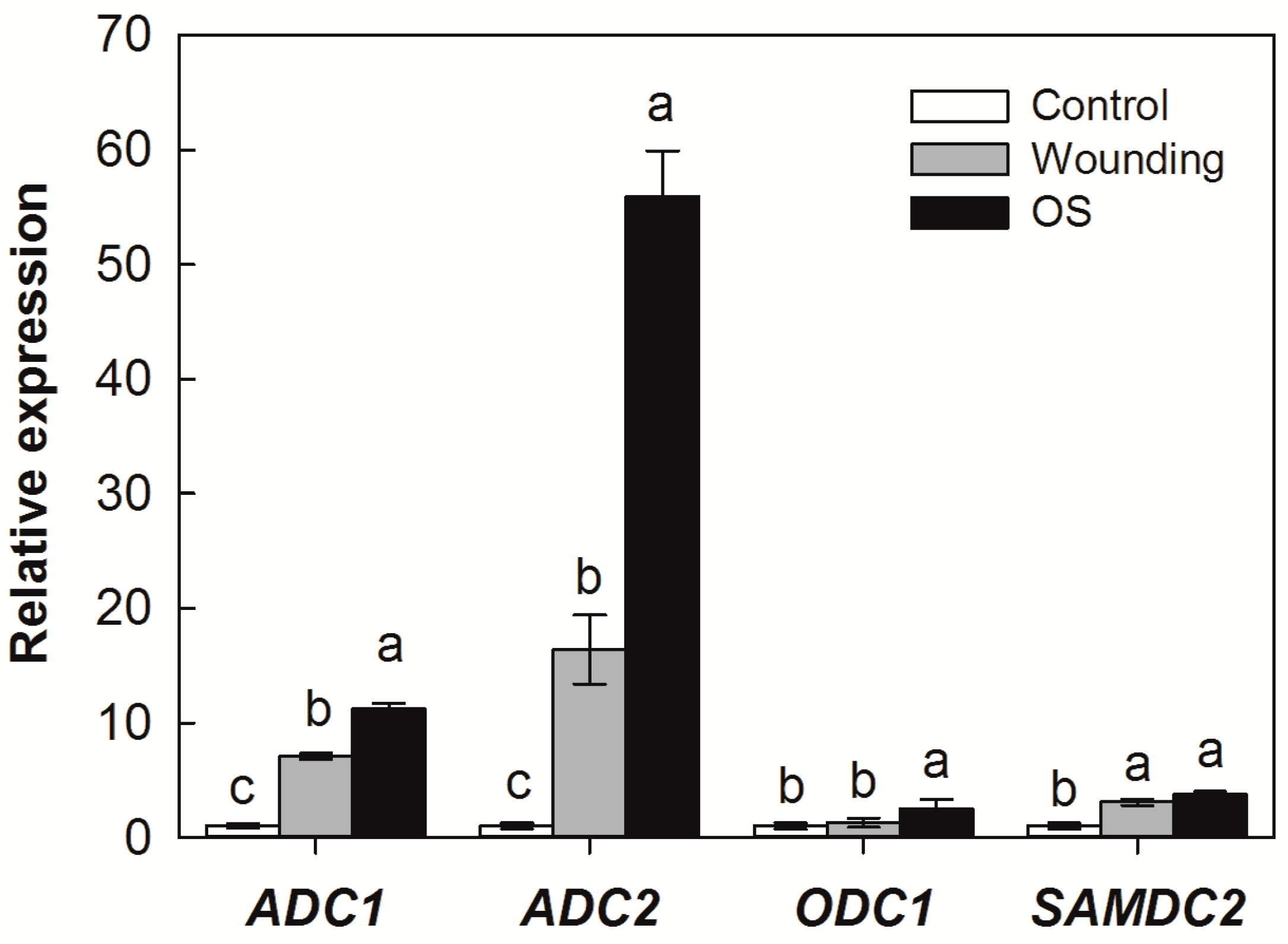
2.3. Effects of SSB Larvae Oral Secretion on the Expression of PA Biosynthesis Genes
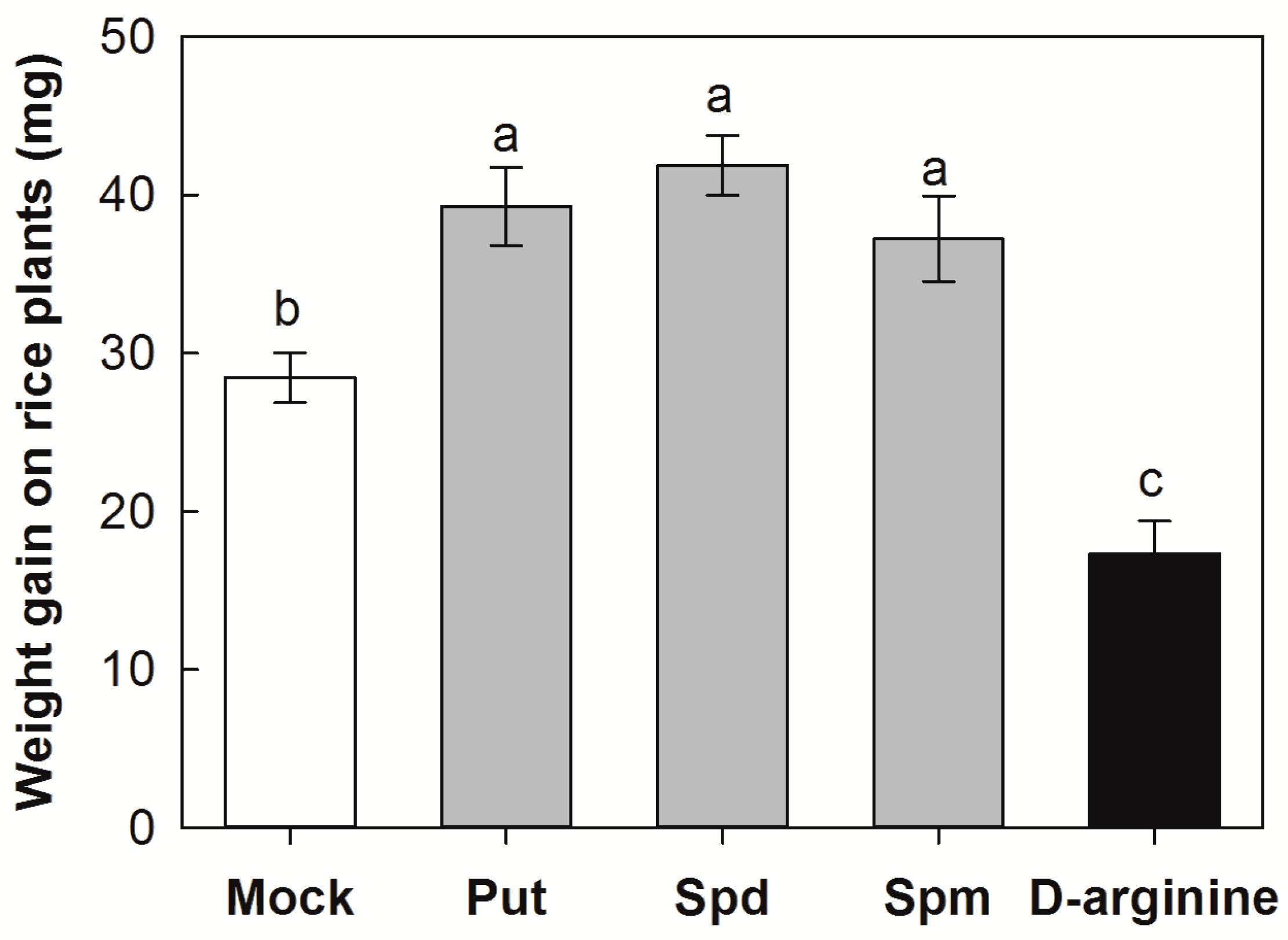
2.4. Effects of Exogenous Application of PAs and D-Arginine on Larval Performance on Rice Plants
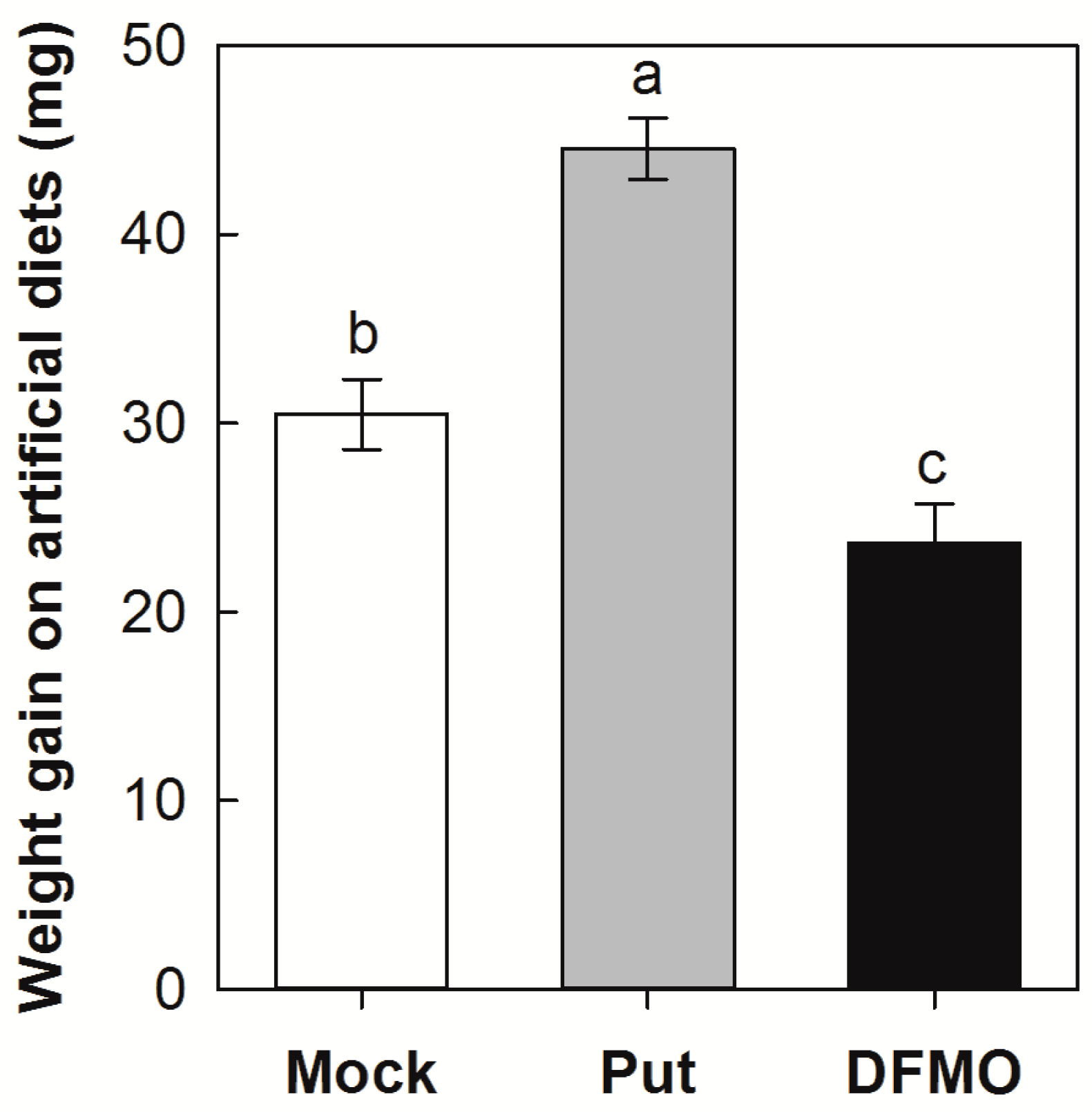
2.5. Effects of Exogenous Application of Put and DMFO (Difluoromethylornithine) on SSB Larval Growth on an Artificial Diet
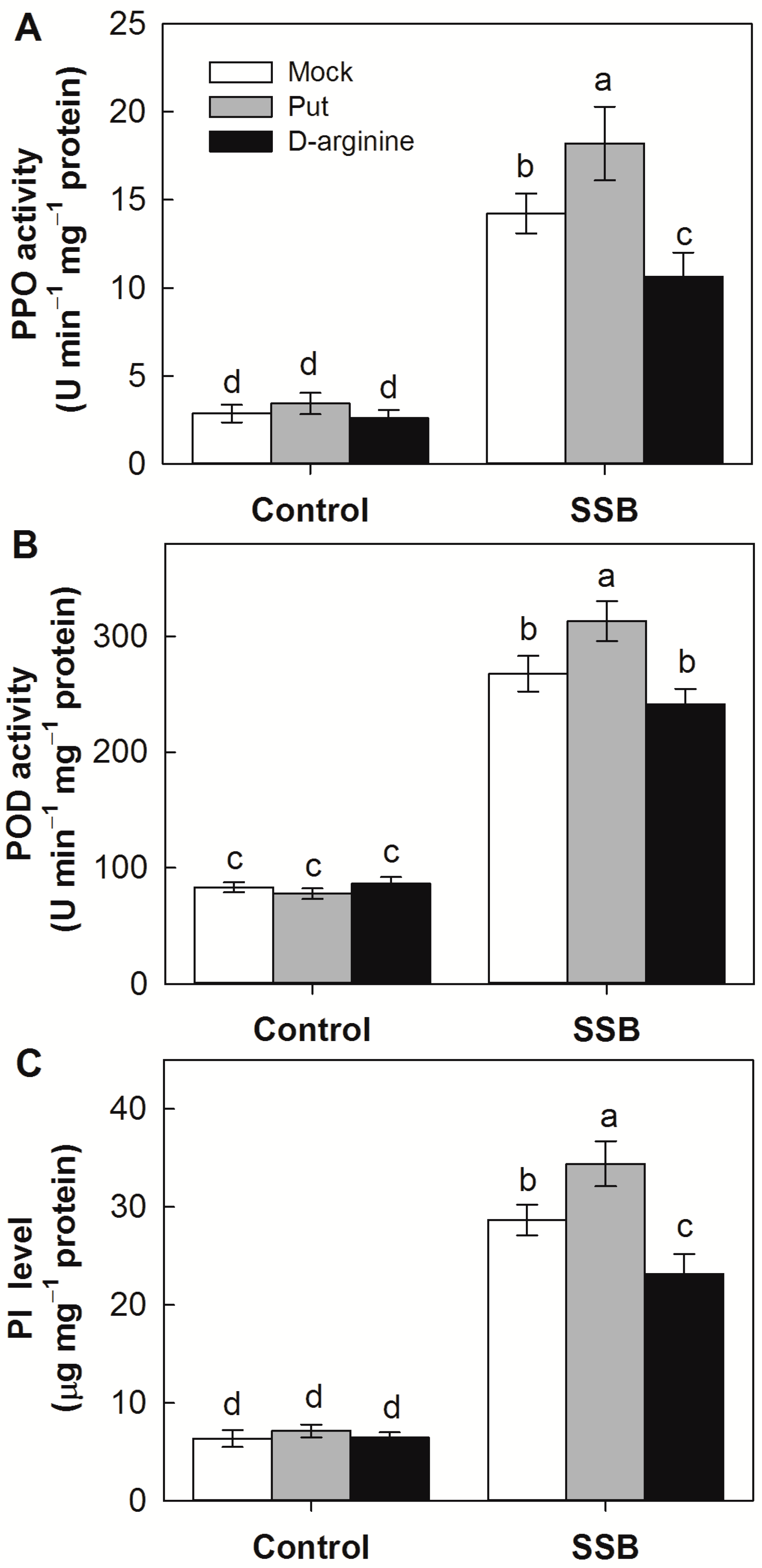
2.6. Effects of Exogenous Application of Put and D-Arginine on Activities of Defense-Related Enzymes in Rice Plants
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Herbivore Treatment
4.3. PA Quantification
4.4. Gene Expression Analysis
4.5. Oral Secretion Treatment
4.6. Exogenous Application of Put and D-Arginine
4.7. Exogenous Application of Put and DFMO
4.8. Enzyme Activity Assays and PI Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, Y.; Zhang, J.; Gao, C.; Su, J.; Chen, J.; Shen, J. Regression analysis of dynamics of insecticide resistance in field populations of Chilo suppressalis (Lepidoptera: Crambidae) during 2002–2011 in China. J. Econ. Entomol. 2013, 106, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, H.; Zhang, X.; He, Y.; Zhang, J.; Guo, X.; Fu, H.; Ye, G.; Shu, Q. Disruption of serotonin biosynthesis increases resistance to striped stem borer without changing innate defense response in rice. J. Pineal Res. 2023, 75, e12895. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shelton, A.; Ye, G.Y. Insect-resistant genetically modified rice in China: From research to commercialization. Annu. Rev. Entomol. 2011, 56, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Strategies for Enhanced crop resistance to insect pests. Annu. Rev. Plant Biol. 2018, 69, 637–660. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, L.; Chen, Q.; Qin, W.; Huang, S.; Jiang, Y.; Qin, H. Chlorantraniliprole resistance and its biochemical and new molecular target mechanisms in laboratory and field strains of Chilo suppressalis (Walker). Pest Manag. Sci. 2018, 74, 1416–1423. [Google Scholar] [CrossRef]
- Gerlin, L.; Baroukh, C.; Genin, S. Polyamines: Double agents in disease and plant immunity. Trends Plant Sci. 2021, 26, 1061–1071. [Google Scholar] [CrossRef]
- Hussain, S.S.; Ali, M.; Ahmad, M.; Siddique, K.H. Polyamines: Natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol. Adv. 2011, 29, 300–311. [Google Scholar] [CrossRef]
- Majumdar, R.; Minocha, R.; Lebar, M.D.; Rajasekaran, K.; Long, S.; Carter-Wientjes, C.; Minocha, S.; Cary, J.W. Contribution of maize polyamine and amino acid metabolism toward resistance against Aspergillus flavus infection and aflatoxin production. Front. Plant Sci. 2019, 10, 692. [Google Scholar] [CrossRef]
- Ramazan, S.; Nazir, I.; Yousuf, W.; John, R. Environmental stress tolerance in maize (Zea mays): Role of polyamine metabolism. Funct. Plant Biol. 2023, 50, 85–96. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, W.; Wu, H.; Gong, X.; Moriguchi, T. Polyamines function in stress tolerance: From synthesis to regulation. Front. Plant Sci. 2015, 6, 827. [Google Scholar] [CrossRef]
- Gonzalez, M.E.; Jasso-Robles, F.I.; Flores-Hernández, E.; Rodríguez-Kessler, M.; Pieckenstain, F.L. Current status and perspectives on the role of polyamines in plant immunity. Ann. Appl. Biol. 2021, 178, 244–255. [Google Scholar] [CrossRef]
- Marini, F.; Betti, L.; Scaramagli, S.; Biondi, S.; Torrigiani, P. Polyamine metabolism is upregulated in response to tobacco mosaic virus in hypersensitive, but not in susceptible, tobacco. New Phytol. 2001, 149, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Mhaske, S.D.; Mahatma, M.K.; Jha, S.; Singh, P.; Ahmad, T. Polyamine metabolism and lipoxygenase activity during Fusarium oxysporum f. sp. ricini-Castor interaction. Physiol. Mol. Biol. Plants 2013, 19, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Wojtasik, W.; Kulma, A.; Namysł, K.; Preisner, M.; Szopa, J. Polyamine metabolism in flax in response to treatment with pathogenic and non-pathogenic Fusarium strains. Front. Plant Sci. 2015, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Martínez, A.I.; Ortega-Amaro, M.A.; Torres, M.; Serrano, M.; Jiménez-Bremont, J.F. Arabidopsis adc-silenced line exhibits differential defense responses to Botrytis cinerea and Pseudomonas syringae infection. Plant Physiol. Biochem. 2020, 156, 494–503. [Google Scholar] [CrossRef]
- Liu, C.; Atanasov, K.E.; Arafaty, N.; Murillo, E.; Tiburcio, A.F.; Zeier, J.; Alcázar, R. Putrescine elicits ROS-dependent activation of the salicylic acid pathway in Arabidopsis thaliana. Plant Cell Environ. 2020, 43, 2755–2768. [Google Scholar] [CrossRef]
- Alcázar, R.; Planas, J.; Saxena, T.; Zarza, X.; Bortolotti, C.; Cuevas, J.; Bitrián, M.; Tiburcio, A.F.; Altabella, T. Putrescine accumulation confers drought tolerance in transgenic Arabidopsis plants over-expressing the homologous Arginine decarboxylase 2 gene. Plant Physiol. Biochem. 2010, 48, 547–552. [Google Scholar] [CrossRef]
- Mo, H.; Wang, X.; Zhang, Y.; Zhang, G.; Zhang, J.; Ma, Z. Cotton polyamine oxidase is required for spermine and camalexin signalling in the defence response to Verticillium dahliae. Plant J. 2015, 83, 962–975. [Google Scholar] [CrossRef]
- Subramanyam, S.; Sardesai, N.; Minocha, S.C.; Zheng, C.; Shukle, R.H.; Williams, C.E. Hessian fly larval feeding triggers enhanced polyamine levels in susceptible but not resistant wheat. BMC Plant Biol. 2015, 15, 3. [Google Scholar] [CrossRef]
- Ozawa, R.; Bertea, C.M.; Foti, M.; Narayana, R.; Arimura, G.; Muroi, A.; Horiuchi, J.; Nishioka, T.; Maffei, M.E.; Takabayashi, J. Exogenous polyamines elicit herbivore-induced volatiles in lima bean leaves: Involvement of calcium, H2O2 and Jasmonic acid. Plant Cell Physiol. 2009, 50, 2183–2199. [Google Scholar] [CrossRef]
- Tebayashi, S.; Horibata, Y.; Mikagi, E.; Kashiwagi, T.; Mekuria, D.B.; Dekebo, A.; Ishihara, A.; Kim, C.S. Induction of resistance against the leafminer, Liriomyza trifolii, by jasmonic acid in sweet pepper. Biosci. Biotech. Biochem. 2007, 71, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Voelckel, C.; Baldwin, I.T. Detecting herbivore-specific transcriptional responses in plants with multiple DDRT-PCR and subtractive library procedures. Physiol. Plant. 2003, 118, 240–252. [Google Scholar] [CrossRef]
- Klose, M.K.; Atkinson, J.K.; Mercier, A.J. Effects of a hydroxy-cinnamoyl conjugate of spermidine on arthropod neuromuscular junctions. J. Comp. Physiol. 2002, 187, 945–952. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, X.; Liu, X.; Qin, N.; Xu, K.; Zeng, R.; Liu, J.; Song, Y. Nitrogen supply alters rice defense against the striped stem borer Chilo suppressalis. Front. Plant Sci. 2021, 12, 691292. [Google Scholar] [CrossRef]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining mysteries of molecular biology: The role of polyamines in the cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef]
- Pál, M.; Szalai, G.; Janda, T. Speculation: Polyamines are important in abiotic stress signaling. Plant Sci. 2015, 237, 16–23. [Google Scholar] [CrossRef]
- Seifi, H.S.; Shelp, B.J. Spermine differentially refines plant defense responses against biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 117. [Google Scholar] [CrossRef]
- Shi, H.; Chan, Z. Improvement of plant abiotic stress tolerance through modulation of the polyamine pathway. J. Integr. Plant Biol. 2014, 56, 114–121. [Google Scholar] [CrossRef]
- Sempruch, C.; Horbowicz, M.; Kosson, R.; Leszczyński, B. Biochemical interactions between triticale (Triticosecale; Poaceae) amines and bird cherry-oat aphid (Rhopalosiphum padi; Aphididae). Biochem. Syst. Ecol. 2012, 40, 162–168. [Google Scholar] [CrossRef]
- Minocha, R.; Majumdar, R.; Minocha, S.C. Polyamines and abiotic stress in plants: A complex relationship. Front. Plant Sci. 2014, 5, 175. [Google Scholar] [CrossRef]
- Dunn, M.F.; Becerra-Rivera, V.A. The Biosynthesis and functions of polyamines in the interaction of plant growth-promoting rhizobacteria with plants. Plants 2023, 12, 2671. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.R. Polyamines and plant disease. Phytochemistry 2003, 64, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Hogenhout, S.A.; Bos, J.I. Effector proteins that modulate plant–insect interactions. Curr. Opin. Plant Biol. 2011, 14, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Guiguet, A.; Dubreuil, G.; Harris, M.O.; Appel, H.M.; Schultz, J.C.; Pereira, M.H.; Giron, D. Shared weapons of blood- and plant-feeding insects: Surprising commonalities for manipulating hosts. J. Insect Physiol. 2016, 84, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Oates, C.N.; Denby, K.J.; Myburg, A.A.; Slippers, B.; Naidoo, S. Insect gallers and their plant hosts: From omics data to systems biology. Int. J. Mol. Sci. 2016, 17, 1891. [Google Scholar] [CrossRef]
- Oliveira, D.C.; Isaias, R.M.S.; Fernandes, G.W.; Ferreira, B.G.; Carneiro, R.G.S.; Fuzaro, L. Manipulation of host plant cells and tissues by gall-inducing insects and adaptive strategies used by different feeding guilds. J. Insect Physiol. 2016, 84, 103–113. [Google Scholar] [CrossRef]
- Larqué, E.; Sabater-Molina, M.; Zamora, S. Biological significance of dietary polyamines. Nutrition 2007, 23, 87–95. [Google Scholar] [CrossRef]
- Davis, G.R.F. Putrescine and spermidine as growth-promoting substances for the saw-toothed grain bettle, Oryzaephilus surinamensis (L.) (coleoptera, silvanidae). Comp. Biochem. Physiol. 1966, 19, 619–627. [Google Scholar] [CrossRef]
- Ye, M.; Song, Y.; Long, J.; Wang, R.; Baerson, S.R.; Pan, Z.; Zhu-Salzman, K.; Xie, J.; Cai, K.; Luo, S.; et al. Priming of jasmonate-mediated antiherbivore defense responses in rice by silicon. Proc. Natl. Acad. Sci. USA 2013, 110, E3631–E3639. [Google Scholar] [CrossRef]
- Montilla-Bascón, G.; Rubiales, D.; Prats, E. Changes in polyamine profile in host and non-host oat-powdery mildew interactions. Phytochem. Lett. 2014, 8, 207–212. [Google Scholar] [CrossRef]
- Chen, D.; Chen, D.; Xue, R.; Long, J.; Lin, X.; Lin, Y.; Jia, L.; Zeng, R.; Song, Y. Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants. J. Hazard. Mater. 2019, 367, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Li, Q.; Guo, R.; Yan, H.; Ju, X.; Liao, L.; Zeng, R.; Song, Y.; Wang, J. Rice defense responses orchestrated by oral bacteria of the rice striped stem borer, Chilo suppressalis. Rice 2023, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cao, B.; Qi, L.; Yin, L.; Wang, S.; Deng, X. Silicon-moderated K-deficiency-induced leaf chlorosis by decreasing putrescine accumulation in sorghum. Ann. Bot. 2016, 118, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shao, M.; Sun, S.; Liu, T.; Zhang, H.; Qin, N.; Zeng, R.; Song, Y. Enhancement of jasmonate-mediated antiherbivore defense responses in tomato by acetic acid, a potent inducer for plant protection. Front. Plant Sci. 2019, 10, 764. [Google Scholar] [CrossRef] [PubMed]


| Gene | Accession Number | Primer |
|---|---|---|
| ADC1 | LOC_Os06g04070 | F: 5′-AGCTCCTGCACTTCCACATT-3′ R: 5′-CAAGCTGTATGCCACGGACA-3′ |
| ADC2 | LOC_Os04g01690 | F: 5′-CCTACCGTGACAGAAGAAAGGA-3′ R: 5′-CACCCGAGGATGTTGTACACT-3′ |
| ODC1 | LOC_Os09g37120 | F: 5′-CCATCTCCATCCCACGCTA-3′ R: 5′-CACGTTGCTAGTGTGTTTGGG-3′ |
| SAMDC2 | LOC_Os02g39795 | F: 5′-CGAGCTGTCCAACAAGGACT-3′ R: 5′-TCACAGCAGCAAGTGGCATA-3′ |
| Actin7 | LOC_Os11g06390 | F: 5′-ACTGTCCCCATCTA TGAAGGA-3′ R: 5′-CTGCTGGAATGTGCTGAGAGA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Gai, C.; Shao, M.; Fang, L.; Li, X.; Song, Y.; Zeng, R.; Chen, D. Herbivory by Striped Stem Borer Triggers Polyamine Accumulation in Host Rice Plants to Promote Its Larval Growth. Plants 2023, 12, 3249. https://doi.org/10.3390/plants12183249
Zhang H, Gai C, Shao M, Fang L, Li X, Song Y, Zeng R, Chen D. Herbivory by Striped Stem Borer Triggers Polyamine Accumulation in Host Rice Plants to Promote Its Larval Growth. Plants. 2023; 12(18):3249. https://doi.org/10.3390/plants12183249
Chicago/Turabian StyleZhang, Hao, Chaoyue Gai, Min Shao, Linzhi Fang, Xinyu Li, Yuanyuan Song, Rensen Zeng, and Daoqian Chen. 2023. "Herbivory by Striped Stem Borer Triggers Polyamine Accumulation in Host Rice Plants to Promote Its Larval Growth" Plants 12, no. 18: 3249. https://doi.org/10.3390/plants12183249
APA StyleZhang, H., Gai, C., Shao, M., Fang, L., Li, X., Song, Y., Zeng, R., & Chen, D. (2023). Herbivory by Striped Stem Borer Triggers Polyamine Accumulation in Host Rice Plants to Promote Its Larval Growth. Plants, 12(18), 3249. https://doi.org/10.3390/plants12183249








