Influence of Increase in Phosphorus Supply on Agronomic, Phenological, and Physiological Performance of Two Common Bean Breeding Lines Grown in Acidic Soil under High Temperature Stress Conditions
Abstract
:1. Introduction
2. Results
2.1. Dynamics of Growth and Development of Different Vegetative Organs under Heat Stress
2.2. Phenological Characteristics, Stomatal Density, and Dynamics of Morphological Development
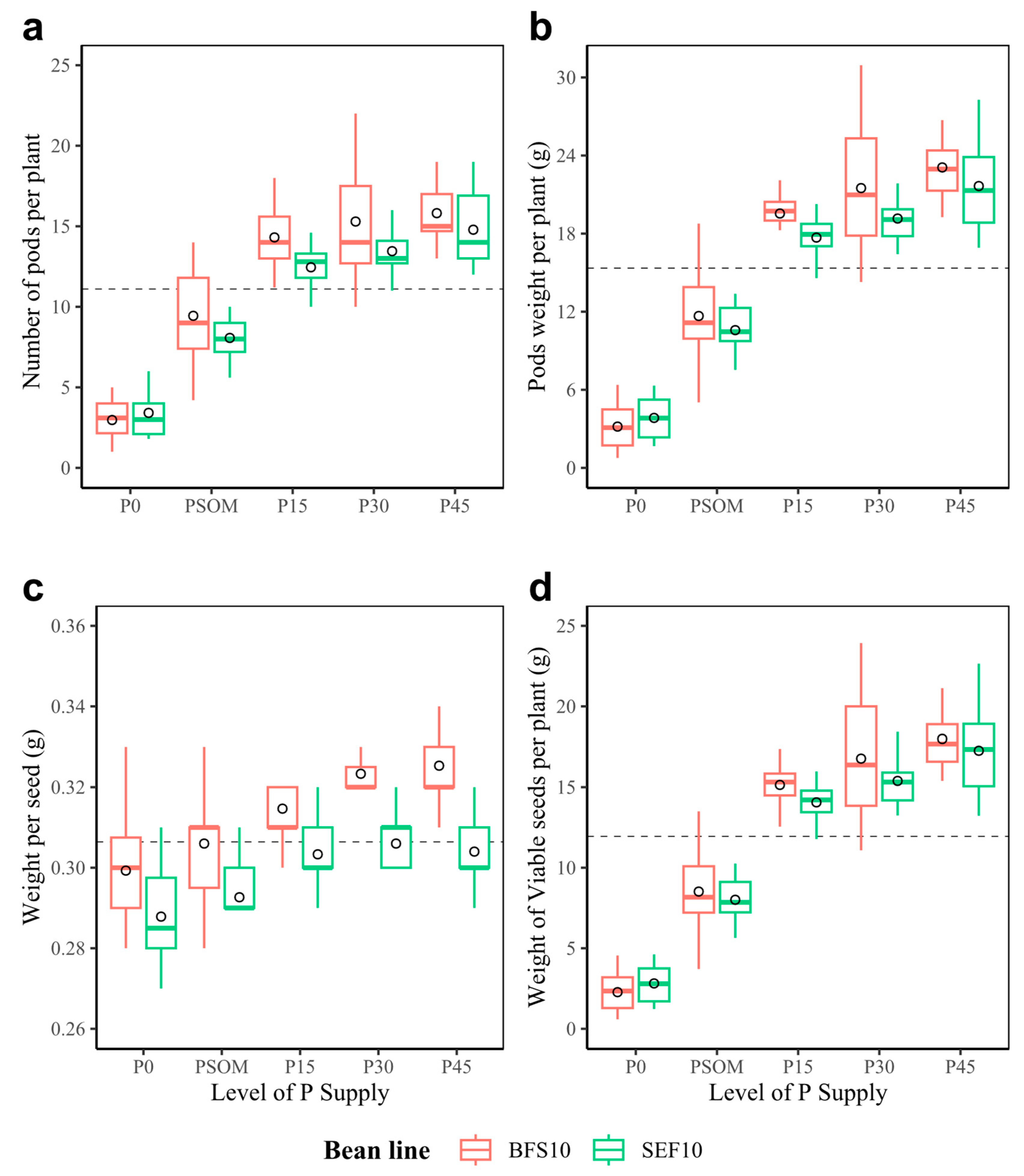
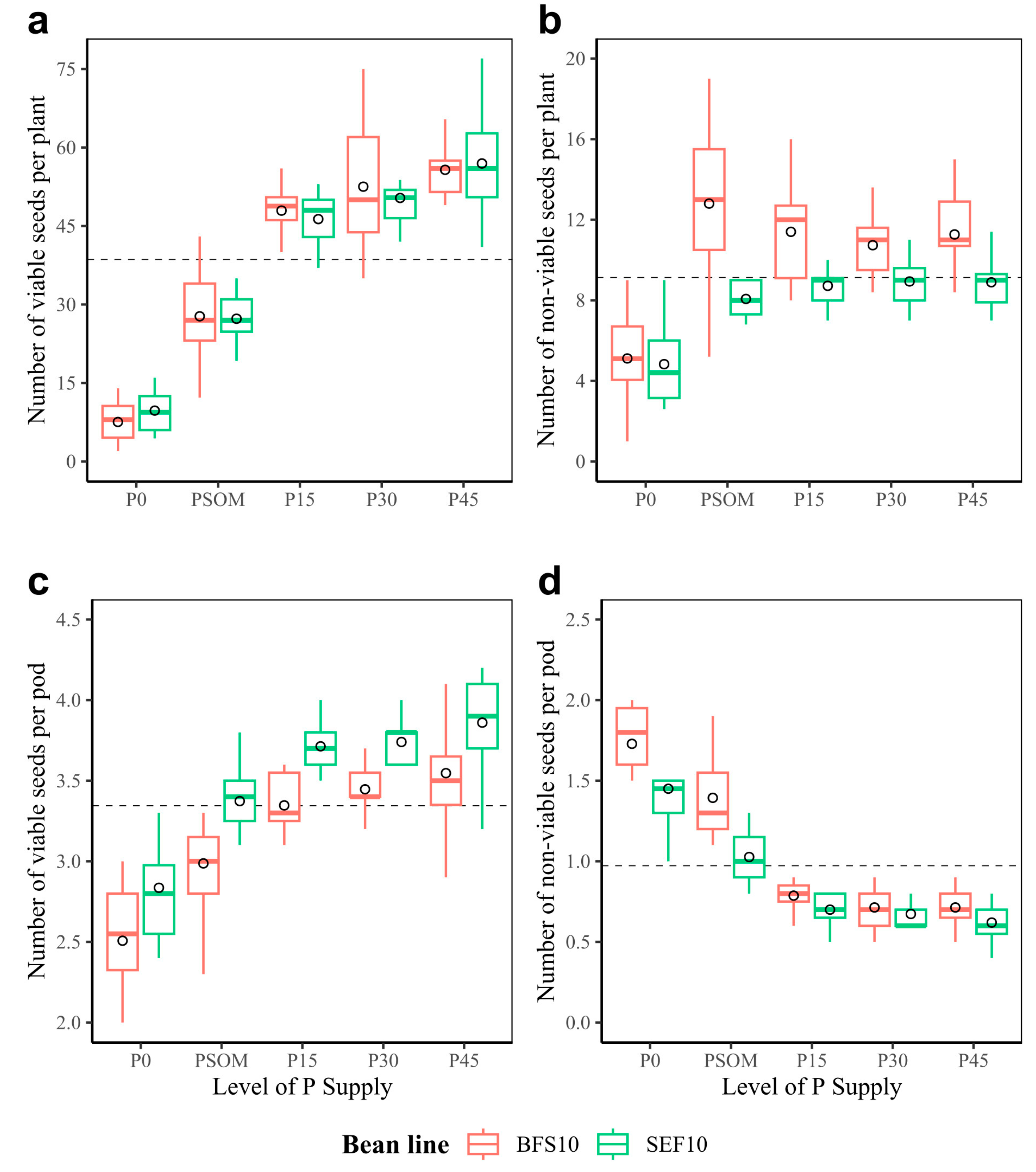
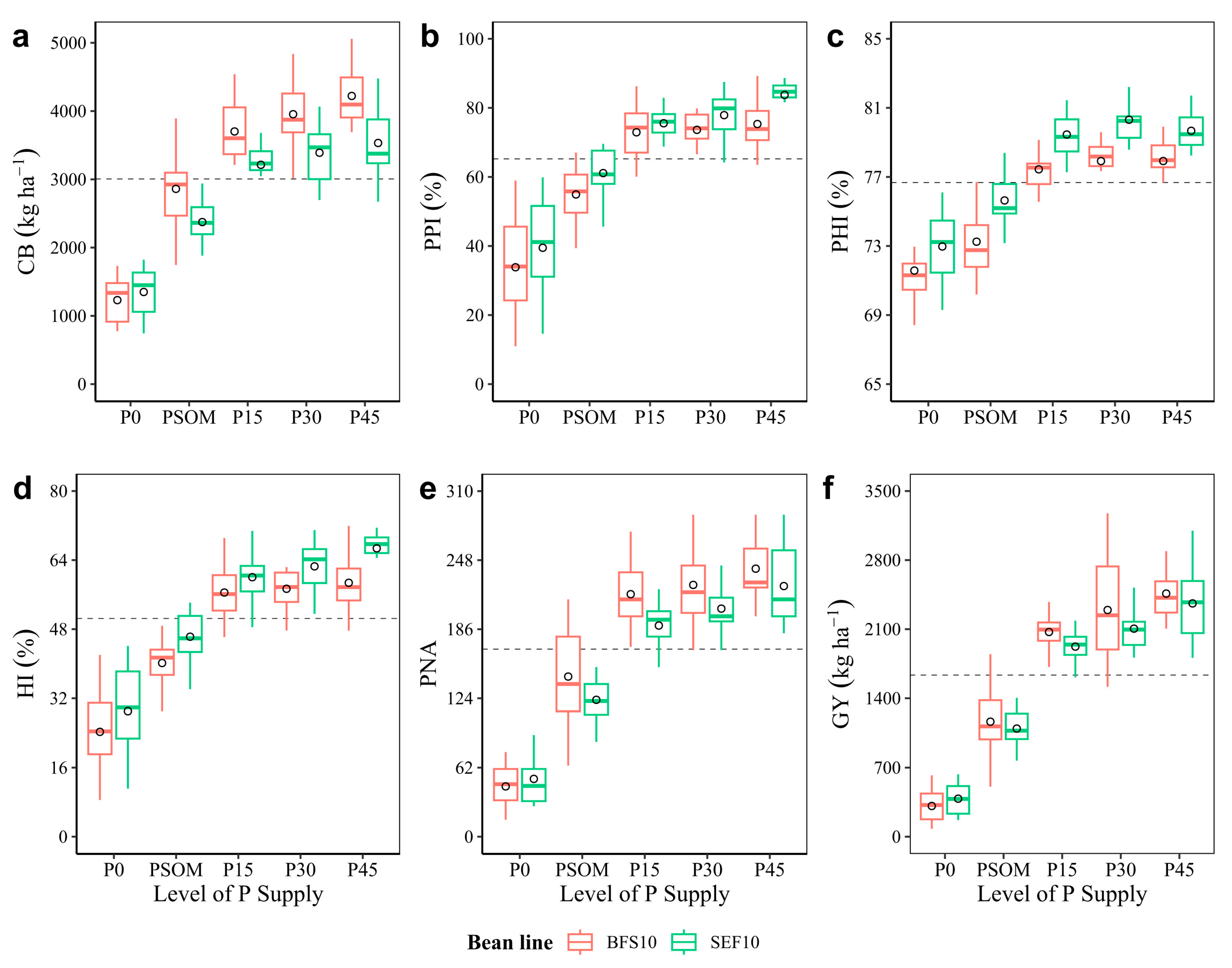
2.3. Efficiency in Assimilate Mobilization: Canopy Biomass, Dry Matter Partitioning, Yield, and Yield Components
2.4. Chla Fluorescence and Images of Chlorophyll (Chla) Parameters under Different Levels of P Supply
3. Discussion
3.1. Increased Phosphorus Supply Alleviated, to Some Extent, the High Temperature Stress Effects on Growth and Development of Reproductive Organs
3.2. Phosphorus Supply Influenced Phenological Characteristics, Stomatal Density, and Dynamics of Morphological Development
3.3. The Ability to Mobilize Photosynthates Contributed to Improved Agronomic Performance under Different Levels of P Supply as an Adaptive Trait to Cope with High Temperatures
3.4. Mechanisms of Energy Dissipation under Heat Stress Conditions under Different Levels of P Supply
3.5. Increase in P Supply Could Act as a Heat Stress Dissipater under Climate Variability Scenarios
4. Materials and Methods
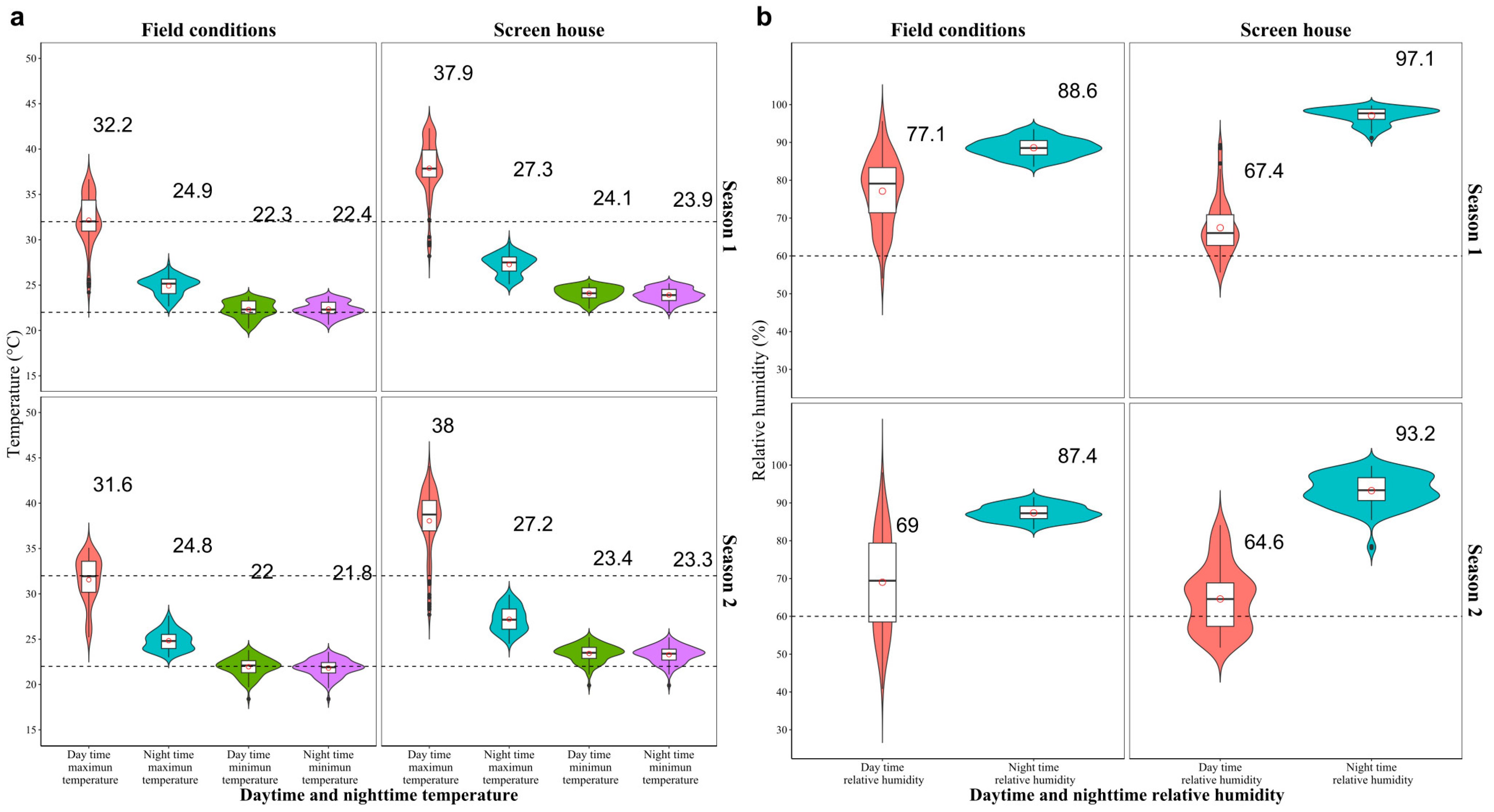
4.1. Study Area and Experimental Setup
4.2. Dynamics of Growth and Development of Different Vegetative Organs
4.3. Biomass Partitioning and Grain Yield
4.4. Phenological Characteristics, Stomatal Density, and Morphological Development
4.5. Chla Fluorescence and Imaging for the Chlorophyll (Chla) Fluorescence Parameters under Different Levels of P Supply
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, H.; Rubio, G.; Yan, X.; Cao, A.; Brown, K.M.; Lynch, J.P. Effect of phosphorus availability on basal root shallowness in common bean. Plant Soil 2001, 232, 69–79. [Google Scholar] [CrossRef]
- Rao, I.M. Digging deep into defining physiological responses to environmental stresses in the tropics: The case of common bean and Brachiaria forage grasses. In Handbook of Plant and Crop Physiology; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 1099–1140. [Google Scholar]
- Rahman, R.; Upadhyaya, H. Aluminium Toxicity and Its Tolerance in Plant: A Review. J. Plant Biol. 2020, 64, 101–121. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Devi, M.J.; Polania, J. Common beans, biodiversity, and multiple stresses: Challenges of drought resistance in tropical soils. Crop Pasture Sci. 2014, 65, 667–675. [Google Scholar] [CrossRef]
- Borch, K.; Bouma, T.J.; Lynch, J.P.; Brown, K.M. Ethylene: A regulator of root architectural responses to soil phosphorus availability. Plant Cell Environ. 1999, 22, 425–431. [Google Scholar] [CrossRef]
- Okada, K.; Kondo, M.; Ando, H.; Kakuda, K.-I. Phosphorus application affects root length distribution and water uptake of upland rice in a column experiment. Soil Sci. Plant Nutr. 2004, 50, 257–261. [Google Scholar] [CrossRef]
- Butare, L.; Rao, I.; Lepoivre, P.; Cajiao, C.; Polania, J.; Cuasquer, J.; Beebe, S. Phenotypic evaluation of interspecific recombinant inbred lines (RILs) of Phaseolus species for aluminium resistance and shoot and root growth response to aluminium–toxic acid soil. Euphytica 2012, 186, 715–730. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. Resumen Para Responsables de Políticas. Calen-Tamiento Global de 1.5 °C Informe Especial Del Ipcc Sobre Los Impactos Del Calentamiento Global de 1.5 °C Con Respecto a Los Niveles Preindustriales Y Las Trayectorias Correspondientes Que Deberían Seguir Las E. 2019. Available online: www.ipcc.ch (accessed on 13 November 2021).
- Perdomo, J.A.; Carmo-Silva, E.; Hermida-Carrera, C.; Flexas, J.; Galmés, J. Acclimation of Biochemical and Diffusive Components of Photosynthesis in Rice, Wheat, and Maize to Heat and Water Deficit: Implications for Modeling Photosynthesis. Front. Plant Sci. 2016, 7, 1719. [Google Scholar] [CrossRef]
- Siebers, M.H.; Yendrek, C.R.; Drag, D.; Locke, A.M.; Acosta, L.R.; Leakey, A.D.B.; Ainsworth, E.A.; Bernacchi, C.J.; Ort, D.R. Heat waves imposed during early pod development in soybean (Glycine max) cause significant yield loss despite a rapid recovery from oxidative stress. Glob. Change Biol. 2015, 21, 3114–3125. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.T.; Yao, Y.T.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef]
- Beebe, S.; Ramirez, J.; Jarvis, A.; Rao, I.M.; Mosquera, G.; Bueno, J.M.; Blair, M.W. Genetic Improvement of Common Beans and the Challenges of Climate Change. In Crop Adaptation to Climate Change; Yadav, S.S., Redden, R.J., Hatfield, J.L., Lotze-Campen, H., Hall, A.E., Eds.; Wiley-Blackwell: Oxford, UK, 2011; pp. 356–369. [Google Scholar]
- Rao, I.M.; Beebe, S.E.; Polania, J.; Grajales, M.; Cajiao, C.; Ricaurte, J.; García, R.; Rivera, M. Evidence for genotypic differences among elite lines of common bean in the ability to remobilize photosynthate to increase yield under drought. J. Agric. Sci. 2017, 155, 857–875. Available online: https://www.cambridge.org/core/journals/journal-of-agricultural-science/article/abs/evidence-for-genotypic-differences-among-elite-lines-of-common-bean-in-the-ability-to-remobilize-photosynthate-to-increase-yield-under-drought/83C9BB85244976B650B0846AD47E275C (accessed on 12 July 2023). [CrossRef]
- Rippke, U.; Ramirez-Villegas, J.; Jarvis, A.; Vermeulen, S.J.; Parker, L.; Mer, F.; Diekkrüger, B.; Challinor, A.J.; Howden, M. Timescales of transformational climate change adaptation in sub-Saharan African agriculture. Nat. Clim. Change 2016, 6, 605–609. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Cajiao, C.; Grajales, M. Selection for Drought Resistance in Common Bean Also Improves Yield in Phosphorus Limited and Favorable Environments. Crop Sci. 2008, 48, 582–592. [Google Scholar] [CrossRef]
- Polania, J.A.; Poschenrieder, C.; Beebe, S.; Rao, I.M. Effective Use of Water and Increased Dry Matter Partitioned to Grain Contribute to Yield of Common Bean Improved for Drought Resistance. Front. Plant Sci. 2016, 7, 660. [Google Scholar] [CrossRef]
- da Silva, D.A.; Esteves, J.A.d.F.; Messias, U.; Teixeira, A.; Gonçalves, J.G.R.; Chiorato, A.F.; Carbonell, S.A.M. Efficiency in the use of phosphorus by common bean genotypes. Sci. Agric. 2014, 71, 232–239. [Google Scholar] [CrossRef]
- Hall, A.E. Comparative ecophysiology of cowpea, common bean, and peanut. In Physiology and Biotechnology Integration for Plant Breeding; Henry, T., Nguyen, A.B., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 271–325. [Google Scholar]
- Deva, C.R.; Urban, M.O.; Challinor, A.J.; Falloon, P.; Svitákova, L. Enhanced Leaf Cooling Is a Pathway to Heat Tolerance in Common Bean. Front. Plant Sci. 2020, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Porch, T.G.; Hall, A.E. Heat Tolerance; Springer: Berlin/Heidelberg, Germany, 2013; pp. 167–202. [Google Scholar] [CrossRef]
- Omae, H.; Kumar, A.; Shono, M. Adaptation to high temperature and water deficit in the common bean (Phaseolus vulgaris L.) during the reproductive period. J. Bot. 2012, 2012, 803413. [Google Scholar]
- Salazar, J.C.S.; Polanía, J.A.; Bastidas, A.T.C.; Suárez, L.R.; Beebe, S.; Rao, I.M. Agronomical, phenological and physiological performance of common bean lines in the Amazon region of Colombia. Theor. Exp. Plant Physiol. 2018, 30, 303–320. [Google Scholar] [CrossRef]
- Suárez, J.C.; Polanía, J.A.; Contreras, A.T.; Rodríguez, L.; Machado, L.; Ordoñez, C.; Beebe, S.; Rao, I.M. Adaptation of common bean lines to high temperature conditions: Genotypic differences in phenological and agronomic performance. Euphytica 2020, 216, 28. [Google Scholar] [CrossRef]
- Suárez, J.C.; Urban, M.O.; Contreras, A.T.; Noriega, J.E.; Deva, C.; Beebe, S.E.; Polanía, J.A.; Casanoves, F.; Rao, I.M. Water Use, Leaf Cooling and Carbon Assimilation Efficiency of Heat Resistant Common Beans Evaluated in Western Amazonia. Front. Plant Sci. 2021, 12, 644010. [Google Scholar] [CrossRef]
- Sofi, P.; Maduraimuthu, D. Variation in some physiological parameters in relation to high temperature stress in common bean (Phaseolus vulgaris L.). SKUAST J. Res. 2017, 19, 251–257. [Google Scholar]
- Xu, G.; Singh, S.K.; Reddy, V.R.; Barnaby, J.Y.; Sicher, R.C.; Li, T. Soybean grown under elevated CO2 benefits more under low temperature than high temperature stress: Varying response of photosynthetic limitations, leaf metabolites, growth, and seed yield. J. Plant Physiol. 2016, 205, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Barrantes, N.F.; Polanía, J.A.; Muñoz-Perea, C.G.; Rao, I.M.; Beebe, S.E. Caracterización fenotípica por resistencia a sequía terminal de germoplasma de frijol común. Agron. Mesoam. 2018, 29, 1–18. [Google Scholar] [CrossRef]
- Tene, T.M.; Sari, H.; Canci, H.; Maaruf, A.; Eker, T.; Toker, C. Traits Related to Heat Stress in Phaseolus Species. Agriculture 2023, 13, 953. [Google Scholar] [CrossRef]
- Ayala, C.C.; Orozco, A.J.; Tatis, H.; Agresott, M.P.; Córdoba, C.V. Respuestas fisiológicas y bioquímicas del fríjol caupí (Vigna unguiculata L. Walp.) bajo déficit hídrico. Rev. Colomb. Cienc. Hortíc. 2015, 8, 250–261. [Google Scholar] [CrossRef]
- Traub, J.; Porch, T.; Naeem, M.; Urrea, C.A.; Austic, G.; Kelly, J.D.; Loescher, W. Screening for Heat Tolerance in Phaseolus spp. Using Multiple Methods. Crop Sci. 2018, 58, 2459–2469. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, S. Broadening the genetic base of grain cereals. In Broadening the Genetic Base of Grain Cereals; Springer: New Delhi, India, 2016; pp. 1–275. [Google Scholar]
- Abel, S.; Ticconi, C.A.; Delatorre, C.A. Phosphate sensing in higher plants. Physiol. Plant. 2002, 115, 1–8. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Turuko, M.; Mohammed, A. Effect of Different Phosphorus Fertilizer Rates on Growth, Dry Matter Yield and Yield Components of Common Bean (Phaseolus vulgaris L.). World J. Agric. Res. 2014, 2, 88–92. [Google Scholar] [CrossRef]
- Timlin, D.J.; Naidu, T.C.M.; Fleisher, D.H.; Reddy, V.R. Quantitative Effects of Phosphorus on Maize Canopy Photosynthesis and Biomass. Crop Sci. 2017, 57, 3156–3169. [Google Scholar] [CrossRef]
- Rao, I.M.; Miles, J.W.; Beebe, S.E.; Horst, W.J. Root adaptations to soils with low fertility and aluminium toxicity. Ann. Bot. 2016, 118, 593–605. [Google Scholar] [CrossRef]
- Ramaekers, L.; Remans, R.; Rao, I.M.; Blair, M.W.; Vanderleyden, J. Strategies for improving phosphorus acquisition efficiency of crop plants. Field Crops Res. 2010, 117, 169–176. [Google Scholar] [CrossRef]
- López-Arredondo, D.L.; Leyva-González, M.A.; González-Morales, S.I.; López-Bucio, J.; Herrera-Estrella, L. Phosphate Nutrition: Improving Low-Phosphate Tolerance in Crops. Annu. Rev. Plant Biol. 2014, 65, 95–123. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Root phenotypes for improved nutrient capture: An underexploited opportunity for global agriculture. New Phytol. 2019, 223, 548–564. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Kai, Y.; Takayanagi, M.; El-Shemy, H.; Adu-Gyamfi, J.J.; Mohapatra, P.K. Genotypic variability of pigeonpea in dis-tribution of photosynthetic carbon at low phosphorus level. Plant Sci. 2004, 166, 641–649. [Google Scholar] [CrossRef]
- Snapp, S.; Koide, R.; Lynch, J. Exploitation of localized phosphorus-patches by common bean roots. Plant Soil 1995, 177, 211–218. [Google Scholar] [CrossRef]
- Nesme, T.; Colomb, B.; Hinsinger, P.; Watson, C.A. Soil Phosphorus Management in Organic Cropping Systems: From Current Practices to Avenues for a More Efficient Use of P Resources; Springer: Dordrecht, The Netherlands, 2014; pp. 23–45. [Google Scholar] [CrossRef]
- Meena, R.; Yadav, R.; Meena, V. Response of groundnut (Arachis hypogaea L.) varieties to sowing dates and NP fertilizers under western dry zone of India. Bangladesh J. Bot. 2015, 43, 169–173. [Google Scholar] [CrossRef]
- Tajini, F.; Drevon, J.-J. Phosphorus Use Efficiency for Symbiotic Nitrogen Fixation Varies among Common Bean Recombinant Inbred Lines under P Deficiency. J. Plant Nutr. 2014, 37, 532–545. [Google Scholar] [CrossRef]
- Singh, S.K.; Reddy, V.R.; Fleisher, D.H.; Timlin, D.J. Growth, nutrient dynamics, and efficiency responses to carbon dioxide and phosphorus nutrition in soybean. J. Plant Interact. 2014, 9, 838–849. [Google Scholar] [CrossRef]
- Khan, B.M.; Asif, M.; Hussain, N.; Aziz, M. Impact of different levels of phosphorus on growth and yield of mung bean geno-types. Asian J. Plant Sci. 2003, 2, 677–679. [Google Scholar]
- Jones, C.A.; Jacobsen, J.S.; Wraith, J.M. The effects of P fertilization on drought tolerance of malt barley. West. Nutr. Manag. Conf. 2003, 5, 88–93. [Google Scholar]
- Jin, J.; Wang, G.; Liu, X.; Pan, X.; Herbert, S.J.; Tang, C. Interaction Between Phosphorus Nutrition and Drought on Grain Yield, and Assimilation of Phosphorus and Nitrogen in Two Soybean Cultivars Differing in Protein Concentration in Grains. J. Plant Nutr. 2007, 29, 1433–1449. [Google Scholar] [CrossRef]
- Traub, J.; Kelly, J.D.; Loescher, W. Early Metabolic and Photosynthetic Responses to Drought Stress in Common and Tepary Bean. Crop Sci. 2017, 57, 1670–1686. [Google Scholar] [CrossRef]
- Rainey, K.; Griffiths, P. Differential Response of Common Bean Genotypes to High Temperature. J. Am. Soc. Hortic. Sci. 2005, 130, 18–23. [Google Scholar] [CrossRef]
- Soltani, A.; Weraduwage, S.M.; Sharkey, T.D.; Lowry, D.B. Elevated temperatures cause loss of seed set in common bean (Phaseolus vulgaris L.) potentially through the disruption of source-sink relationships. BMC Genom. 2019, 20, 312. [Google Scholar] [CrossRef]
- Mourice, S.K.; Tryphone, G.M.; Weigel, H. Evaluation of Common Bean (Phaseolus vulgaris L.) Genotypes for Adaptation to Low Phosphorus. Int. Sch. Res. Not. 2012, 2012, 309614. [Google Scholar] [CrossRef]
- CIAT. Developing Beans That Can Beat the Heat; Tech. Report; International Center for Tropical Agriculture: Cali, Colombia, 2015. [Google Scholar]
- Zafar, M.; Abbasi, M.K.; Rahim, N.; Khaliq, A.; Shaheen, A.; Jamil, M.; Shahid, M. Influence of integrated phosphorus supply and plant growth promoting rhizobacteria on growth, nodulation, yield and nutrient uptake in Phaseolus vulgaris. Afr. J. Biotechnol. 2011, 10, 16781–16792. Available online: https://www.ajol.info/index.php/ajb/article/view/97756 (accessed on 13 November 2022).
- Bhattacharya, A. Plant Growth Hormones in Plants under Low-Temperature Stress: A Review; Springer: Singapore, 2022; pp. 517–627. [Google Scholar] [CrossRef]
- Hageman, A.N.; Urban, M.O.; Van Volkenburgh, E. Sensitivity of leaflet growth rate to drought predicts yield in common bean (Phaseolus vulgaris). Funct. Plant Biol. 2020, 47, 792. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Tsukaguchi, T.; Takeda, H.; Egawa, Y. Decrease of pollen stainability of green bean at high temperatures and rela-tionship to heat tolerance. J. Am. Soc. Hortic. Sci. 2001, 126, 571–574. Available online: https://journals.ashs.org/jashs/view/journals/jashs/126/5/article-p571.xml (accessed on 13 November 2021). [CrossRef]
- Porch, T.G.; Jahn, M. Effects of high-temperature stress on microsporogenesis in heat-sensitive and heat-tolerant genotypes of Phaseolus vulgaris. Plant Cell Environ. 2001, 24, 723–731. [Google Scholar] [CrossRef]
- Trujillo, I.; Rivas, M.; Castrillo, M. Leaf recovery responses during rehydration after water deficit in two bean (Phaseolus vulgaris L.) cultivars. J. Plant Interact. 2013, 8, 360–369. [Google Scholar] [CrossRef]
- White, J.W.; Rojas, C.M. Variation in parameters related to leaf thickness in common bean (Phaseolus vulgaris L.). Field Crop Res. 2005, 91, 7–21. [Google Scholar] [CrossRef]
- Cabeza, R.A.; Liese, R.; Lingner, A.; von Stieglitz, I.; Neumann, J.; Salinas-Riester, G.; Pommerenke, C.; Dittert, K.; Schulze, J. RNA-seq transcriptome profiling reveals that Medicago truncatula nodules acclimate N2 fixation before emerging P deficiency reaches the nodules. J. Exp. Bot. 2014, 65, 6035–6048. [Google Scholar] [CrossRef]
- Mitran, T.; Meena, R.S.; Lal, R.; Layek, J.; Kumar, S.; Datta, R. Role of Soil Phosphorus on Legume Production; Springer: Singapore, 2018; pp. 487–510. [Google Scholar] [CrossRef]
- de Oliveira Maia, S., Jr.; de Andrade, J.R.; dos Santos, C.M.; Silva, J.A.C.; dos Santos, K.P.O.; Silva, J.V.; Endres, L. Leaf thickness and gas exchange are indicators of drought stress tolerance of sugarcane. Emir. J. Food Agric. 2019, 31, 29–38. Available online: https://www.ejfa.me/index.php/journal/article/view/1897 (accessed on 12 July 2023).
- Lima, J.; Mosquim, P.; Da Matta, F. Leaf Gas Exchange and Chlorophyll Fluorescence Parameters in Phaseolus Vulgaris as Affected by Nitrogen and Phosphorus Deficiency. Photosynthetica 1999, 37, 113–121. [Google Scholar] [CrossRef]
- Nielsen, K.L.; Eshel, A.; Lynch, J.P. The effect of phosphorus availability on the carbon economy of contrasting common bean (Phaseolus vulgaris L.) genotypes. J. Exp. Bot. 2001, 52, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Grusak, M.A.; Pomper, K.W. Influence of Pod Stomatal Density and Pod Transpiration on the Calcium Concentration of Snap Bean Pods. J. Am. Soc. Hortic. Sci. 1999, 124, 194–198. [Google Scholar] [CrossRef]
- Polania, J.A.; Chater, C.C.C.; Covarrubias, A.A.; Rao, I.M. Phaseolus Species Responses and Tolerance to Drought; Springer: Singapore, 2020; pp. 319–336. [Google Scholar] [CrossRef]
- Singh, B.; Pandey, R. Differences in Root Exudation Among Phosphorus-Starved Genotypes of Maize and Green Gram and Its Relationship with Phosphorus Uptake. J. Plant Nutr. 2007, 26, 2391–2401. [Google Scholar] [CrossRef]
- Polania, J.; Rao, I.M.; Cajiao, C.; Rivera, M.; Raatz, B.; Beebe, S. Physiological traits associated with drought resistance in Andean and Mesoamerican genotypes of common bean (Phaseolus vulgaris L.). Euphytica 2016, 210, 17–29. [Google Scholar] [CrossRef]
- Polania, J.; Rao, I.M.; Cajiao, C.; Grajales, M.; Rivera, M.; Velasquez, F.; Raatz, B.; Beebe, S.E. Shoot and Root Traits Contribute to Drought Resistance in Recombinant Inbred Lines of MD 23–24 × SEA 5 of Common Bean. Front. Plant Sci. 2017, 8, 296. [Google Scholar] [CrossRef]
- Deresa, S. Response of common bean (Phaseolus vulgaris L.) varieties to rates of blended NPS fertilizer in Adola district, Southern Ethiopia. Afr. J. Plant Sci. 2018, 12, 164–179. Available online: https://academicjournals.org/journal/AJPS/article-abstract/7E10A5758318 (accessed on 12 July 2023).
- Bechtaoui, N.; Rabiu, M.K.; Raklami, A.; Oufdou, K.; Hafidi, M.; Jemo, M. Phosphate-Dependent Regulation of Growth and Stresses Management in Plants. Front. Plant Sci. 2021, 12, 679916. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Sareen, S.; Munjal, R.; Singh, N.; Singh, B.; Verma, R.; Meena, B.; Shoran, J.; Sarial, A.; Singh, S. Genotype × environment interaction and AMMI analysis for heat tolerance in wheat. Cereal Res. Commun. 2012, 40, 267–276. [Google Scholar] [CrossRef]
- Yao, X.; Zhou, H.; Zhu, Q.; Li, C.; Zhang, H.; Wu, J.-J.; Xie, F. Photosynthetic Response of Soybean Leaf to Wide Light-Fluctuation in Maize-Soybean Intercropping System. Front. Plant Sci. 2017, 8, 1695. [Google Scholar] [CrossRef]
- Pérez-Bueno, M.L.; Pineda, M.; Barón, M. Phenotyping Plant Responses to Biotic Stress by Chlorophyll Fluorescence Imaging. Front. Plant Sci. 2019, 10, 1135. [Google Scholar] [CrossRef]
- Horton, P.; Johnson, M.P.; Perez-Bueno, M.L.; Kiss, A.Z.; Ruban, A.V. Photosynthetic acclimation: Does the dynamic structure and macro-organisation of photosystem II in higher plant grana membranes regulate light harvesting states? FEBS J. 2008, 275, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. Available online: https://academic.oup.com/jxb/article/64/13/3983/436509 (accessed on 7 April 2022). [CrossRef]
- Stirbet, A. Govindjee On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Carstensen, A.; Herdean, A.; Schmidt, S.B.; Sharma, A.; Spetea, C.; Pribil, M.; Husted, S. The Impacts of Phosphorus Deficiency on the Photosynthetic Electron Transport Chain. Plant Physiol. 2018, 177, 271–284. [Google Scholar] [CrossRef]
- Pan, C.; Ahammed, G.J.; Li, X.; Shi, K. Elevated CO2 Photosynthesis under high temperature by attenuating the functional limitations to energy fluxes, electron transport and redox homeostasis in tomato leaves. Front. Plant Sci. 2018, 871, 1739. [Google Scholar] [CrossRef]
- Morosinotto, T.; Baronio, R.; Bassi, R. Dynamics of Chromophore Binding to Lhc Proteins in Vivo and in Vitro during Operation of the Xanthophyll Cycle. J. Biol. Chem. 2002, 277, 36913–36920. [Google Scholar] [CrossRef]
- Yang, S.; Meng, D.-Y.; Hou, L.-L.; Li, Y.; Guo, F.; Meng, J.-J.; Wan, S.-B.; Li, X.-G. Peanut violaxanthin de-epoxidase alleviates the sensitivity of PSII photoinhibition to heat and high irradiance stress in transgenic tobacco. Plant Cell Rep. 2015, 34, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, U. Photosynthesis and resource distribution through plant canopies. Plant Cell Environ. 2007, 30, 1052–1071. [Google Scholar] [CrossRef]
- Kanazawa, A.; Ostendorf, E.; Kohzuma, K.; Hoh, D.; Strand, D.D.; Sato-Cruz, M.; Savage, L.; Cruz, J.A.; Fisher, N.; Froehlich, J.E.; et al. Chloroplast ATP Synthase Modulation of the Thylakoid Proton Motive Force: Implications for Photosystem I and Photosystem II Photoprotection. Front. Plant Sci. 2017, 8, 719. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Li, X.-P.; Niyogi, K.K. Non-Photochemical Quenching. A Response to Excess Light Energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Høgh-Jensen, H.; Schjoerring, J.K.; Soussana, J. The Influence of Phosphorus Deficiency on Growth and Nitrogen Fixation of White Clover Plants. Ann. Bot. 2002, 90, 745–753. [Google Scholar] [CrossRef]
- Zhong, Y.; Tian, J.; Li, X.; Liao, H. Cooperative interactions between nitrogen fixation and phosphorus nutrition in legumes. New Phytol. 2023, 237, 734–745. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Change 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, N.; Verma, A.; Singh, H.; Siddique, K.H.M.; Singh, N.P. Novel approaches to mitigate heat stress impacts on crop growth and development. Plant Physiol. Rep. 2020, 25, 627–644. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Hauptvogel, P.; Misheva, S.; Kocheva, K.; Yang, X.; Li, X.; Allakhverdiev, S.I. Wheat plant selection for high yields entailed improvement of leaf anatomical and biochemical traits including tolerance to non-optimal temperature conditions. Photosynth. Res. 2018, 136, 245–255. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. Available online: https://www.mdpi.com/1422-0067/14/5/9643/htm (accessed on 12 July 2023). [CrossRef]
- Yang, D.; Peng, S.; Wang, F. Response of Photosynthesis to High Growth Temperature Was Not Related to Leaf Anatomy Plasticity in Rice (Oryza sativa L.). Front. Plant Sci. 2020, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Vadez, V. Physiological traits for crop yield improvement in low N and P environments. Plant Soil 2002, 245, 1–15. [Google Scholar] [CrossRef]
- Kumar, M.; Swarup, A.; Patra, A.; Chandrakala, J.; Manjaiah, K. Effect of elevated CO2 and temperature on phosphorus efficiency of wheat grown in an Inceptisol of subtropical India. Plant Soil Environ. 2012, 58, 230–235. [Google Scholar] [CrossRef]
- Thomas, J.M.G.; Boote, K.J.; Pan, D.; Allen, L.H.J. Elevated temperature delays onset of reproductive growth and reduces seed growth rate of soybean. J. Agron. Crop Sci. 2010, 1, 19–32. [Google Scholar]
- Beebe, S.E.; Rao, I.M.; Blair, M.W.; Acosta-Gallegos, J.A. Phenotyping common beans for adaptation to drought. Front. Physiol. 2013, 4, 35. [Google Scholar] [CrossRef]
- Ditta, A.; Muhammad, J.; Imtiaz, M.; Mehmood, S.; Qian, Z.; Tu, S. Application of rock phosphate enriched composts increases nodulation, growth and yield of chickpea. Int. J. Recycl. Org. Waste Agric. 2018, 7, 33–40. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; ter Steege, H.; Morgan, H.D.G.; Van der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. Available online: www.publish.csiro.au/journals/ajb (accessed on 17 February 2021).
- Kuhlgert, S.; Austic, G.; Zegarac, R.; Osei-Bonsu, I.; Hoh, D.; Chilvers, M.I.; Roth, M.G.; Bi, K.; TerAvest, D.; Weebadde, P.; et al. MultispeQ Beta: A tool for large-scale plant phenotyping connected to the open PhotosynQ network. R. Soc. Open Sci. 2016, 3, 160592. [Google Scholar] [CrossRef]
- Brooks, M.D.; Niyogi, K.K. Use of a Pulse-Amplitude Modulated Chlorophyll Fluorometer to Study the Efficiency of Photosynthesis in Arabidopsis Plants. In Chloroplast Research in Arabidopsis; Jarvis, R.P., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 775, pp. 299–310. ISBN 978-1-61779-236-6. [Google Scholar]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components—Calculation of qP and Fv-/Fm-; without measuring Fo-. Photosynth. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New Fluorescence Parameters for the Determination of QaRedox State and Excitation Energy Fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Rios, V.S.; Rios, J.A.; Aucique-Pérez, C.E.; Silveira, P.R.; Barros, A.V.; Rodrigues, F. Leaf gas exchange and chlorophyll a fluorescence in soybean leaves infected by Phakopsora pachyrhizi. J. Phytopathol. 2018, 166, 75–85. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.Z.J. Visualization of a Correlation Matrix. R package version 0.73. Statistician 2017, 230, 11–15. Available online: https://peerj.com/articles/9945/Supplemental_Data_S10.pdf (accessed on 14 November 2021).
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. Circlize implements enhances circular visualization in R. Bioinformatics 2014, 30, 2811–2812. Available online: https://academic.oup.com/bioinformatics/article/30/19/2811/2422259 (accessed on 14 November 2021). [CrossRef]
- R Development Core Team. R, Version 4.2.0; Vigorous Calisthenics, Platform: x86_64-apple-darwin17.0 (64-bit); The R Foundation for Statistical Computing: Vienna, Austria, 2023. Available online: https://www.r-project.org (accessed on 22 April 2022).





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez, J.C.; Urban, M.O.; Anzola, J.A.; Contreras, A.T.; Vanegas, J.I.; Beebe, S.E.; Rao, I.M. Influence of Increase in Phosphorus Supply on Agronomic, Phenological, and Physiological Performance of Two Common Bean Breeding Lines Grown in Acidic Soil under High Temperature Stress Conditions. Plants 2023, 12, 3277. https://doi.org/10.3390/plants12183277
Suárez JC, Urban MO, Anzola JA, Contreras AT, Vanegas JI, Beebe SE, Rao IM. Influence of Increase in Phosphorus Supply on Agronomic, Phenological, and Physiological Performance of Two Common Bean Breeding Lines Grown in Acidic Soil under High Temperature Stress Conditions. Plants. 2023; 12(18):3277. https://doi.org/10.3390/plants12183277
Chicago/Turabian StyleSuárez, Juan Carlos, Milan O. Urban, José Alexander Anzola, Amara Tatiana Contreras, José Iván Vanegas, Stephen E. Beebe, and Idupulapati M. Rao. 2023. "Influence of Increase in Phosphorus Supply on Agronomic, Phenological, and Physiological Performance of Two Common Bean Breeding Lines Grown in Acidic Soil under High Temperature Stress Conditions" Plants 12, no. 18: 3277. https://doi.org/10.3390/plants12183277
APA StyleSuárez, J. C., Urban, M. O., Anzola, J. A., Contreras, A. T., Vanegas, J. I., Beebe, S. E., & Rao, I. M. (2023). Influence of Increase in Phosphorus Supply on Agronomic, Phenological, and Physiological Performance of Two Common Bean Breeding Lines Grown in Acidic Soil under High Temperature Stress Conditions. Plants, 12(18), 3277. https://doi.org/10.3390/plants12183277





